Abstract
Context:
Vitamin D is increasingly recognized as an important immunomodulator. Lower levels of 25-hydroxyvitamin D (25[OH]D) and 1,25-dihydroxyvitamin D (1,25[OH]2D) are observed in persons living with HIV.
Objective:
The purpose of this study was to evaluate the relationship of 25(OH)D, and 1,25(OH)2D to HIV viral load, and CD4+ T cells in HIV-infected adults.
Design:
This was a cross-sectional study completed between January 2010 and April 2011.
Setting:
This study was conducted with volunteers who received HIV care in Wisconsin at either a University-based HIV clinic or an urban community HIV clinic.
Patients:
One hundred twelve adults with HIV infection participated in this study.
Main Outcome Measures:
The primary outcome for this study was the relationship between 1,25(OH)2D and HIV viral load. Secondary outcomes included relationships between 25(OH)D and HIV viral load, 25(OH)D and 1,25(OH)2D to CD4+ T cells, and predictors of vitamin D deficiency.
Results:
The 112 volunteers included 24 women and 3 transgender individuals; 68% were from the university clinic, and 32% were from the urban clinic. Mean age was 44.2 years. The mean 25(OH)D level was 22.5 ng/mL; mean 1,25(OH)2D level was 23.5 pg/mL. Twenty-two percent had 25(OH)D ≤10 ng/mL; 53% had values <20 ng/mL, and 73% were ≤30 ng/mL. There was no association between vitamin D and CD4. A nonlinear relationship between viral load and 1,25(OH)2D was found. For 1,25(OH)2D below 32 pg/mL, for each 10 pg/mL decrease in 1,25(OH)2D, (log10) viral load increased by 0.84 (95% CI: 0.16–1.51, P = .015). For 1,25(OH)2D above 32 pg/mL, for each 10 pg/mL increase in 1,25(OH)2D, (log10) viral load increased by 0.36 (95% CI: 0.15–0.57, P = .0009).
Conclusion:
Vitamin D deficiency was common in this HIV population, as seen in other HIV cohorts. A novel, U-shaped relationship between 1,25(OH)2D and viral load, with the lowest and highest 1,25(OH)2D levels seen with high viral loads, was found and deserves further study.
Vitamin D plays a well-known, central role in calcium, phosphorous, and bone metabolism and is well described as an immunomodulator (1–3). Vitamin D receptors can be found on multiple cells of the immune system, including T and B lymphocytes, monocytes, and dendritic cells (3–7) Activated immune cells locally covert vitamin D to its active form, 1,25-dihydroxyvitamin D (1,25[OH]2D), via the activating enzyme 25-hydroxyvitamin D-1-α-hydroxylase (8). Emerging research highlights its potential role in aiding the human immune system with serious infections, including HIV (2, 9–11). Activated monocytes and macrophages produce the 1,25(OH)2D-dependent antimicrobial peptide, cathelicidin (9). Recent work defines the central role of cathelicidin in the immune response to tuberculosis, and possibly other viral infections, including HIV, and in vitro studies demonstrate cathelicidin's ability to inhibit HIV replication in CD4+ T cells and macrophages (9, 10, 12–14).
Vitamin D deficiency refers to a low serum 25-hydroxyvitamin D (25[OH]D) concentration, whereas levels of 1,25(OH)2D are usually maintained at normal or elevated levels in the face of low 25(OH)D status (15–17). The cut points chosen to define deficiency or inadequacy are controversial (15, 16). Factors that likely contribute to low vitamin D in the body include season and latitude, skin color, and sun exposure and are complicated by polymorphisms in the vitamin D receptor and variations in vitamin D binding protein (1, 8, 18). Vitamin D deficiency is widespread among those living with HIV/AIDS (19–24) and recent reports associate low 25(OH)D levels with more rapid progression of disease, higher all-cause mortality, and increased mother-to-child transmission of HIV (24, 25). Decreased serum concentrations of 1,25(OH)2D are usually only seen with chronic renal disease or in rare conditions involving mutations in the converting enzyme (17). However, past studies find low levels of 1,25(OH)2D among HIV cohorts, including an associated increase in mortality, and a positive correlation between levels of 1,25(OH)2D and CD4+ T cells, the primary targets for HIV infection (21–23). Decreased levels of 1,25(OH)2D in the setting of HIV infection may be related to increased levels of TNF-α or other inflammatory mediators, decreasing the conversion of 25(OH)D to 1,25(OH)2D via 1-α-hydroxylase, effects of antiretroviral therapy (ART), and possibly increased utilization (2, 26, 27).
There is little known about the relationship between 25(OH)D and 1,25(OH)2D levels and HIV viral load. We hypothesized that low levels of 1,25(OH)2D and/or 25(OH)D would be associated with a lack of virologic suppression, as evidenced by elevated HIV viral loads. To evaluate this hypothesis, we undertook a cross-sectional study among HIV/AIDS patients receiving care at 2 sites, a university-based HIV clinic in Madison, Wisconsin, and a community-based, inner city HIV clinic in Milwaukee, Wisconsin.
Materials and Methods
We conducted a cross-sectional, observational study among adults living with HIV. The study was reviewed and approved by the University of Wisconsin Health Sciences Internal Review Board. From each volunteer, we obtained informed consent, followed by a single blood draw for 25(OH)D and 1,25(OH)2D. Electronic medical records were reviewed for current CD4 cell count (absolute number and percentage) and HIV viral load, which are the standard immunologic and virologic measures used for clinically monitoring patients with HIV infection, as well as descriptive data, and factors hypothesized as potential predictors of low vitamin D metabolite levels or confounders in the relationship between vitamin D and HIV viral load. These factors included age, gender, race, ethnicity, AIDS diagnosis, duration of HIV infection, ART, diabetes, hepatitis C, renal disease (defined as glomerular filtration rate <60 or known renal disease), mental health diagnosis, substance abuse, diagnosis of osteopenia or osteoporosis, documented vitamin D supplementation, and type of insurance. Data on weight and height were not available for all volunteers and therefore were not included.
Settings
Volunteers were recruited from 2 sites. The university-based clinic cares for approximately 600 nonincarcerated adults living with HIV/AIDS, 20% of which are women and 20% of which receive care through the federally funded Title III Ryan White Grant. The second site was a community-based HIV clinic, housed in a federal funding–qualified community health center in Milwaukee, Wisconsin and serves an urban population of adults living with HIV/AIDS, most of which receive care through federally and or state-funded programs. The clinic serves approximately 180 patients: 90% are African-American and 25% of the patients are women.
All nonpregnant, nonincarcerated adults over the age of 18, able to give informed consent, living with HIV/AIDS, and receiving care at one of these clinics were eligible for the study. Recruitment took place at the university clinic between January 2010 and April 2011 and at the community clinic between January 2011 and April 2011, and data collection continued through October 2011. The sample size of 112 subjects was chosen to provide 80% power to detect a correlation of 0.268 between 1,25(OH)2D level and HIV viral load using a 2-sided 5% level test.
Study procedures included a single blood draw, which was usually done in conjunction with routine HIV monitoring laboratory tests, and a self-administered questionnaire regarding vitamin D dietary intake and UV light exposure. All 25(OH)D levels were determined using HPLC (University of Wisconsin Hospital and Clinics Clinical Laboratory, Madison, Wisconsin) and all 1,25(OH)2D levels were analyzed by quantitative RIA (ARUP Laboratories, Salt Lake City, Utah).
Statistical analysis
Participant characteristics were compared between clinics using 2 sample t tests for continuous variables and χ2 tests for categorical variables (Table 1). For the purpose of this study, 25(OH)D levels were defined as sufficient (≥30 ng/mL, 75 nmol/L), insufficient (21–29 ng/mL, 52.5–72.5 nmol/L), inadequate (≤20 ng/mL, 50 nmol/L), and deficient (≤10 ng/mL, 25 nmol/L).
Table 1.
Descriptive Characteristics of Study Volunteers: Total Study Population and Results Based on Enrollment Site
| Total Study Population (n = 112) |
University Clinic (n = 76) |
Community Clinic (n = 36) |
P Value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Demographics | |||||||
| Season | <.0001 | ||||||
| Winter | 67 | 59.8 | 32 | 42.1 | 35 | 97.2 | |
| Spring | 22 | 19.6 | 21 | 27.6 | 1 | 2.8 | |
| Summer | 10 | 8.9 | 10 | 13.2 | 0 | 0.0 | |
| Fall | 13 | 11.6 | 13 | 17.1 | 0 | 0.0 | |
| Age, mean/SD | 44.2 | 11.1 | 44.7 | 10.7 | 43.2 | 12.1 | .51 |
| Sex | .10 | ||||||
| Female | 24 | 21.4 | 12 | 15.8 | 12 | 33.3 | |
| Male | 85 | 75.9 | 62 | 81.6 | 23 | 63.9 | |
| Transgender | 3 | 2.7 | 2 | 2.6 | 1 | 2.8 | |
| Race | <.0001 | ||||||
| White or Asian | 68 | 60.7 | 67 | 88.2 | 1 | 2.8 | |
| Black | 44 | 39.3 | 9 | 11.8 | 35 | 97.2 | |
| Ethnicity | |||||||
| Hispanic | 8 | 7.1 | 7 | 9.2 | 1 | 2.8 | .22 |
| Comorbidities | |||||||
| AIDS | 54 | 48.2 | 40 | 52.6 | 14 | 38.9 | .17 |
| Diabetes | 11 | 9.8 | 4 | 5.3 | 7 | 19.4 | .02 |
| Hepatitis C | 8 | 7.1 | 2 | 2.6 | 6 | 16.7 | .007 |
| Renal disease | 5 | 4.5 | 2 | 2.6 | 3 | 8.3 | .17 |
| Mental health disease | 48 | 42.9 | 27 | 35.5 | 21 | 58.3 | .02 |
| Substance abuse | 27 | 24.1 | 5 | 6.6 | 22 | 61.1 | <.0001 |
| Osteoporosis | 7 | 6.3 | 7 | 9.2 | 0 | 0.0 | .06 |
| Treatment-related factors | |||||||
| Current ARV, Y/N | 91 | 81.3 | 63 | 82.9 | 28 | 77.8 | .52 |
| Duration of HIV, y (mean/SD) | 10.8 | 7.6 | 10.9 | 7.8 | 10.6 | 7.4 | .83 |
| Insurance | <.0001 | ||||||
| Public | 57 | 50.9 | 28 | 36.8 | 29 | 80.6 | |
| Private or Medicare | 55 | 49.1 | 48 | 63.2 | 7 | 19.4 | |
HIV viral load, CD4+ T cell absolute number, and CD4% were modeled as smooth, nonlinear functions of vitamin D levels using generalized additive models (28). The following variables were evaluated as potential confounders of these relationships: age, season, race, clinic, sex, insurance, Hispanic ethnicity, duration of HIV, diabetes, hepatitis C, renal disease, mental health, substance abuse, osteoporosis, antiretrovirus, and current use of vitamin D supplements. Stepwise variable selection based on Aikaike Information Criterion (AIC) was used to identify potential confounders associated with vitamin D levels first (29). From this reduced list of potential confounders, a final list of confounders for each outcome (viral load, absolute CD4+ T-cell count, and CD4%) was then identified using stepwise selection based on AIC. These variables were then included as adjustment variables in the final models for viral load, CD4+ T cell, and CD4%. Change point models were used to characterize significant nonlinear relationships of vitamin D levels with these outcomes (30).
A nominal P value of .05 was regarded as statistically significant. Analyses were performed in R version 2.13.1 (31) (R Foundation for Statistical Computing, Vienna, Austria). Generalized additive models were fit using the mgcv package (32).
Results
Volunteers
Seventy-six volunteers were recruited from the university clinic (68%) and 36 volunteers were recruited from the urban community clinic (32%) for a total of 112 patients enrolled between January 2010 and April 2011. Demographic and descriptive factors of the volunteers are listed in Table 1, and CD4, HIV viral load, and vitamin D metabolite values are summarized in Table 2. The volunteers were 76% men, and 81% were taking ART at the time of the study. Compared with the university clinic, the urban community clinic volunteers had more comorbid disease (diabetes and hepatitis C), a greater percentage of volunteers with mental health disease and substance abuse, and a higher percentage of participants receiving federal or state subsidized insurance coverage based on need (not including Medicare recipients). African-American volunteers made up approximately 39% of the total study population; however, the majority came from the urban community clinic, whose participants were 97% African-American.
Table 2.
Results: Mean Values for CD4, CD4%, Viral Load, and Vitamin D Levels
| Total Study Population (n = 112) |
University Clinic (n = 76) |
Community Clinic (n = 36) |
P Value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| CD4, cells/uL | 454.2 | 306.7 | 488.0 | 315.0 | 382.9 | 279.5 | .09 |
| CD4, % | 23.3 | 11.8 | 25.9 | 12.0 | 17.8 | 9.4 | .0005 |
| HIV viral load, log10 | 2.79 | 1.4 | 1.59 | 2.54 | 2.72 | 1.70 | .08 |
| 25(OH)D, ng/mL | 22.5 | 14.3 | 28.1 | 13.3 | 10.2 | 7.4 | <.0001 |
| 1,25(OH)2D, pg/mL | 42.6 | 21.8 | 46.7 | 22.1 | 33.8 | 18.9 | .003 |
Vitamin D levels
The average 25(OH)D level was 22.5 ng/mL (SD 14.3 ng/mL) among all participants. The mean 1,25(OH)2D level was 23.5 pg/mL (SD 21.8 pg/mL) (Table 2). Of volunteers, 52.7% had inadequate vitamin D with 25(OH)D of 20 ng/mL or less, and 22% of all participants had 25(OH)D ≤10 ng/mL, representing deficiency. Vitamin D insufficiency (25[OH]D of 21–29 ng/mL) was seen in 20.5% of volunteers, whereas 26.8% had sufficient vitamin D levels (≥30 ng/mL). From the community clinic, 58% of volunteers were deficient (25[OH]D levels ≤10 ng/mL), whereas only 5.3% of volunteers from the university clinic had values ≤10 ng/mL (Figure 1). Community clinic participants had 25(OH)D and 1,25(OH)2D levels 17 ng/mL and 13 pg/mL lower than those from the university clinic (P < .0001 and <0.0034, respectively). Overall, 25(OH)D and 1,25(OH)2D levels were highly correlated (CORR 0.49, P < .0001). Stepwise selection using AIC identified age, season, race, clinic (university-based or community-based inner city), Hispanic ethnicity, renal disease, osteoporosis, and current use of ART as correlates of 25(OH)D, and season as a correlate of 1,25(OH)2D.
Figure 1.
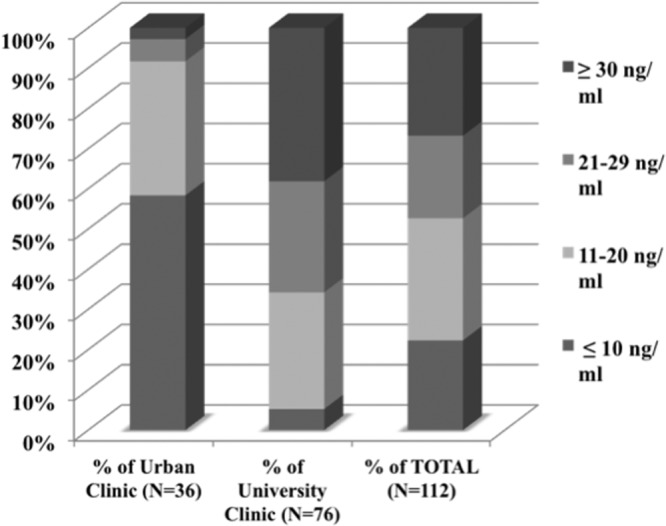
25-Hydroxyvitamin D levels (ng/mL). Illustrating the percentage of volunteers with levels less than or equal to 10 ng/mL, 11–20 ng/mL, 21–29 ng/mL, and 30 ng/mL or more, at each site and for the entire study population.
Vitamin D levels and CD4+ T cells and CD4%
From the above correlates of 25(OH)D and 1,25(OH)2D, stepwise variable selection identified season, clinic, osteoporosis, and Hispanic ethnicity as predictors of absolute number of CD4+ T cells or CD4%. Adjusting for these factors, there was no association between CD4 count and 25(OH)D (difference in mean CD4 count per 10 ng/mL increase in 25[OH]D: 8.4, 95% CI −41.1, 57.9, P = .74); nor between CD4 and 1,25(OH)2D (difference in mean CD4 count per 10 pg/mL increase in 1,25[OH]2D: 2.3, 95% CI −25.3, 30.1, P = .87); CD4% and 25(OH)D (difference in mean CD4% per 10 ng/mL increase in 25[OH]D: 0.6, 95% CI −1.2, 2.4, P = .53); nor CD4% and 1,25 (OH)2D (difference in mean CD4% per 10 pg/mL increase in 1,25[OH]2D: 0.3, 95% CI −0.7, 1.3, P = .56). There was no evidence for nonlinear relationships between CD4 cell counts (absolute and percentage) and 25(OH)D nor 1,25(OH)2D (P = .99, P = .39, P = .88, P = .09, respectively).
Vitamin D levels and viral load
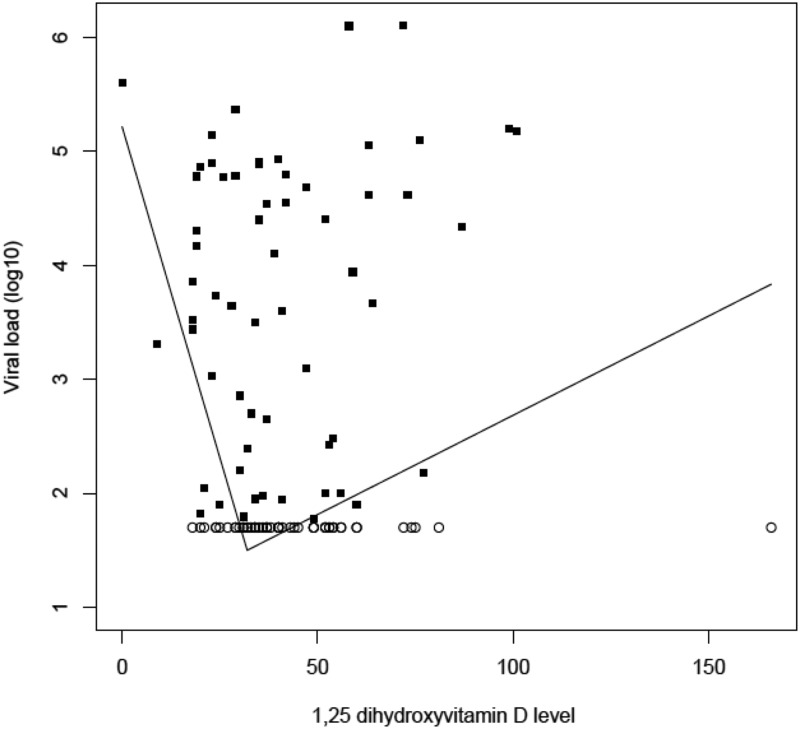
From the correlates of 25(OH)D and 1,25(OH)2D, stepwise variable selection identified age, season, clinic, and use of ART as predictors of viral load. Adjusting for these factors, there was strong evidence for a nonlinear relationship between 1,25(OH)2D and viral load (log-transformed) (P = .009). Follow-up analyses identified a change point of 32 pg/mL. For 1,25(OH)2D below 32 pg/mL, each 10 pg/mL decrease in 1,25(OH)2D, (log10) viral load increased by 0.84 (95% CI: 0.16–1.51, P = .015). For 1,25(OH)2D above 32 pg/mL, for each 10 pg/mL increase in 1,25(OH)2D, (log10) viral load increased by 0.36 (95% CI: 0.15–0.57, P = .0009). The estimated relationship between 1,25(OH)2D levels to log-transformed viral load is illustrated in Figure 2.
Figure 2.
Nonlinear relationship of 1,25-dihydroxyvitamin D (pg/mL) and viral load (log10): high viral loads seen with the lowest and highest 1,25-dihydroxyvitamin D levels, with a change point identified at 32 pg/mL. Open circles, viral load values equal to the lower limit of detection, 1.7 (log10); closed squares, exact values.
Similar results were seen in both clinics. At the university-based clinic, for each 10 pg/mL decrease in 1,25(OH)2D below 32 pg/mL, the (log10) viral load increased by 0.79 (95% CI: 0.33–2.12 P = .016). For each 10 pg/mL increase in 1,25(OH)2D above 32 pg/mL, the (log10) viral load increased by 0.35 (95% CI: 0.09–0.60, P = .009). At the inner city clinic, for each 10 pg/mL decrease in 1,25(OH)2D below 32 pg/mL, the (log10) viral load increased by 0.82 (95% CI: 0.25–1.40, P = .005). For each 10 pg/mL increase in 1,25(OH)2D above 32 pg/mL, the (log10) viral load increased by 0.42 (95% CI: 0.13–0.70, P = .004).
The analysis was repeated separately for African-American and non-African-American volunteers to account for race (skin color) as a possible confounder. Within the African-American study population, for each 10 pg/mL decrease in 1,25(OH)2D below 32 pg/mL, the (log10) viral load increased by 0.65 (95% CI: 0.05–1.24, P = .033). For each 10 pg/mL increase in 1,25(OH)2D above 32 pg/mL, the (log10) viral load increased by 0.34 (95% CI: 0.04–0.63, P = .027). In non-African-Americans, for each 10 pg/mL decrease in 1,25(OH)2D below 32 pg/mL, the (log10) viral load increased by 1.96 (95% CI: 1.37–2.56, P = .016). For each 10 pg/mL increase in 1,25(OH)2D above 32 pg/mL, the (log10) viral load increased by 0.40 (95% CI: 0.11–0.69, P = .006).
A nonsignificant decrease in viral load was associated with increasing 25(OH)D levels (difference in mean [log10] viral load per 10 point increase in 25[OH]D: −0.16, 95% CI −0.51, 0.19 P = .36). There was no evidence for a nonlinear relationship (P = .70).
Vitamin D levels and antiretroviral therapy
Multivariate analysis revealed that the following antiretroviral agents predicted vitamin D levels (25[OH]D, 1,25[OH]2D, or both): efavirenz, emtricitabine, tenofovir, darunavir, ritonavir, atazanavir, lamivudine, etravirine, and lopinavir. When treated as a dichotomous variable, any use of current ART predicted 25(OH)D levels and viral loads, but not 1,25(OH)2D levels. Use of vitamin D supplementation was not predictive of 25(OH)D, 1,25(OH)2D, or other outcomes.
Discussion
Our study found vitamin D (25[OH]D) inadequacy (≤20 ng/mL) in 53% and deficiency (≤10 ng/mL) in 22% of this mixed population of HIV/AIDS ambulatory patients. Applying the often recommended cut point of 30 ng/mL, 73.2% of volunteers in our study had vitamin D insufficiency. Our results are consistent with the recent reports in the literature of HIV cohorts, which find vitamin deficiency rates between 60% and 90% (19, 20, 24).
The high degree of inadequate vitamin D levels among African-Americans in our study is consistent with the general population as 97% of non-Hispanic blacks are estimated to have vitamin D insufficiency or deficiency (33). The final model for relating 1,25(OH)2D levels and viral load did not include race, as this was not found to predict viral load on its own. However, the enrollment site (clinic) was found to predict viral load and was in the final model. Milwaukee's community clinic volunteers were overwhelmingly African-American (97%). Therefore, race was likely accounted for with the inclusion of this variable. To be certain, we ran the model separately for each clinic and for African-American versus non-African-American volunteers and the results did not change dramatically. However, the relationship between 1,25(OH)2D and HIV viral load appeared to be stronger for non-African-Americans (for each 10 pg/mL below 32 pg/mL, log 10 viral load increased by 0.65 in African-Americans vs 1.96 in non-African-Americans). One explanation for this difference is a possible decreased responsiveness to 1,25(OH)2D in African-Americans, leading to a less potent antiviral or supportive immunomodulatory role for vitamin D in this population. Further large studies are needed to clarify this potential difference.
Various antiretrovirals are now well known to interact with vitamin D metabolism (26, 27). Our study found multiple antiretrovirals to be correlates of vitamin D levels. However, we hesitate to make any robust conclusions given the small sample size and the fact that the agents were analyzed independently of one another rather than accounting for coformulations and coadministration. Current ART (any) predicted 25(OH)D but not 1,25(OH)2D, the active form of vitamin D.
Although recent publications have linked lower 25(OH)D to lower CD4+ T cell counts and higher viral loads (34), as well as increased morbidity and mortality, the mechanisms behind these relationships remain unclear. The role of 1,25(OH)2D, the active form of vitamin D, as an immune modulator acting at the level of the immune cell is now well described (8). Emerging research highlights vitamin D's role in the human immune response to HIV. Recent work describes inhibition of HIV replication in macrophages via intracrine conversion of vitamin D to its active form (1,25[OH]2D), activation of the antimicrobial peptide cathelicidin, and induction of autophagy (14). We found an inverse relationship of low 1,25(OH)2D levels associated with high viral loads. We anticipated 1,25(OH)2D levels to be low in the face of high viral loads as, perhaps, the hormone is depleted in response to virus triggering 1,25(OH)2D-dependent immune defense mechanisms. Alternatively, the low levels could be related to an inability to covert 25(OH)D to its active form in response to HIV due to extremely low 25(OH)D levels (≤4 ng/mL) (35). However, only 4 of the 112 volunteers had levels 25(OH)D levels ≤4 ng/mL, making this an unlikely explanation. Finally, renal conversion of 25(OH)D to 1,25(OH)2D may be inhibited by the increased levels of TNF-α associated with HIV viremia, indirectly linking viral load to 1,25(OH)2D levels (23, 36–39).
Surprisingly, increasing 1,25(OH)2D levels were associated with higher viral loads as well, creating a U-shaped association with a change point of 32 pg/mL (Figure 2). It is unclear exactly why 1,25(OH)2D levels should be high in the setting of high amounts of detectable virus. The U-shaped curve found in this study is likely reflective of a complex role for vitamin D in HIV infection, which is not yet well understood and deserves further study.
One possible explanation for the association of increasing 1,25(OH)2D levels with higher HIV viral loads relates to immune activation. With increasing HIV viremia, systemic activation of the immune system, including macrophages, is expected (40). Liu and colleagues' recent work demonstrates increased expression of 25-hydroxyvitamin D-1-α-hydroxylase in activated macrophages, resulting in local conversion of 25(OH)D to 1,25(OH)2D and subsequent induction of cathelicidin (9). Therefore, higher HIV viral loads and the related immune activation could lead to greater conversion of vitamin D to its active form, increasing levels of 1,25(OH)2D. Theoretically, stores would eventually be depleted, contributing to the low 25(OH)D and 1,25(OH)2D levels common in HIV patients, explaining the negative association found between low 1,25(OH)2D levels and high viral load, and thus creating a U-shaped curve.
An alternative explanation for the association between increasing 1,25(OH)2D levels and HIV viral load is that of a possible positive effect of 1,25(OH)2D on HIV replication. Older in vitro studies demonstrated increased replication of HIV in promonocytic cell lines in the presence of 1,25(OH)2D, possibly related to the maturation effects of 1,25(OH)2D on monocytes and influence of TNF-α (2, 41, 42). However, these results were not consistent among studies and recent work strengthens the argument for an antiviral role for vitamin D in HIV infection (2, 10, 14, 43).
We found 25(OH)D levels and HIV viral load to be only weakly associated and not statistically significant (P = .36), consistent with the understanding that 25(OH)D metabolism in HIV infection is complex. Factors beyond viral load, and those factors accounted for in our model, likely influence 25(OH)D levels as well. For example, we were unable to include measures for obesity in our analysis due to lack of available data, and obesity could be an important confounder. Additionally, individual antiretroviral agents were not included in the final multivariate model, namely efavirenz, which is associated with 25(OH)D deficiency in HIV populations (27, 36, 44). Finally, having an undetectable HIV viral load was associated with a lower odds ratio of vitamin D deficiency in a recent large, cohort study (34). Therefore, it is possible a larger sample size is needed to demonstrate a significant relationship between HIV viral load and 25(OH)D.
Our study had several limitations. First, the enrollment occurred across seasons and, although this was accounted for in the analysis, an ideal study would be done in a tighter timeframe to minimize the effect of season on vitamin D levels. Body mass index, found as a risk factor for low vitamin D status in HIV patients, was not accounted for in the analysis because weight and height measurements were not consistently recorded in the electronic medical records (20).
The volunteers for this study were recruited from 2 distinct and different populations. Notably, the Milwaukee clinic had 25(OH)D and 1,25(OH)2D levels 17 ng/mL and 13 pg/mL lower than those from the university clinic (P < .0001 and <.0034, respectively) and 58% of the volunteers from that site had 25(OH)D levels ≤10 ng/mL. The reason for 2 sites was to enhance the diversity of the study population and meet recruitment goals. We attempted to identify any potential confounding due to clinic in the analysis by including it in the models and analyzing the clinic populations separately as well as analyzing based on African-American vs non-African-American, given the community clinic was 97% African-American.
Finally, our study was small and cross-sectional and included only a single blood draw, thus limiting the ability to draw robust conclusions from the findings. The small sample size likely contributed to the wide confidence intervals found. A larger, prospective study evaluating 1,25(OH)2D levels and viral loads at multiple time points and studies evaluating the effect of vitamin D supplementation on viral load would help to understand better the complex and important role vitamin D likely plays in HIV infection.
Acknowledgments
We acknowledge the dedication of the research team who helped complete this study: Lotoyus Hines, RN; Dawn Boh, RN; Sandy Olsen, MS; Jim Stahl, PhD; and Ellen Cook, PhD.
Current affiliation for A.B.: University of Southern California, Keck School of Medicine–Los Angeles, California.
This work was supported by The Wisconsin Women's Health Foundation (PI: Safdar), and by Grant 1UL1RR025011 from the Clinical and Translational Science Award program, previously through the National Institutes of Health National Center for Research Resources, and now by the National Institutes of Health National Center for Advancing Translational Sciences, Grant 9U54TR000021. Additional support through the University of Wisconsin's Center for Women's Health Research and the Veteran's Affairs Women's Health Fellowship.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- AIC
- Aikaike Information Criterion
- ART
- antiretroviral therapy
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 25(OH)D
- 25-hydroxyvitamin D.
References
- 1. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 2. Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64:226–233 [DOI] [PubMed] [Google Scholar]
- 3. Hayes CE, Nashold FE, Spach KM, Pedersen LB. The immunological functions of the vitamin D endocrine system. Cell Mol Biol (Noisy-le-grand). 2003;49:277–300 [PubMed] [Google Scholar]
- 4. Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–1310 [DOI] [PubMed] [Google Scholar]
- 5. Brennan A, Katz DR, Nunn JD, et al. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457–461 [PMC free article] [PubMed] [Google Scholar]
- 6. Morgan JW, Morgan DM, Lasky SR, Ford D, Kouttab N, Maizel AL. Requirements for induction of vitamin D-mediated gene regulation in normal human B lymphocytes. J Immunol. 1996;157:2900–2908 [PubMed] [Google Scholar]
- 7. Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183 [DOI] [PubMed] [Google Scholar]
- 8. Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012;76:315–325 [DOI] [PubMed] [Google Scholar]
- 9. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773 [DOI] [PubMed] [Google Scholar]
- 10. Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5:410–415 [DOI] [PubMed] [Google Scholar]
- 13. Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–7198 [DOI] [PubMed] [Google Scholar]
- 14. Campbell GR, Spector SA. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem. 2011;286:18890–18902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenbaum LA. Rickets and hypervitaminosis D. Nelson Textbook of Pediatrics. 19th ed Philadelphia: Elsevier; 2007:200–209.e201 [Google Scholar]
- 18. Binkley N, Krueger D, Drezner MK. Low vitamin D status: time to recognize and correct a Wisconsin epidemic. WMJ. 2007;106:466–472 [PubMed] [Google Scholar]
- 19. Mueller NJ, Fux CA, Ledergerber B, et al. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS. 2010;24:1127–1134 [DOI] [PubMed] [Google Scholar]
- 20. Dao CN, Patel P, Overton ET, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405 [DOI] [PubMed] [Google Scholar]
- 21. Teichmann J, Stephan E, Lange U, et al. Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. J Infect. 2003;46:221–227 [DOI] [PubMed] [Google Scholar]
- 22. Haug C, Muller F, Aukrust P, Froland SS. Subnormal serum concentration of 1,25-vitamin D in human immunodeficiency virus infection: correlation with degree of immune deficiency and survival. J Infect Dis. 1994;169:889–893 [DOI] [PubMed] [Google Scholar]
- 23. Haug CJ, Aukrust P, Haug E, Morkrid L, Muller F, Froland SS. Severe deficiency of 1,25-dihydroxyvitamin D3 in human immunodeficiency virus infection: association with immunological hyperactivity and only minor changes in calcium homeostasis. J Clin Endocrinol Metab. 1998;83:3832–3838 [DOI] [PubMed] [Google Scholar]
- 24. Viard JP, Souberbielle JC, Kirk O, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25:1305–1315 [DOI] [PubMed] [Google Scholar]
- 25. Mehta S, Hunter DJ, Mugusi FM, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200:1022–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17:513–520 [DOI] [PubMed] [Google Scholar]
- 27. Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15:425–429 [DOI] [PubMed] [Google Scholar]
- 28. Hastie TJ. Generalized additive models. In: Chambers JM, Hastie T, eds. Statistical Models in S. Pacific Grove, CA: Wadsworth & Brooks/Cole; 1992:249–307 [Google Scholar]
- 29. Akaike H. A new look at the statistical model identification. IEEE Trans Autom Contr. 1974;19:716–723 [Google Scholar]
- 30. Bacon DW, Watts DG. Estimating the transition between two intersecting straight lines. Biometrika. 1971;58:525–534 [Google Scholar]
- 31. R Development Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011 [Google Scholar]
- 32. Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc (B). 2011;73:3–36 [Google Scholar]
- 33. Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adeyemi OM, Agniel D, French AL, et al. Vitamin D deficiency in HIV-infected and HIV-uninfected women in the United States. J Acquir Immune Defic Syndr. 2011;57:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Need AG, O'Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BEC. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res. 2008;23:1859–1863 [DOI] [PubMed] [Google Scholar]
- 36. Lake JE, Adams JS. Vitamin D in HIV-infected patients. Curr HIV/AIDS Rep. 2011;8:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hanevold CD, Yamaguchi DT, Jordan SC. Tumor necrosis factor alpha modulates parathyroid hormone action in UMR-106-01 osteoblastic cells. J Bone Miner Res. 1993;8:1191–1200 [DOI] [PubMed] [Google Scholar]
- 38. Norris PJ, Pappalardo BL, Custer B, Spotts G, Hecht FM, Busch MP. Elevations in IL-10, TNF-α, and IFN-γ from the earliest point of HIV type 1 infection. AIDS Res Human Retroviruses. 2006;22:757–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vyakarnam A, McKeating J, Meager A. Tumour necrosis factors (alpha, beta) induced by HIV-1 in peripheral blood mononuclear cells potentiate virus replication. AIDS. 1990;4:21–27 [DOI] [PubMed] [Google Scholar]
- 40. Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–147 [DOI] [PubMed] [Google Scholar]
- 41. Locardi C, Petrini C, Boccoli G, et al. Increased human immunodeficiency virus (HIV) expression in chronically infected U937 cells upon in vitro differentiation by hydroxyvitamin D3: roles of interferon and tumor necrosis factor in regulation of HIV production. J Virol. 1990;64:5874–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pauza CD, Kornbluth R, Emau P, Richman DD, Deftos LJ. Vitamin D3 compounds regulate human immunodeficiency virus type 1 replication in U937 monoblastoid cells and in monocyte-derived macrophages. J Leukoc Biol. 1993;53:157–164 [DOI] [PubMed] [Google Scholar]
- 43. Goletti D, Kinter AL, Biswas P, Bende SM, Poli G, Fauci AS. Effect of cellular differentiation on cytokine-induced expression of human immunodeficiency virus in chronically infected promonocytic cells: dissociation of cellular differentiation and viral expression. J Virol. 1995;69:2540–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gyllensten K, Josephson F, Lidman K, Sääf M. Severe vitamin D deficiency diagnosed after introduction of antiretroviral therapy including efavirenz in a patient living at latitude 59 degrees N. AIDS. 2006;20:1906–1907 [DOI] [PubMed] [Google Scholar]