Abstract
Context:
Type 2 diabetes (T2D) has features of disordered lipid and glucose metabolism, due in part to reduced mitochondrial content.
Objective:
Our objective was to investigate effects of different types of exercise on mitochondrial content and substrate oxidation in individuals with T2D (ancillary study of the randomized controlled trial Health Benefits of Aerobic and Resistance Training in Individuals with Type 2 Diabetes, HART-D).
Intervention:
T2D individuals were randomized to aerobic training (AT, n = 12), resistance training (RT, n = 18), combination training (ATRT, n = 12), or nonexercise control (n = 10). Blood draws, peak oxygen consumption tests, dual-energy x-ray absorptiometry scans and muscle biopsies of vastus lateralis were performed before and after 9 months. Ex vivo substrate oxidations (14CO2), mitochondrial content, and enzyme activities were measured. Glycated hemoglobin A1c and free fatty acids were also determined.
Results:
Mitochondrial content increased after RT and ATRT. Octanoate oxidation increased after AT and ATRT, whereas palmitate, pyruvate, and acetate oxidations increased in all exercise groups. Exercise-induced responses in mitochondrial DNA were associated with improvements in peak oxygen consumption, β-hydroxyacyl-coenzyme A dehydrogenase activity, and palmitate oxidation.
Conclusions:
Nine months of AT and RT significantly improved most aspects of skeletal muscle mitochondrial content and substrate oxidation, whereas the combination improved all aspects. These exercise responses were associated with clinical improvements, indicating that long-term training, especially combination, is an effective lifestyle therapy for individuals with T2D by way of improving muscle substrate metabolism.
Increased sedentary behavior, coupled with early onset of obesity and type 2 diabetes (T2D), has popularized exercise interventions as both an investigative tool of the health benefits associated with physical activity and as a feasible lifestyle modification. Physical inactivity and obesity, rather than age, are the true culprits of insulin resistance (1–3). We recently demonstrated that 9 months of combined aerobic training (AT) and resistance training (RT) reduced glycated hemoglobin A1c (HbA1c) levels to a greater degree compared with either AT or RT alone in a population of men and women with T2D (4). However, the underlying mechanism was not investigated. Given the impact of exercise training on muscle metabolism, we here investigated whether adaptations in muscle could underlie these differences observed in the parent study Health Benefits of Aerobic and Resistance Training in Individuals with Type 2 Diabetes (HART-D) (4).
It is suggested that individuals with T2D have reduced mitochondrial content within their skeletal muscle, accompanied by an abnormal morphology and impaired bioenergetic capacity (5, 6). Skeletal muscle homogenates of obese women exhibit blunted fatty acid oxidation compared with their lean counterparts (7) and ex vivo skeletal muscle fatty acid oxidation is elevated in trained individuals (8). Therefore, interventions aimed at increasing skeletal muscle mitochondrial content and tissue oxidative capacity are critical to improvements of the metabolic perturbations observed in obesity and T2D.
Contemporary studies into prolonged exercise interventions have made significant contributions to this area of research. Toledo and colleagues (9) revealed that 16 weeks of AT, with concomitant weight loss, in sedentary overweight/obese individuals greatly improved aerobic capacity, mitochondrial content, and electron transport chain activity. Moreover, a mere 10 days of AT significantly increased skeletal muscle fatty acid oxidation in obese women (10). In general, RT increases muscle cross-sectional area (∼10%) (11) and stimulates mitochondrial biogenesis (12) in elderly adults. Pesta and colleagues (13) elegantly demonstrated that 10 weeks of RT enhanced mitochondrial respiration to the same extent as AT in skeletal muscle fibers of lean, previously sedentary adults.
Most of these studies are performed in lean and obese, but otherwise healthy, individuals. Therefore, we here investigated the effects of AT and RT alone, and in combination, on mitochondrial content and substrate oxidation in the skeletal muscle of individuals with T2D in a well-controlled study of longer duration (ie, >6 months). We can then use that knowledge to further treat (and possibly prevent) T2D more effectively with feasible lifestyle modifications.
We hypothesized that 9 months of combined exercise would be superior to either AT or RT alone in enhancing skeletal muscle substrate oxidation in a subpopulation of men and women with T2D from the aforementioned parent study HART-D (4). We answered this question by assessing mitochondrial content as well as the oxidation to 14CO2 of 14C-labeled medium-chain (octanoate) and long-chain (palmitate) fatty acids, as well as β-oxidation- and glycolytic-derived substrates (pyruvate and acetate), in skeletal muscle homogenates. We then associated these exercise responses of the skeletal muscle with measures of aerobic fitness [peak oxygen consumption (VO2peak)] and glucose control (HbA1c). These data provide insight into the clinical efficacy of long-term AT, RT, or combined training on resting skeletal muscle substrate oxidation in individuals with T2D.
Subjects and Methods
Participants and intervention
Fifty-two men (n = 23) and women aged 57.6 ± 7.5 years participated in the present ancillary study to the HART-D study (4). These samples sizes are adequate to detect significant changes in the primary outcome based on previous studies (7, 8, 10). Details of enrollment are in Supplemental Materials (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). HART-D participants interested in the present study had an additional muscle biopsy at baseline and ∼1 week after their last exercise bout. All participants were asked to adhere to their normal activity level outside of the intervention, record their daily steps, fill out monthly medical symptoms questionnaire forms, track daily blood sugars, maintain their normal dietary regime, and attend a monthly one-on-one visit with a Certified Diabetes Educator. The study was approved by the Pennington Biomedical Research Center Institutional Review Board, and written informed consents were obtained. HART-D participants were randomized to 9 months of AT, RT, the combination (ATRT), or a nonexercise control group. Because inclusion to this substudy was done before randomization, unequal numbers of participants were randomized to the intervention groups (Table 1).
Table 1.
Baseline Clinical Characteristicsa
| Characteristic | Control | AT | RT | ATRT | P Value |
|---|---|---|---|---|---|
| Age, y | 60.8 ± 8.0 | 54.2 ± 6.0 | 60.4 ± 7.3 | 54.1 ± 6.2 | .01b |
| Ethnicity (CA/AA) | 6/4 | 9/3 | 14/4 | 6/6 | NS |
| Sex (M/F) | 2/8 | 6/6 | 9/9 | 6/6 | NS |
| T2D duration, y | 5.4 ± 3.3 | 6.9 ± 6.5 | 9.4 ± 6.8 | 6.3 ± 4.1 | NS |
| BMI, kg/m2 | 34.6 ± 4.0 | 33.4 ± 5.8 | 33.9 ± 5.2 | 37.1 ± 6.8 | NS |
| % fat | 40.3 ± 4.9 | 33.2 ± 9.4 | 37.1 ± 8.1 | 38.2 ± 7.0 | NS |
| FFM, kg | 54.1 ± 12.2 | 63.1 ± 8.1 | 61.5 ± 11.1 | 64.6 ± 14.0 | NS |
| HbA1c, % | 7.84 ± 1.95 | 7.02 ± 1.15 | 7.12 ± 1.00 | 7.00 ± 1.15 | NS |
| FFA, mmol/L | 0.60 ± 0.20 | 0.67 ± 0.24 | 0.60 ± 0.23 | 0.65 ± 0.26 | NS |
| VO2peak, ml/kg FFM/min | 29.7 ± 5.4 | 34.1 ± 6.2 | 31.2 ± 4.7 | 30.8 ± 3.0 | NS |
Abbreviations: AA, African-American; BMI, body mass index; CA, Caucasian; F, female; M, male; NS, not significant.
All data are presented as mean ± SD.
AT different from control and RT.
Nonexercise control
The nonexercise control group was offered weekly stretching and relaxation classes but was asked to maintain their level of activity during the 9-month study period.
Aerobic exercise training
Each exercise intervention was designed to have approximately equal time requirements across groups. All exercise sessions were supervised in our exercise training center. We chose to standardize the exercise prescription to body weight and estimated that 150 min/wk moderate intensity exercise is equivalent to 10 to 12 kcal/kg body weight/wk (KKW). We chose an exercise intensity range of 50% to 80% of VO2 peak, which allowed for flexibility in the exercise prescription. We selected an aerobic dose of 12 KKW for the AT group and 10 KKW for the ATRT group.
Participants were weighed each week to calculate their KKW target, and we gradually built up to their full exercise dose over the first month. During the 12th and 24th week, the exercise dose was reduced by one third to give participants a break. Before and after each session, participant's blood pressure and glucose were assessed for safety reasons. Each exercise session had a 5-minute warm-up and cool-down period. Standard equations from the American College of Sports Medicine were used to estimate caloric expenditure rate and, hence, the amount of time required per session (14). During the AT sessions, speed, grade, heart rate, and rate of perceived exertion (Borg scale) were recorded every 5 minutes.
Resistance exercise training
For the RT group, we selected a 3-d/wk protocol with each session consisting of 2 sets of 4 upper body exercises (bench press, seated row, shoulder press, and lat pull down), 3 sets of 3 leg exercises (leg press, extension, and flexion), and 2 sets each of abdominal crunches and back extensions. This program took approximately 45 to 50 minutes per session. For each exercise, the number of repetitions and amount of weight were recorded. The ATRT group had 2 RT sessions per week with each session consisting of 1 set of each of the above 9 exercises. For both RT and ATRT, each set consisted of 10 to 12 repetitions. Once the participant was able to complete 12 repetitions for each set of exercises on 2 consecutive exercise sessions, the prescribed weight was increased.
VO2 peak and body composition
VO2 peak testing was conducted at baseline and at least 48 hours after the last exercise bout of a 9-month intervention on a treadmill as previously described (4). VO2peak is reported relative to fat-free mass (FFM). Body composition was measured by dual-energy x-ray absorptiometry (QDR 4500A; Hologic, Inc, Waltham, Massachusetts).
Blood analyses
Fasting blood samples were obtained by venipuncture. HbA1c was assessed as previously described (4), and free fatty acids (FFAs) were assayed by established enzymatic procedures (Beckman Synchron CX7; Beckman Coulter, Brea, California) using the Wako (Richmond, Virginia) FFA reagents.
Muscle tissue handling
Muscle samples were obtained under local anesthesia from the vastus lateralis using the Bergstrom technique (15) and processed freshly for ex vivo substrate oxidation or snap frozen in liquid nitrogen for future analyses.
14C-labeled ex vivo substrate oxidation
Substrate oxidation studies on muscle homogenates were determined by measuring production of 14CO2 (complete oxidation) as previously described (7, 8). Briefly, muscle tissue samples were homogenized in buffer containing (in mM) 250 sucrose, 10 Tris-HCl, 1 EDTA, and 2 ATP (pH 7.4). This method provides intact mitochondria for metabolic studies (16). Homogenates were plated in triplicate into a modified 48-well trapping device (17). Reactions were started with the addition of a mixture containing one of the 4 labels ([1-14C]palmitate, [1-14C]octanoate, [1-14C]pyruvate, and [1-14C]acetate) and yielding final concentrations of (in mM) 100 sucrose, 10 Tris-HCl, 5 potassium phosphate, 80 potassium chloride, 1 magnesium chloride, 0.1 malate, 2 ATP, 1 dithiothreitol, 0.2 EDTA, 1 l-carnitine, 0.05 coenzyme A (CoA), and 0.5% fatty-acid–free BSA and incubated for 2 hours at 37°C. Reactions were terminated by addition of 70% perchloric acid. 14CO2 was trapped in the adjoining well in 1N NaOH. Remaining muscle homogenates were spun twice for measurement of 14C-labeled acid-soluble metabolites (ASMs). Radioactivity was measured on a scintillation counter (LS6500; Beckman Coulter) in 5 mL of Uniscint BD (National Diagnostics, Atlanta, Georgia). Data were normalized to protein content.
DNA isolation and mitochondrial DNA copy number
Total DNA was isolated from ∼10 mg of skeletal muscle tissue using DNeasy blood and tissue extraction kit (QIAGEN, Valencia, California) as previously described (18). Quantity and integrity of the DNA was confirmed by a Nanodrop spectrophotometer (NanoDrop Technologies, Inc, Wilmington, Delaware). All primers and probes were designed using Primer Express version 2.1 (Applied Biosystems, Roche, Branchburg, New Jersey). Sequences are shown in Supplemental Table 1. Relative amounts of mitochondrial DNA (mtDNA) and nuclear DNA were determined by real-time quantitative PCR as previously described (19).
Western immunoblotting
Western blots were performed in protein lysates from whole muscle homogenates as previously described (20). Coomassie Brilliant Blue staining was used to determine total protein content. Subsequently, Western blotting was performed using 10% SDS-PAGE gels. Equal protein loading was confirmed by Western blotting of sarcomeric-actin. Nitrocellulose membranes were incubated with antibodies against OXPHOS (MitoSciences, Eugene, Oregon) and sarcomeric-actin (Sigma, St Louis, Missouri) and probed with IRDye800-conjugated or IRDye700-conjugated secondary antibodies (LICOR Biosciences, Leusden, The Netherlands). Bands were quantified using the Odyssey infrared imaging system (LICOR Biosciences).
Enzymatic activity
β-Hydroxyacyl-CoA degydrogenase (BHAD) and citrate synthase (CS) activities were measured by spectrophotometric analysis as previously described (21).
Statistical analyses
ANOVA was used to assess baseline group differences. To test for sex and ethnicity differences, we performed Fisher's exact χ2 tests. For analyses of group changes (after minus before), we used a mixed model with analysis of covariance test and used baseline values as covariates. Post hoc analyses for differences between groups were Tukey-adjusted. For variables that did not have a normal distribution, we performed nonparametric tests as indicated in the figure legends accordingly. Spearman correlations were used to determine relationships among change (before vs after) values because the mtDNA data were not normally distributed. Statistical significance was set at P < .05, and data were reported as mean ± SEM. Statistical analyses were performed using JMP version 9.0 (SAS Institute, Inc, Cary, North Carolina). Figures were generated using GraphPad Prism version 5 (GraphPad Software, Inc, La Jolla, California).
Results
Body composition and blood profiles improved with exercise training
Baseline characteristics are presented in Table 1. As a result of the design for the parent study HART-D (4), the 4 intervention groups are not balanced in number, and there are significant random differences in age between the AT group and the control and RT groups (54.2 ± 6.0 vs 60.8 ± 8.0 and 60.4 ± 7.3 years, respectively; P = .01). No other differences exist across groups at baseline, including sex and ethnicity (data not shown).
Changes in the clinical characteristics after 9 months are presented in Table 2. The combined training (ATRT) group significantly decreased body mass index (−0.74 ± 0.3 kg/m2, P < .05), which was in tandem with a significant decrease in percent body fat (−1.4 ± 0.5%, P < .05) as well as circulating FFAs (−0.12 ± 0.06 mmol/L, P < .05). As expected, the RT group significantly increased FFM (1.4 ± 0.4 kg, P < .05) and significantly decreased percent body fat (−1.0 ± 0.4%, P < .05). All groups decreased HbA1c levels in a manner similar to that of the reductions observed in the entire cohort of the parent study, HART-D, in which HbA1c levels of the ATRT group were significantly lower (4). Changes in HbA1c levels in the present ancillary did not reach statistical significance. Similar to HART-D findings, the 2 groups with an AT component to the intervention increased aerobic capacity (VO2peak, 1.28 ± 0.99 and 1.56 ± 1.05 ml/kg FFM/min; P = .10 and P = .12 for AT and ATRT, respectively), but this did not reach statistical significance.
Table 2.
Group Changes in Clinical Characteristicsa
| Characteristics | Control | AT | RT | ATRT |
|---|---|---|---|---|
| BMI, kg/m2 | 0.13 ± 0.2 | −0.49 ± 0.3 | 0.17 ± 0.2 | −0.74 ± 0.3b |
| % fat | −0.4 ± 0.6 | −0.1 ± 0.5 | −1.0 ± 0.4b | −1.4 ± 0.5b |
| FFM, kg | −0.1 ± 0.6 | −0.5 ± 0.5 | 1.4 ± 0.4b | −0.3 ± 1.5 |
| HbA1c, % | −0.3 ± 0.2 | −0.5 ± 0.2 | −0.1 ± 0.2 | −0.5 ± 0.2 |
| FFA, mmol/L | 0.03 ± 0.07 | 0.05 ± 0.06 | −0.02 ± 0.05 | −0.12 ± 0.06b |
| VO2peak, ml/kg FFM/min | −0.81 ± 1.22 | 1.28 ± 0.99 | −0.19 ± 0.84 | 1.56 ± 1.05 |
Abbreviation: BMI, body mass index.
All data are presented as mean ± sem and are baseline adjusted.
P < 0.05 vs baseline.
Nine months of RT and ATRT increased skeletal muscle mitochondrial content
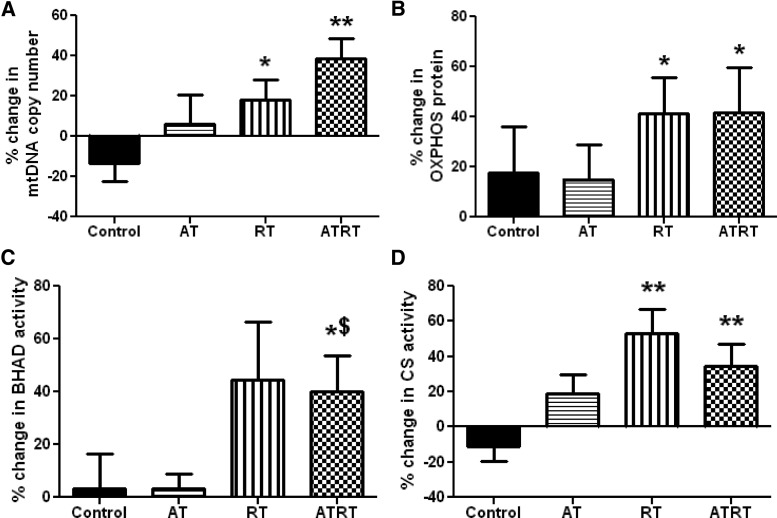
No baseline differences in markers of mitochondrial density were observed among the groups. The mtDNA copy number significantly increased after 9 months of RT and ATRT compared with the control group (17.95% ± 10.24% and 38.72% ± 10.09% vs −13.31% ± 9.27%, P < .05 for RT and P < .01 for ATRT; Figure 1A). Protein content of the subunits of the 5 complexes of the mitochondrial electron transport system (denoted as OXPHOS) was significantly increased after 9 months of training in both the RT and ATRT groups and compared with controls (41.39% ± 14.21% and 41.74% ± 17.94% vs 17.76% ± 18.41%, P < .05 for both, respectively; Figure 1B).
Figure 1.
Skeletal muscle mitochondrial content. Fifty-two individuals with T2D were randomized to 1 of 4 groups for a 9-month intervention and underwent skeletal muscle biopsies at baseline and 9 months: nonexercise control (n = 10), AT (n = 12), RT (n = 18), and ATRT (n = 12). A and B, Mitochondrial content was assessed by mtDNA copy number via quantitative PCR (A) and protein content of electron transport system complexes (OXPHOS) via Western immunoblotting (B). C and D, BHAD (C), a key enzyme in fatty acid oxidation, and CS (D), a critical enzyme in the TCA cycle, were measured in skeletal muscle homogenates. Data are presented as mean ± SEM. The mtDNA copy number was not normally distributed. *P < .05; **P < .01 vs control; $P < .05 vs AT.
Mitochondrial enzyme activities increased with RT and ATRT
Activity of BHAD, a key regulatory enzyme of fatty acid oxidation, significantly increased with 9 months of ATRT compared with controls and with AT (40.14% ± 13.66% vs. 3.14% ± 13.53% and 3.14% ± 5.72%, respectively, P < .05; Figure 1C). In addition, activity of CS, an enzyme critical to tricarboxylic acid (TCA) cycle function, was significantly up-regulated with 9 months of either RT or ATRT and compared with the control group (52.88% ± 13.97% and 34.78% ± 12.29% vs −10.88% ± 8.60%, P < .01 for both, respectively; Figure 1D).
Long- and medium-chain fatty acid oxidation improved with exercise training
We measured 14C-radiolabeled substrate oxidation using an exogenously supplied long-chain (palmitate) or medium-chain (octanoate) fatty acid (Figure 2). The results indicate that after 9 months of AT, RT, or ATRT, skeletal muscle significantly improves its capacity to oxidize palmitate completely to 14CO2 compared with the control group (239.9% ± 85.5%, 599.6% ± 243.0%, and 323.9% ± 153.9%, P < .05 for all, respectively; Figure 2A). Additionally, RT and ATRT significantly improved the ratio of complete (14CO2) to incomplete oxidation (14C-labeled ASMs) of palmitate compared with controls (240.4% ± 120.5%, P < .05 and 465.1% ± 177.1%, P < .01, respectively; Figure 2B), whereas AT trended to increase this ratio compared with the control group (139.6% ± 87.2% vs 44.1% ± 69.1, P = .08; Figure 2B). These ASMs are primarily comprised of TCA cycle and lipid intermediates (22). The findings were similar with oxidation of the medium-chain fatty acid (octanoate) to 14CO2, except there was no significant effect of RT compared with the control group. AT and ATRT were both significantly different from the control group (176.4% ± 54.3% and 375.8% ± 134.9% vs 40.6% ± 54.9%, P < .05 and P < .01, respectively; Figure 2C), and ATRT was significantly different from the RT group as well (375.8% ± 134.9% vs 91.2% ± 54.8%, P < .05; Figure 2C).
Figure 2.
Skeletal muscle fatty acid oxidation. Fifty-two individuals with T2D were randomized to 1 of 4 groups for a 9-month intervention and underwent skeletal muscle biopsies at baseline and 9 months: nonexercise control (n = 10), AT (n = 12), RT (n = 18), and ATRT (n = 12). A and B, Long-chain fatty acid oxidation to CO2 was assessed using [1-14C]palmitate for complete oxidation (A) and the ratio of complete to incomplete oxidation (B). C, Medium-chain fatty acid oxidation to CO2 was measured using [1-14C]octanoate. Data are presented as mean ± SEM. All substrate oxidation data were not normally distributed. *P < .05; **P < .01 vs control; $P < .05 vs RT.
TCA cycle flux robustly increased with all 3 types of exercise training
We next investigated whether 9 months of different types of training was sufficient to improve oxidation to CO2 of pyruvate, the end-product of glycolysis before TCA cycle entry, as well as acetate, a substrate derived from either β-oxidation or decarboxylation of pyruvate before TCA cycle entry. All 3 exercise modalities significantly improved pyruvate oxidation to 14CO2 compared with the control group (517.7% ± 135.1%, 479.9% ± 101.2%, and 653.5% ± 254.9% vs 28.9% ± 35.6%, P < .01 for all; AT, RT, ATRT, and control, respectively; Figure 3A). Likewise, acetate oxidation to 14CO2 significantly increased in all 3 exercise groups (614.9% ± 436.1%, 533.7% ± 182.2%, and 356.2% ± 143.9% vs −24.8% ± 23.1%, P < .01 for all; AT, RT, ATRT, and control, respectively; Figure 3B).
Figure 3.
Skeletal muscle TCA cycle flux. Fifty-two individuals with T2D were randomized to 1 of 4 groups for a 9-month intervention and underwent skeletal muscle biopsies at baseline and 9 months: nonexercise control (n = 10), AT (n = 12), RT (n = 18), and ATRT (n = 12). A and B, To investigate the flux through the TCA cycle, oxidation to CO2 was assessed using [1-14C]pyruvate (A) and [1-14C]acetate (B). Data are presented as mean ± SEM. All substrate oxidation data were not normally distributed. *P < .05; **P < .01 vs control.
Changes in mitochondrial content associated with clinical and functional outcomes in skeletal muscle after 9 months of exercise
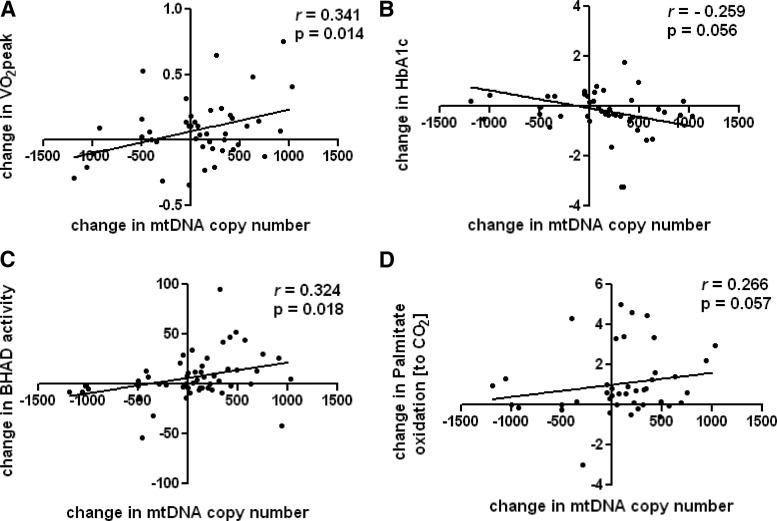
After collapsing all 3 exercise groups, we found that the change in mitochondrial content (as measured by mtDNA copy number) was significantly related to the change in VO2peak (r = 0.341, P = .014; Figure 4A) and was borderline for an inverse association with the change in HbA1c levels (r = −0.259, P = .056; Figure 4B). Both the change in BHAD activity (r = 0.324, P = .018; Figure 4C) and the change in palmitate oxidation to CO2 (r = 0.266, P = .057; Figure 4D) were positively related with the change in mtDNA copy number. In aggregate, these results build on evidence that exercise-induced adaptations in mitochondrial content positively contribute to substrate metabolism and glucose homeostasis (20, 23).
Figure 4.
Changes in mitochondrial content related to changes in clinical and functional outcomes. Fifty-two individuals with T2D were randomized to 1 of 4 groups for a 9-month intervention and underwent skeletal muscle biopsies at baseline and 9 months. We collapsed the exercise groups and performed Spearman correlation analyses: AT (n = 12), RT (n = 18), and ATRT (n = 12). A–D, We present the relationships between the changes in mtDNA copy number and the changes in VO2peak (A), HbA1c (B), BHAD activity (C), and palmitate oxidation to CO2 (D). The mtDNA copy number and palmitate oxidation data were not normally distributed.
Discussion
Conflicting studies have been published on the topic of mitochondrial (dys)function, its relationship with insulin resistance and T2D, and how either (or both) can be alleviated (for review see Ref. 24). The aim of this ancillary study was to fill a void in our understanding of how different types of exercise training would improve muscle mitochondrial content and substrate utilization in T2D and whether these improvements would subsequently be related to the exercise-induced in vivo improvements in these same individuals.
The first significant finding of this investigation was the clear demonstration of an RT-induced increase in mitochondrial content in the skeletal muscle of T2D individuals after 9 months of training. We used 3 different surrogates for mitochondrial content (mtDNA copy number, protein content of OXPHOS, and CS activity) to consistently show this response (Figure 1). ATRT also increased content of these 3 markers; however, the AT did not significantly impact these markers of mitochondrial content. We concluded that this increase in mitochondrial content was primarily due to the RT component of the exercise training, which is in line with findings of others (12, 13).
T2D is as much a disease of disordered lipid metabolism as it is disordered glucose metabolism (25). As such, the second major finding of our study was the significant improvement in complete oxidation of exogenously supplied long-chain fatty acids (palmitate) to CO2 after 9 months of all 3 types of exercise as well as the improvement in the ratio of complete to incomplete oxidation (CO2:ASMs), which indicates a more efficient lipid metabolism (Figure 2) (22). However, it is important to note that AT, in contrast to RT and ATRT, improved fatty acid oxidation (both long- and medium-chain) without significantly increasing mitochondrial content. This finding fits with the view that the quality of the mitochondria, and not necessarily the quantity, impacts oxidative capacity and muscle metabolism. For example, Pesta and colleagues (13) demonstrated that the enhanced lipid oxidative capacity in young, sedentary individuals after endurance training was mainly (∼87%) due to qualitative mitochondrial changes increasing the relative capacity for fatty acid oxidation in the absence of a significant increase in mtDNA content. Along these lines, Finley and colleagues (26) recently demonstrated that mitochondrial biogenesis in skeletal muscle was not necessary for the whole-body metabolic benefits of calorie restriction.
A third finding of our investigation was the significant improvement in TCA cycle flux with all 3 types of exercise. We determined flux through the TCA cycle by measuring the oxidation to CO2 of both pyruvate and acetate and found that AT, RT, and ATRT all significantly increased CO2 production from both substrates (Figure 3). Flux through the TCA cycle links β-oxidation and glycolysis with the electron transport chain for the generation of ATP. Increased pyruvate oxidation suggested a higher capacity for carbohydrate oxidation, which may reduce acidification of the intracellular compartment, thereby providing a feed-forward mechanism to maintain the electron transport chain proton gradient and enhance ATP synthase activity. Entry of acetyl-CoA (derived from either fatty acid or glycolytic substrate) into the TCA cycle is indicative of healthy mitochondrial function, a process known to be disrupted in the skeletal muscle of T2D individuals (27, 28). Moreover, acetyl-CoA synthetase (AceCS) catalyzes the conversion of acetate to acetyl-CoA. We therefore concluded that the exercise-induced increase in acetate oxidation indicated an increased AceCS activity. It is tempting to then speculate that increased AceCS activity, which plays a prominent role in conditions of nutrient deprivation (29), likely improved the ketogenic conditions often seen in these individuals with T2D.
A fourth, and critical, component of our investigation was the relationship of the change in mitochondrial content with exercise responses in clinical and functional outcomes. Lower mitochondrial content correlates with lower fasting lipid oxidation and metabolic inflexibility, suggesting it may be intrinsically linked to abnormal fuel utilization patterns of obesity-associated insulin resistance and T2D (20, 23, 30, 31). As such, we found that the exercise-induced augmentations of mitochondrial content were associated with improvements in fitness, lipid oxidation, and glucose control (Figure 4). In this context, it is tempting to speculate that the content of mitochondria is a critical readout for training efficiency/response whereby the change in mtDNA relates to VO2peak and can perhaps determine oxidation rates.
Strengths of the study are that this is an efficacy study, using tightly controlled exercise regimens, with all exercise completed in a laboratory with extensive monitoring of training. However, the fact that this was an ancillary study to a larger trial (4) imposed a few limitations. The population was diverse in age, sex, ethnicity, and comorbidities making our findings generalizable, yet complicated, at the level of the skeletal muscle tissue function. Specifically, the lack of significant change in VO2peak with AT is likely due to the large SE, which likely results from the population diversity.
In conclusion, we found that 9 months of RT and ATRT significantly improved mitochondrial content in individuals with T2D and that these exercise-induced changes were significantly related to improvements in the clinic (ie, VO2peak and HbA1c). Interestingly, augmentation of mitochondrial content was not necessary to observe clinical and functional improvements in muscle substrate utilization, as was the case with the AT group as well as the findings of others (13). Furthermore, ATRT improved all measures of lipid and carbohydrate oxidation as well as mitochondrial content and enzyme activity. In the context of our parent study HART-D (4) in which ATRT was most effective at reducing HbA1c levels, the results of our ancillary study indicate a combined exercise program as a more effective lifestyle therapy to improve resting muscle substrate metabolism in sedentary individuals with T2D.
Supplementary Material
Acknowledgments
We acknowledge the volunteers from the ancillary HART-D study for their participation as well as Shantele C. Thomas from the Sanford-Burnham Medical Research Institute for her contributions to the biopsy collections and 14C oxidation assays.
This work was partially funded by the National Institutes of Health Pennington Biomedical Research Center Nutrition/Obesity Research Center Grant 2P30DK072476 (to S.R.S.). Costs of analyses were partially supported by a VICI (Grant 918.96.618) for innovative research from The Netherlands Organization for Scientific Research (NWO) (to P.S.) and an unrestricted research funding grant from The Coca-Cola Company (to T.S.C.). Further funding was contributed by Dr. Donna Ryan from the Pennington Biomedical Research Center Clinical and Translational Research Fund.
Parts of this article were presented as an oral presentation at the Seventh Annual Scientific Meeting of The American Diabetes Association, Philadelphia, PA, June 8–12, 2012.
L.M.S. researched data; contributed to the study concept, design, analysis, and interpretation of the data; and wrote the manuscript. N.M.J. researched data, contributed to the analysis and interpretation of the data, and reviewed and edited the manuscript. T.S.C. contributed to the study concept and design and reviewed and edited the manuscript. E.M.-K. researched data. C.M. researched data and contributed to the study concept and design. M.K.C.H. reviewed and edited the manuscript. S.R.S. contributed to the study concept, design, analysis, and interpretation of the data and reviewed and edited the manuscript. P.S. contributed to the interpretation of the data and reviewed and edited the manuscript.
Current address for C.M.: Inserm 1048, Obesity Research Laboratory, Institute of Metabolic and Cardiovascular Diseases, Paul Sabatier University, Toulouse, France.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AceCS
- acetyl-CoA synthetase
- ASM
- acid-soluble metabolite
- AT
- aerobic training
- BHAD
- β-hydroxyacyl-CoA dehydrogenase
- CS
- citrate synthase
- CoA
- coenzyme A
- FFA
- free fatty acid
- FFM
- fat-free mass
- HART-D
- Health Benefits of Aerobic and Resistance Training in Individuals with Type 2 Diabetes
- HbA1c
- glycated hemoglobin A1c
- KKW
- kcal/kg body weight/wk
- mtDNA
- mitochondrial DNA
- RT
- resistance training
- TCA
- tricarboxylic acid
- T2D
- type 2 diabetes
- VO2peak
- peak oxygen consumption.
References
- 1. Alibegovic AC, Sonne MP, Hojbjerre L, et al. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am J Physiol Endocrinol Metab. 2010;299:E752–E763 [DOI] [PubMed] [Google Scholar]
- 2. Hansen KB, Vilsboll T, Bagger JI, Holst JJ, Knop FK. Reduced glucose tolerance and insulin resistance induced by steroid treatment, relative physical inactivity, and high-calorie diet impairs the incretin effect in healthy subjects. J Clin Endocrinol Metab. 2010;95:3309–3317 [DOI] [PubMed] [Google Scholar]
- 3. Amati F, Dube JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care. 2009;32:1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ritov VB, Menshikova EV, Azuma K, et al. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab. 2010;298:E49–E58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14 [DOI] [PubMed] [Google Scholar]
- 7. Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–E1044 [DOI] [PubMed] [Google Scholar]
- 8. Jong-Yeon K, Hickner RC, Dohm GL, Houmard JA. Long- and medium-chain fatty acid oxidation is increased in exercise-trained human skeletal muscle. Metabolism. 2002;51:460–464 [DOI] [PubMed] [Google Scholar]
- 9. Toledo FG, Menshikova EV, Azuma K, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57:987–994 [DOI] [PubMed] [Google Scholar]
- 10. Cortright RN, Sandhoff KM, Basilio JL, et al. Skeletal muscle fat oxidation is increased in African-American and white women after 10 days of endurance exercise training. Obesity (Silver Spring). 2006;14:1201–1210 [DOI] [PubMed] [Google Scholar]
- 11. Staron RS, Leonardi MJ, Karapondo DL, et al. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J Appl Physiol. 1991;70:631–640 [DOI] [PubMed] [Google Scholar]
- 12. Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol. 2001;90:1663–1670 [DOI] [PubMed] [Google Scholar]
- 13. Pesta D, Hoppel F, Macek C, et al. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1078–R1087 [DOI] [PubMed] [Google Scholar]
- 14. Medicine ACoS ACSM's Guidelines for Exercise Testing and Prescription. 7th ed Philadelphia, PA: Lippincott Williams, Wilkins; 2006 [Google Scholar]
- 15. Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616 [PubMed] [Google Scholar]
- 16. Scholte HR, Yu Y, Ross JD, Oosterkamp II, Boonman AM, Busch HF. Rapid isolation of muscle and heart mitochondria, the lability of oxidative phosphorylation and attempts to stabilize the process in vitro by taurine, carnitine and other compounds. Mol Cell Biochem. 1997;174:61–66 [PubMed] [Google Scholar]
- 17. Ukropcova B, McNeil M, Sereda O, et al. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest. 2005;115:1934–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phielix E, Schrauwen-Hinderling VB, Mensink M, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–1399 [DOI] [PubMed] [Google Scholar]
- 20. Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sparks LM, Xie H, Koza RA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933 [DOI] [PubMed] [Google Scholar]
- 22. Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 23. Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53:1714–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holloszy JO. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr. 2009;89:463S–466S [DOI] [PubMed] [Google Scholar]
- 25. McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770 [DOI] [PubMed] [Google Scholar]
- 26. Finley LW, Lee J, Souza A, et al. Skeletal muscle transcriptional coactivator PGC-1α mediates mitochondrial, but not metabolic, changes during calorie restriction. Proc Natl Acad Sci U S A. 2012;109:2931–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schrauwen P, Hesselink MK. Reduced tricarboxylic acid cycle flux in type 2 diabetes mellitus? Diabetologia. 2008;51:1694–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimazu T, Hirschey MD, Huang JY, Ho LT, Verdin E. Acetate metabolism and aging: an emerging connection. Mech Ageing Dev. 2010;131:511–516 [DOI] [PubMed] [Google Scholar]
- 30. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 31. Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.