Abstract
Context:
Stress fractures are common in endurance athletes. Whereas studies have described distal tibia bone structure in athletes, there are few data regarding hip geometric parameters. Hip structural analysis (HSA) using dual-energy x-ray absorptiometry is a validated technique to assess hip bone structure.
Objectives:
The purpose of this study was to compare hip geometry in young oligoamenorrheic athletes (AAs), eumenorrheic athletes (EAs), and nonathletes using HSA. We hypothesized that AAs would have impaired bone structure compared with that of EAs.
Design:
This was a cross-sectional study.
Setting:
The setting was a clinical research center.
Subjects:
We enrolled 55 AAs, 24 EAs, and 23 nonathletes of normal weight who were 14 to 22 years old. Athletes ran ≥20 miles/wk or were engaged in weight-bearing sports for ≥4 hours/wk.
Main Outcome Measures:
Dual-energy x-ray absorptiometry was used for HSA and hip areal bone mineral density (aBMD).
Results:
Hip aBMD Z-scores were lower in AAs and in nonathletes than in EAs (P = .002). A larger proportion of AAs than EAs and nonathletes had hip Z-scores <−1 (30.9, 4.2, 17.4%, P = .01). At the narrow neck, trochanteric region, and femoral shaft, subperiosteal width, cross-sectional moment of inertia, and section modulus were higher in EAs than in nonathletes; values in AAs did not differ from those of nonathletes. Cross-sectional area was lower in AAs and in nonathletes than in EAs. Groups did not differ for cortical thickness or buckling ratio. Group differences were lost after adjustment for lean mass but not aBMD.
Conclusions:
In an eugonadal state, athletic activity confers benefits for hip structure independent of aBMD. This advantage is lost in AAs, who do not differ from nonathletes for most parameters and fare worse than EAs for cross-sectional area.
Stress fractures are common in endurance athletes and often involve the foot, tibia, and femur (1). Recent studies have described the microarchitecture of the distal tibia and radius in oligoamenorrheic and eumenorrheic athletes and nonathletes (2). Using high-resolution peripheral quantitative computed tomography (HRpQCT), we have shown that at the distal tibia, oligoamenorrheic athletes (AAs) have lower trabecular number and greater trabecular separation than eumenorrheic athletes (EAs) and nonathletes (2). We have also shown, using finite element analysis, that estimates of bone strength such as stiffness and failure load are higher at the distal tibia in EAs than in nonathletes, whereas these measures do not differ in AAs versus nonathletes (3). Body mass index (BMI), lean mass, height, bone age, and menarchal age are important predictors of trabecular and cortical parameters. However, there are few data regarding microarchitecture and estimates of bone strength at the hip in athletes and nonathletes and particularly in amenorrheic athletes (who are hypogonadal) versus eumenorrheic athletes (who are eugonadal). Because hip fractures are associated with significant morbidity, bone strength at the hip is important to determine. Quantitative computed tomography (QCT) measurements of the hip are associated with significant radiation exposure, limiting their use in adolescents and young adults.
Hip structural analysis (HSA) is a technique that uses the properties of dual energy x-ray absorptiometry (DXA) images to derive geometric parameters for the hip that are associated with bone strength (4). This is a validated technique to assess hip bone geometry, which avoids the significant radiation associated with QCT at this site. Strong correlations have been reported between HSA and QCT of the hip for cross-sectional area, cross-sectional moment of inertia, and section modulus (5). Several studies have now demonstrated that HSA measures predict hip fractures (6–8). In a study of 7474 women with 635 incident hip fractures over 13 years, women with fractures (compared with those without fractures) had greater neck-shaft angles, subperiosteal and endosteal diameters, and buckling ratios and lower areal hip bone mineral density (BMD), cross-sectional area, cross-sectional moment of inertia, section modulus, and cortical thickness (8). Similarly, another large study of postmenopausal women reported that trochanteric diameter and femoral shaft outer diameter (subperiosteal diameter), as well as buckling ratio, were independent predictors of fracture risk even after controlling for age, body size, clinical risk factors, and areal BMD (9). HSA is now being used in studies assessing the impact of exercise and mechanical loading on bone (4, 10) and has been used in large and longitudinal studies in children (11, 12).
We used HSA to determine bone geometric parameters at the hip (at the narrow neck, trochanteric region, and femoral shaft) in AAs, EAs, and nonathletes and hypothesized that AAs (who are hypogonadal) would have impaired bone geometry and strength estimates at the hip compared with those of EAs (who are eugonadal).
Subjects and Methods
Subject selection
We enrolled 55 AAs, 24 EAs, and 23 nonathletes between 14 and 22 years old and with a bone age of at least 15 years (indicating less than 1% of remaining statural growth). Enrolled athletes ran at least 20 miles every week or were engaged in weight-bearing aerobic sports for at least 4 hours/week for at least 6 months preceding the study, whereas nonathlete participants were not engaged in any organized sports and exercised for less than 2 hours/week. All athletes and nonathletes had a BMI between the 10th and 90th percentiles. We defined oligoamenorrhea (for AAs) as the absence of menses for at least 3 months within a period of oligomenorrhea (cycle length >6 wk) for at least 6 months or absence of menarche at 16 years or older. We defined eumenorrhea (for EAs and nonathletes) as at least 9 menses (cycle length 21−35 days) in the preceding year. Subjects were recruited through advertisements in medical clinics, local newspapers, and colleges. Exclusion criteria included conditions other than exercise-induced amenorrhea and use of medications other than calcium and vitamin D supplements that may affect bone metabolism, and other causes of amenorrhea such as premature ovarian failure, hyperprolactinemia, thyroid dysfunction, and hyperandrogenism, which were ruled out with a history, physical examination, and screening laboratory tests. The institutional review board of Partners HealthCare approved the study. Informed consent was obtained from subjects ≥18 years and parents of subjects <18 years. Informed assent was obtained from subjects <18 y. Clinical characteristics and hip bone density (but not HSA data) of 17 AAs, 18 EAs, and 16 nonathletes have been published previously (2, 3).
Experimental protocol
Subjects were studied at the clinical research center of our institution. Anthropometric measurements were obtained on the same electronic scale (to the nearest 0.1 kg) and wall-mounted stadiometer (to the nearest 0.1 cm). Subjects were asked to complete a 4-day food diary to assess daily caloric intake and intake of calcium and vitamin D using Nutrient Data System for Research software (version 2008; developed by the Nutrition Coordinating Center University of Minnesota, Minneapolis, Minnesota) (13–15). Subjects also completed the Bouchard 3-day activity record, a validated method to assess 24-hour energy expenditure (16). This questionnaire provides activity information for a 3-day period of typical activity but does not assess energy expenditure through exercise/athletic activities over a prolonged period. Therefore, we also assessed mean hours per week of exercise/athletic activity for the preceding 6 and 12 months using information obtained from subjects by recall. Resting energy expenditure was measured using the VMAX Encore Metabolic Cart (Care Fusion Corporation, Yorba Linda, California) (17). Subjects underwent a treadmill test with peak aerobic capacity (VO2peak) measurements to determine exercise tolerance and fitness. Subjects had a wrist and hand X-ray to assess bone age. We used a chemiluminescent immunoassay to measure fasting 25-hydroxyvitamin D [25(OH)D] (sensitivity, 4 ng/ml; intraassay coefficient of variation, 2.9%–5.5%; DiaSorin, Stillwater, Minnesota).
Bone density and HSA
DXA (Hologic QDR-Discovery A, Apex software version 13.3; Hologic Inc, Waltham, Massachusetts) was used to assess total hip and femoral neck BMD and body composition and to perform HSA. The coefficients of variation for total hip BMD, fat mass, and lean mass for our institution are 0.8% to 1.1%, 2.1%, and 1.0%, respectively. The same scanner and software version were used for all participants. The HSA program performs its analysis at 3 femoral sites using averages from 5 parallel lines 1 pixel apart across the cross-section of these sites: (1) narrow neck, which is the narrowest point of the femoral neck; (2) the trochanteric region, along the bisector of the angle of the axes of the neck and femoral shaft; and (3) the femoral shaft, a site across the shaft at a distance of 1.5 cm minimum neck width distal to the intersection of the neck and shaft axes (9). The program provides the following measurements at the 3 sites: (1) subperiosteal (or outer) diameter; (2) estimated endosteal (or inner) diameter; (3) cross-sectional area, an index of resistance to axial forces (excludes soft spaces in marrow and pores); (4) estimated cortical thickness; (5) cross-sectional moment of inertia, which is an estimate of resistance to bending forces in a cross-section; (6) section modulus, which is an index of strength of bending; (7) buckling ratio, an index of susceptibility to local cortical buckling under compressive loads; (8) neck shaft angle; and (9) hip axis length (9, 18).
Statistical analysis
We used JMP (version 10; SAS Institute, Inc., Cary, North Carolina) for all analyses and report data as means ± SD. A value of P < .05 indicates significance. For 3-group comparisons, we performed an overall ANOVA, followed by a Tukey-Kramer analysis to assess differences between groups while controlling for multiple comparisons. We confirmed equal variances for the groups for HSA parameters before running the ANOVA. Variances were similar across groups for all HSA parameters except cross-sectional moment of inertia and buckling ratio of the narrow neck. Comparisons for these parameters were also performed using tests of unequal variance, and the results did not differ. Pearson correlations were used to assess associations of hip geometry parameters with BMI, lean mass, bone age, and menarchal age. Finally, we used multivariate analysis to determine whether differences among groups persisted after adjustment for potential confounders. Normality of the groups was confirmed before use of parametric tests to compare differences across groups.
Results
Clinical characteristics
Table 1 shows the clinical characteristics of athletes and nonathletes. Although all subjects were of normal weight, BMI and fat mass were lower in AAs than in EAs and nonathletes. Lean mass was higher in EAs than in AAs and nonathletes. The groups did not differ for height. Reported caloric intake did not differ among groups; however, hours per week of exercise was higher in both groups of athletes than in nonathletes. The nature of weight-bearing athletic activity did not differ between AAs and EAs, and 94.4% of AAs and 95.8% of EAs were involved in activities that require protracted periods of running, namely cross-country, recreational long distance running, soccer, basketball, lacrosse, track, and field hockey. The remainder reported activities such as volleyball and dance. 25(OH)D levels were highest in AAs, followed by those in EAs and nonathletes (P < .0001).
Table 1.
Clinical Characteristics of AAs, EAs, and Nonathletes
| AAs (n = 55) | EAs (n = 24) | Nonathletes (n = 23) | P | |
|---|---|---|---|---|
| Age, y | 19.3 ± 2.1 | 18.0 ± 2.1 | 19.1 ± 1.7 | .04 |
| Bone age, y | 17.4 ± 1.0 | 17.2 ± 1.2 | 17.5 ± 1.1 | NS |
| Weight, kg | 54.6 ± 7.5 | 62.0 ± 10.7 | 57.7 ± 8.0 | .002a |
| Height, cm | 164.9 ± 6.5 | 165.8 ± 8.2 | 162.8 ± 7.2 | NS |
| Age at menarche, y | 13.9 ± 1.8 | 12.8 ± 1.4 | 12.2 ± 1.5 | .0001a,b |
| Duration since last menses, mo | 8.2 ± 12.4 | |||
| BMI, kg/m2 | 20.1 ± 2.2 | 22.4 ± 2.4 | 21.7 ± 2.5 | <.0001a,b |
| Fat mass, kg | 11.8 ± 3.8 | 15.1 ± 4.4 | 16.0 ± 4.9 | <.0001a,b |
| Lean body mass, kg | 42.3 ± 5.7 | 46.3 ± 7.7 | 40.5 ± 4.4 | .003a,c |
| Body fat, % | 20.8 ± 5.2 | 23.5 ± 3.8 | 27.0 ± 5.2 | <.0001b,c |
| Hip BMD, g/cm2 | 0.95 ± 0.12 | 1.04 ± 0.12 | 0.95 ± 0.09 | .005a,c |
| Hip BMD Z-score | −0.26 ± 1.16 | 0.65 ± 1.12 | −0.30 ± 0.81 | .002a,c |
| Femoral neck BMD, g/cm2 | 0.84 ± 0.12 | 0.90 ± 0.12 | 0.83 ± 0.11 | .05 |
| Femoral neck BMD Z-score | −0.49 ± 1.12 | 0.19 ± 1.18 | −0.56 ± 0.99 | .03a |
| Hip Z-score <−1, % | 30.9 | 4.2 | 17.4 | .01 |
| Femoral neck Z-score <−1, % | 34.6 | 16.7 | 34.8 | NS |
| Resting energy expenditure, cal | 1246 ± 185 | 1444 ± 219 | 1255 ± 193 | .0003a,c |
| Total energy expenditure (Bouchard), cal | 2591 ± 617 | 2973 ± 1160 | 2291 ± 395 | .02c |
| Exercise (past 6 mo), h/wk | 10.8 ± 7.2 | 11.4 ± 5.4 | 0.7 ± 0.8 | <.0001b,c |
| Exercise (past 12 mo), h/wk | 11.0 ± 7.3 | 11.0 ± 4.9 | 0.8 ± 0.8 | <.0001b,c |
| 25(OH)D, ng/mL | 39.9 ± 12.5 | 30.5 ± 13.8 | 21.1 ± 7.8 | <.0001a,b,c |
| Vitamin D intake, μg/d | 5.3 ± 3.0 | 4.9 ± 3.2 | 4.6 ± 2.3 | NS |
| Calcium intake, mg/d | 1228 ± 512 | 1018 ± 483 | 865 ± 315 | .02b |
| Vo2peak % predicted, ml/kg/min | 115.2 ± 34.4 | 115.9 ± 32.6 | 83.1 ± 23.2 | .0009b,c |
| All fractures, % | 40 | 16.7 | 8.7 | .007b |
Abbreviations: NS: not significant; Vo2peak, peak oxygen consumption.
P < .05 AA vs EA.
P < .05 AA vs nonathletes.
P < .05 EA vs nonathletes.
Hip Bone Density and HSA
Total hip BMD and BMD Z-scores were lower in AAs and nonathletes than in EAs (Table 1). However, a larger proportion of AAs than EAs and nonathletes had Z-scores of <−1 at this site (30.9% versus 4.2% and 17.4%, respectively, P = .01). For HSA parameters (Table 2), at the narrow neck, trochanteric region, and femoral shaft, subperiosteal width, cross-sectional moment of inertia and section modulus were higher in EAs than in nonathletes, whereas parameters in AAs did not differ from those in nonathletes. Cross-sectional area was lower in AAs and nonathletes than in EAs (Figure 1). In addition, at the trochanteric region, endocortical width was higher in EAs than in nonathletes, whereas that in AAs did not differ from that in nonathletes. The groups did not differ in cortical thickness, buckling ratio, and hip axis length.
Table 2.
HSA Parameters in AAs, EAs, and Nonathletes
| AAs (n = 55) | EAs (n = 24) | Nonathletes (n = 23) | P | Pa | Pb | |
|---|---|---|---|---|---|---|
| Subperiosteal width, cm | ||||||
| Narrow neck | 2.90 ± 0.37 | 3.08 ± 0.43 | 2.80 ± 0.37 | .04c | .05c | NS |
| Trochanter | 4.56 ± 0.78 | 4.86 ± 0.76 | 4.16 ± 0.72 | .009c | .02c | NS |
| Femoral shaft | 2.27 ± 0.60 | 2.57 ± 0.56 | 2.07 ± 0.59 | .02c | .03c | NS |
| Endocortical width, cm | ||||||
| Narrow neck | 2.48 ± 0.39 | 2.65 ± 0.46 | 2.40 ± 0.43 | .06 | .04c | NS |
| Trochanter | 3.58 ± 0.90 | 3.85 ± 0.94 | 3.18 ± 0.83 | .04c | .02c | NS |
| Femoral shaft | 1.12 ± 0.57 | 1.38 ± 0.53 | 1.02 ± 0.59 | NS | .03c | NS |
| Cross-sectional area, cm2 | ||||||
| Narrow neck | 2.89 ± 0.54 | 3.18 ± 0.47 | 2.68 ± 0.41 | .003c,d | .05e | NS |
| Trochanter | 4.27 ± 1.05 | 5.04 ± 1.05 | 3.90 ± 0.94 | .0008c,d | NS | NS |
| Femoral shaft | 3.36 ± 0.98 | 3.94 ± 0.91 | 2.93 ± 0.80 | .001c,d | .03c | NS |
| Cross-sectional moment of inertia, cm4 | ||||||
| Narrow neck | 2.03 ± 0.73 | 2.37 ± 0.68 | 1.62 ± 0.45 | .001c,e | .01c,e | NS |
| Trochanter | 8.40 ± 4.48 | 10.84 ± 4.83 | 6.39 ± 3.96 | .004c | .05c | NS |
| Femoral shaft | 1.96 ± 1.39 | 2.70 ± 1.38 | 1.45 ± 1.23 | .008c | .04c | NS |
| Section modulus, cm3 | ||||||
| Narrow neck | 1.30 ± 0.38 | 1.43 ± 0.30 | 1.11 ± 0.30 | .006c | .03e | NS |
| Trochanter | 3.06 ± 1.21 | 3.82 ± 1.23 | 2.55 ± 1.11 | .002c | .05c | NS |
| Femoral shaft | 1.47 ± 0.76 | 1.87 ± 0.74 | 1.17 ± 0.66 | .006c | .05c | NS |
| Cortical thickness, cm | ||||||
| Narrow neck | 0.21 ± 0.036 | 0.21 ± 0.032 | 0.20 ± 0.040 | NS | .009d | NS |
| Trochanter | 0.48 ± 0.99 | 0.51 ± 0.12 | 0.49 ± 0.088 | NS | NS | NS |
| Femoral shaft | 0.58 ± 0.14 | 0.59 ± 0.16 | 0.53 ± 0.13 | NS | NS | NS |
| Bucking ratio | ||||||
| Narrow neck | 7.77 ± 2.41 | 8.00 ± 2.41 | 8.19 ± 3.98 | NS | NS | NS |
| Trochanter | 5.69 ± 1.98 | 5.78 ± 1.95 | 5.01 ± 1.52 | NS | .01c | NS |
| Femoral shaft | 2.15 ± 0.71 | 2.38 ± 0.62 | 2.10 ± 0.59 | NS | .02c,d | NS |
| Neck shaft angle | 130.5 ± 5.0 | 129.6 ± 3.7 | 130.7 ± 3.3 | NS | NS | NS |
| Hip axis length | 107.5 ± 5.5 | 108.3 ± 7.8 | 105.4 ± 6.3 | NS | NS | NS |
Abbreviation: NS: not significant.
Adjusted for areal hip BMD Z-scores.
Adjusted for lean mass.
P < .05 EA vs nonathletes.
P < .05 AA vs EA.
P < .05 AA vs nonathletes.
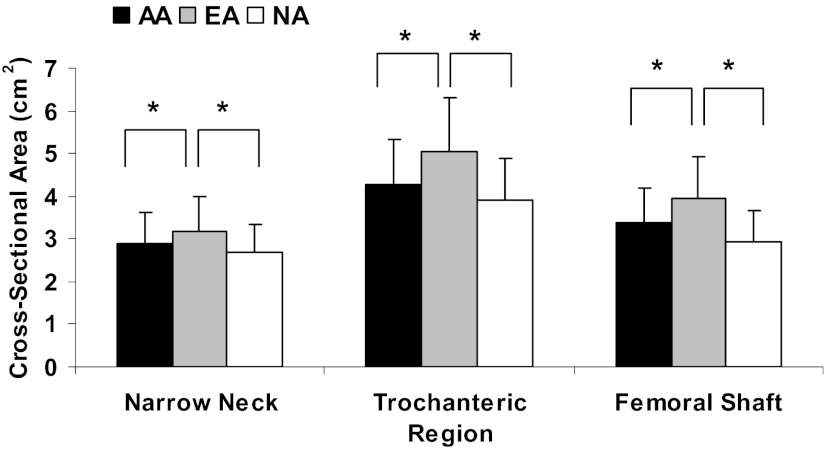
Figure 1.
Cross-sectional area of the narrow neck, trochanteric region, and femoral shaft in athletes and nonathletes. EAs, who are eugonadal, had greater cross-sectional area (a measure of resistance to axial forces) at all regions than nonathletes (NA). In contrast, AAs, who are hypogonadal, did not differ from nonathletes and had lower cross-sectional area than EAs. *P < .05.
Associations of measures of HSA with areal bone density, body composition, bone age, and menarchal age
Subjects with hip BMD Z-scores <−1 had lower cross-sectional area, cross-sectional moment of inertia, section modulus, cortical thickness, and buckling ratio at the narrow neck and trochanteric region than subjects with BMD Z-scores of >−1, whereas subperiosteal and endocortical width did not differ (Table 3). On multivariate analysis, after controlling for total hip BMD Z-scores, differences persisted between EAs and nonathletes for most parameters.
Table 3.
HSA Parameters in Subjects With Hip Z-Scores ≥−1 vs <−1.
| Hip Z-Score ≥−1 (n = 80) | Hip Z-Score <−1 (n = 22) | P | |
|---|---|---|---|
| Subperiosteal width, cm | |||
| Narrow neck | 2.91 ± 0.39 | 2.97 ± 0.41 | NS |
| Trochanter | 4.55 ± 0.79 | 4.47 ± 0.81 | NS |
| Femoral shaft | 2.32 ± 0.61 | 2.22 ± 0.61 | NS |
| Endocortical width, cm | |||
| Narrow neck | 2.47 ± 0.41 | 2.62 ± 0.44 | NS |
| Trochanter | 3.53 ± 0.91 | 3.66 ± 0.93 | NS |
| Femoral shaft | 1.16 ± 0.54 | 1.16 ± 0.70 | NS |
| Cross-sectional area, cm2 | |||
| Narrow neck | 3.03 ± 0.50 | 2.46 ± 0.26 | <.0001 |
| Trochanter | 4.61 ± 1.07 | 3.50 ± 0.66 | <.0001 |
| Femoral shaft | 3.55 ± 0.99 | 2.84 ± 0.72 | .002 |
| Cross-sectional moment of inertia, cm4 | |||
| Narrow neck | 2.10 ± 0.73 | 1.70 ± 0.51 | .02 |
| Trochanter | 9.03 ± 4.82 | 6.68 ± 3.58 | .04 |
| Femoral shaft | 2.13 ± 1.47 | 1.61 ± 1.10 | NS |
| Section modulus, cm3 | |||
| Narrow neck | 1.35 ± 0.36 | 1.06 ± 0.26 | .0006 |
| Trochanter | 3.31 ± 1.29 | 2.44 ± 0.89 | .004 |
| Femoral shaft | 1.57 ± 0.80 | 1.23 ± 0.59 | NS |
| Cortical thickness, cm | |||
| Narrow neck | 0.218 ± 0.03 | 0.170 ± 0.02 | <.0001 |
| Trochanter | 0.517 ± 0.1 | 0.403 ± 0.07 | <.0001 |
| Femoral shaft | 0.582 ± 0.15 | 0.526 ± 0.11 | NS |
| Bucking ratio | |||
| Narrow neck | 7.36 ± 2.23 | 9.95 ± 3.73 | <.0001 |
| Trochanter | 5.25 ± 1.7 | 6.70 ± 2.3 | .001 |
| Femoral shaft | 2.15 ± 0.6 | 2.36 ± 1.0 | NS |
Abbreviation: NS: not significant.
BMI was associated positively with most HSA measures except subperiosteal and endosteal diameter of the narrow neck, buckling ratio for all sites, neck shaft angle, and hip axis length (Table 4). Lean mass was strongly and positively correlated with most measures of HSA (except buckling ratio and neck shaft angle), and correlations were stronger with lean mass than BMI (Table 4). Differences among groups for HSA were lost after controlling for lean mass. There were no associations of HSA parameters with bone age, menarchal age, 25(OH)D levels, and calcium and vitamin D intake, except for a positive association of menarchal age with hip axis length.
Table 4.
Associations of HSA Measures With Body Composition Parameters, Hours per Week of Exercise for the Past Year, Age at Menarche, and 25(OH)D Level
| BMI |
Fat Mass |
Lean Mass |
Hours per Week of Exercise |
Menarchal Age |
25(OH)D |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |
| Subperiosteal width | ||||||||||||
| Narrow neck | 0.16 | NS | 0.16 | NS | 0.41 | <.0001 | 0.15 | NS | 0.05 | NS | 0.02 | NS |
| Trochanter | 0.25 | .01 | 0.19 | .06 | 0.37 | .0002 | 0.20 | .05 | −0.00 | NS | 0.10 | NS |
| Femoral shaft | 0.30 | .003 | 0.21 | .03 | 0.33 | .0007 | 0.14 | NS | −0.06 | NS | 0.08 | NS |
| Endocortical width | ||||||||||||
| Narrow neck | .08 | NS | 0.11 | NS | 0.34 | .0006 | 0.14 | NS | 0.06 | NS | 0.01 | NS |
| Trochanter | 0.19 | .05 | 0.14 | NS | 0.28 | .005 | 0.18 | .08 | −0.01 | NS | 0.09 | NS |
| Femoral shaft | 0.22 | .03 | 0.18 | .07 | 0.23 | .02 | 0.13 | NS | −0.00 | NS | −0.06 | NS |
| Cross-sectional area | ||||||||||||
| Narrow neck | 0.46 | <.0001 | 0.33 | .0009 | 0.61 | <.0001 | 0.13 | NS | 0.00 | NS | 0.10 | NS |
| Trochanter | 0.47 | <.0001 | 0.34 | .0006 | 0.58 | <.0001 | 0.12 | NS | −0.06 | NS | 0.10 | NS |
| Femoral shaft | 0.44 | <.0001 | 0.31 | .002 | 0.51 | <.0001 | 0.16 | NS | −0.06 | NS | 0.11 | NS |
| Cross-sectional moment of inertia | ||||||||||||
| Narrow neck | 0.31 | .002 | 0.20 | .045 | 0.61 | <.0001 | 0.19 | NS | 0.10 | NS | 0.09 | NS |
| Trochanter | 0.39 | <.0001 | 0.28 | .004 | 0.54 | <.0001 | 0.17 | NS | −0.02 | NS | 0.13 | NS |
| Femoral shaft | 0.36 | .0003 | 0.25 | .01 | 0.46 | <.0001 | 0.15 | NS | −0.04 | NS | 0.05 | NS |
| Section modulus | ||||||||||||
| Narrow neck | 0.33 | .0009 | 0.19 | .06 | 0.55 | <.0001 | 0.15 | NS | 0.10 | NS | 0.10 | NS |
| Trochanter | 0.44 | <.0001 | 0.31 | .002 | 0.56 | <.0001 | 0.16 | NS | −0.03 | NS | 0.13 | NS |
| Femoral shaft | 0.38 | .0001 | 0.26 | .009 | 0.46 | <.0001 | 0.16 | NS | −0.06 | NS | 0.07 | NS |
| Cortical thickness | ||||||||||||
| Narrow neck | 0.39 | <.0001 | 0.26 | .01 | 0.28 | .005 | −0.00 | NS | −0.06 | NS | 0.04 | NS |
| Trochanter | 0.17 | .09 | 0.13 | NS | 0.24 | .02 | −0.03 | NS | 0.04 | NS | 0.05 | NS |
| Femoral shaft | 0.20 | .048 | 0.08 | NS | 0.24 | .02 | 0.05 | NS | −0.13 | NS | 0.13 | NS |
| Buckling ratio | ||||||||||||
| Narrow neck | −0.12 | NS | −0.03 | NS | −0.00 | NS | .03 | NS | 0.03 | NS | −0.05 | NS |
| Trochanter | 0.00 | NS | 0.01 | NS | 0.05 | NS | 0.18 | NS | 0.00 | NS | 0.00 | NS |
| Femoral shaft | 0.07 | NS | 0.08 | NS | 0.12 | NS | 0.14 | NS | 0.05 | NS | −0.06 | NS |
| Hip axis length | 0.12 | NS | 0.05 | NS | 0.56 | <.0001 | 0.16 | NS | 0.38 | .0001 | 0.18 | .09 |
Abbreviation: NS, not significant. Significant associations are in bold; values of P < 0.10 are shown.
Discussion
Our data indicate that in a hypogonadal state, athletic activity confers significant benefits at the hip for most structural parameters independent of areal bone density. However, this advantage is lost in AAs, who do not differ from nonathletes for most parameters and fare worse than EAs for cross-sectional area.
Stress fractures are a cause of significant morbidity in athletes, particularly in those engaged in endurance sports involving repetitive impact, such as running as well as jumping (1). In long distance runners and jumpers, sites prone to sustain stress fractures include the metatarsals (8.0%–24.6%), tarsals (7.0%–25.3%), tibia (16.0%–49.1%), fibula (1.3%–12.1%), femoral neck (4.2%–48.0%), and femoral shaft and pelvis (1.3%–5.6%) (for review, see ref. 1). Thus, the lower extremity is predominantly involved, although the upper extremity may be involved in sports such as gymnastics and cheerleading (19). Because stress fractures are associated with significant morbidity, it is important to determine the factors that contribute to an increased risk for fractures.
In studies using HRpQCT, we have reported that eumenorrheic endurance athletes have higher estimates of bone strength, namely stiffness and failure load, at the distal tibia than nonathletes, whereas amenorrheic athletes do not differ from nonathletes (3). These data and reports of a higher prevalence of fractures in amenorrheic compared with eumenorrheic athletes (20, 21) suggest that benefits of mechanical loading on bone through exercise may be attenuated in the amenorrheic (hypogonadal) state.
There are very few data examining hip geometry (which effectively predicts fracture risk in postmenopausal women) (6–8) in athletes and nonathletes and particularly in amenorrheic compared with eumenorrheic female athletes. One study reported greater bone strength estimates using HSA in 18- to 35-year-old athletes versus nonathletes but did not compare amenorrheic with eumenorrheic athletes (4). There are also limited data in adolescence and young adulthood, a time critical for bone accrual toward attainment of peak bone mass.
Our study shows that EAs have improved bone structural parameters at the narrow neck, trochanteric region, and femoral shaft compared with nonathletes. This result is consistent with mechanical loading being beneficial for bone at weight-bearing sites, particularly in a eugonadal state. EAs have greater subperiosteal and endosteal diameter, cross-sectional area, cross-sectional moment of inertia, and section modulus, indicating greater resistance to axial and bending forces compared with nonathletes. A longitudinal study in healthy adolescents similarly reported positive effects of physical activity on cross-sectional area and section modulus at the narrow neck (22), although this effect was lost after controlling for lean mass. Our data are also consistent with studies in pre- and peripubertal children (23), young adult athletes (24), and late adolescent soccer players (25), in whom physical activity was associated with positive effects on HSA parameters. We found strong positive associations of lean mass with hip geometry, similar to the study by Forwood et al (22), and differences between EAs and nonathletes were lost after controlling for lean mass. In addition, there were positive associations of BMI and fat mass (surrogates for energy availability) with HSA parameters in athletes and nonathletes. Importantly, differences between groups persisted after controlling for hip BMD Z-scores, indicating that HSA may provide information regarding bone strength at the hip beyond that provided by areal BMD.
In addition to the specific sports activity, oligoamenorrhea in female athletes is a risk factor for fractures (20, 21, 26). In our current study, the beneficial effect of mechanical loading at the hip was lost in AAs, who did not differ from nonathletes for HSA measures. These data suggest that gonadal status can have an impact on hip structural parameters and estimates of strength. Although there have been no other studies of HSA in amenorrheic versus eumenorrheic athletes, our data for the hip are consistent with reported data for microarchitecture at the distal tibia (using HRpQCT), which show that benefits of mechanical loading on bone strength measures in EAs are lost in AAs (3). Of interest, menarchal age was not a significant predictor of any HSA measure in our study, in contrast to studies of microarchitecture of the distal tibia and distal radius in athletes, which found that increasing menarchal age was associated with greater impairment of bone density and structure (2, 3).
Girls involved in ≥16 hours/wk of moderate to vigorous activity were noted to have a higher risk of stress fractures in one study, suggesting that greater volume or intensity of exercise is deleterious to bone health, despite the effects of mechanical loading (19). In our study, increased volume of physical activity was not associated inversely with hip structural measures. However, it is possible that our subjects did not reach the volume or intensity of exercise necessary to observe this correlation. Finally, a higher intake of vitamin D has been associated with a lower risk for fractures (27), but in our study vitamin D intake or levels were not associated with hip geometry and HSA measures.
Limitations of the study include those inherent to HSA. This technique makes assumptions of shape and the proportion of measured bone in the cortex, whereas these typically vary among individuals (9). However, despite these assumptions, measures derived from HSA perform well in predicting hip fracture risk (6–8) and do provide a mechanical explanation for why low hip BMD predicts fractures. Another limitation is that this is a cross-sectional study with training, dietary, and menstrual history obtained from questionnaires and self report, with the potential for recall bias, which probably explains the lower fat mass (indicative of lower energy stores and a state of lower energy availability) in amenorrheic than in eumenorrheic athletes despite reports of similar total energy intake and expenditure. Food records tend to be biased toward overreporting by people who are underweight and underreporting by those who are overweight (28, 29). In addition, energy expenditure is difficult to assess reliably using questionnaires, and accelerometers have been demonstrated to have significant variability when prediction equations are used (30). Of note, in addition to completing questionnaires, each subject was also interviewed to verify answers and improve accuracy. Finally, the absence of a normal-weight, healthy, amenorrheic control group is a potential study limitation. However, other causes of hypothalamic amenorrhea in nonathletes such as low weight (from anorexia nervosa and gastrointestinal conditions) and systemic disease also affect bone subsequent to chronic inflammation and hormonal alterations associated with low weight. For this reason, we limited our control subjects to normal-weight healthy girls and young women.
We thus demonstrate that eugonadal adolescent and young adult athletes have improved HSA parameters including greater resistance to axial and bending stress than nonathletes. This result appears to be related to greater lean mass in athletes, and differences between the groups disappear after controlling for lean mass. Benefits of mechanical loading on hip structural parameters are lost in amenorrheic (hypogonadal) athletes, who do not differ from nonathletes for these parameters.
Acknowledgments
This work was supported by the National Institutes of Health (Grants 1 UL1 RR025758, 1 R01 HD060827, and 1 K24 HD071843).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- oligoamenorrheic athlete
- BMD
- bone mineral density
- BMI
- body mass index
- DXA
- dual energy x-ray absorptiometry
- EA
- eumenorrheic athlete
- HRpQCT
- high-resolution peripheral quantitative computed tomography
- HSA
- hip structural analysis
- 25(OH)D
- 25-hydroxyvitamin D
- QCT
- quantitative computed tomography.
References
- 1. Liong SY, Whitehouse RW. Lower extremity and pelvic stress fractures in athletes. Br J Radiol. 2012;85:1148–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ackerman KE, Nazem T, Chapko D, et al. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab. 2011;96:3123–3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ackerman KE, Putman M, Guereca G, et al. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone. 2012;51:680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hind K, Gannon L, Whatley E, Cooke C. Sexual dimorphism of femoral neck cross-sectional bone geometry in athletes and non-athletes: a hip structural analysis study. J Bone Miner Metab. 2012;30:454–460 [DOI] [PubMed] [Google Scholar]
- 5. Ramamurthi K, Ahmad O, Engelke K, et al. An in vivo comparison of hip structure analysis (HSA) with measurements obtained by QCT. Osteoporos Int. 2012;23:543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Faulkner KG, Wacker WK, Barden HS, et al. Femur strength index predicts hip fracture independent of bone density and hip axis length. Osteoporos Int. 2006;17:593–599 [DOI] [PubMed] [Google Scholar]
- 7. Leslie WD, Pahlavan PS, Tsang JF, Lix LM. Prediction of hip and other osteoporotic fractures from hip geometry in a large clinical cohort. Osteoporos Int. 2009;20:1767–1774 [DOI] [PubMed] [Google Scholar]
- 8. Kaptoge S, Beck TJ, Reeve J, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23:1892–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LaCroix AZ, Beck TJ, Cauley JA, et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int. 2010;21:919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petit MA, Beck TJ, Lin HM, Bentley C, Legro RS, Lloyd T. Femoral bone structural geometry adapts to mechanical loading and is influenced by sex steroids: the Penn State Young Women's Health Study. Bone. 2004;35:750–759 [DOI] [PubMed] [Google Scholar]
- 11. Alwis G, Karlsson C, Stenevi-Lundgren S, Rosengren BE, Karlsson MK. Femoral neck bone strength estimated by hip structural analysis (HSA) in Swedish Caucasians aged 6–90 years. Calcif Tissue Int. 2012;90:174–185 [DOI] [PubMed] [Google Scholar]
- 12. Jackowski SA, Kontulainen SA, Cooper DM, Lanovaz JL, Baxter-Jones AD. The timing of BMD and geometric adaptation at the proximal femur from childhood to early adulthood in males and females: a longitudinal study. J Bone Miner Res. 2011;26:2753–2761 [DOI] [PubMed] [Google Scholar]
- 13. Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–1271 [PubMed] [Google Scholar]
- 14. Schakel SF. Maintaining a nutrient database in a changing marketplace: keeping pace with changing food products—a research perspective. J Food Compost Anal. 2001;14:315–322 [Google Scholar]
- 15. Schakel SF, Buzzard IM, Gebhardt SE. Procedures for estimating nutrient values for food composition databases. J Food Compost Anal. 1997;10:102–114 [Google Scholar]
- 16. Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43:449–456 [DOI] [PubMed] [Google Scholar]
- 17. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beck TJ. Extending DXA beyond bone mineral density: understanding hip structure analysis. Curr Osteoporos Rep. 2007;5:49–55 [DOI] [PubMed] [Google Scholar]
- 19. Loud KJ, Gordon CM, Micheli LJ, Field AE. Correlates of stress fractures among preadolescent and adolescent girls. Pediatrics. 2005;115:e399–e406 [DOI] [PubMed] [Google Scholar]
- 20. Duckham RL, Peirce N, Meyer C, Summers GD, Cameron N, Brooke-Wavell K. Risk factors for stress fracture in female endurance athletes: a cross-sectional study. BMJ Open. 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wentz L, Liu PY, Ilich JZ, Haymes EM. Dietary and training predictors of stress fractures in female runners. Int J Sport Nutr Exerc Metab. 2012;22:374–382 [DOI] [PubMed] [Google Scholar]
- 22. Forwood MR, Baxter-Jones AD, Beck TJ, Mirwald RL, Howard A, Bailey DA. Physical activity and strength of the femoral neck during the adolescent growth spurt: a longitudinal analysis. Bone. 2006;38:576–583 [DOI] [PubMed] [Google Scholar]
- 23. Janz KF, Gilmore JM, Levy SM, Letuchy EM, Burns TL, Beck TJ. Physical activity and femoral neck bone strength during childhood: the Iowa Bone Development Study. Bone. 2007;41:216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Breban S, Chappard C, Jaffre C, Khacef F, Briot K, Benhamou CL. Positive influence of long-lasting and intensive weight-bearing physical activity on hip structure of young adults. J Clin Densitom. 2011;14:129–137 [DOI] [PubMed] [Google Scholar]
- 25. Ferry B, Lespessailles E, Rochcongar P, Duclos M, Courteix D. Bone health during late adolescence: effects of an 8-month training program on bone geometry in female athletes. Joint Bone Spine. 2013;80:57–63 [DOI] [PubMed] [Google Scholar]
- 26. Christo K, Prabhakaran R, Lamparello B, et al. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics. 2008;121:1127–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sonneville KR, Gordon CM, Kocher MS, et al. Vitamin D, calcium, and dairy intakes and stress fractures among female adolescents. Arch Pediatr Adolesc Med. 2012;166:595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pietilainen KH, Korkeila M, Bogl LH, et al. Inaccuracies in food and physical activity diaries of obese subjects: complementary evidence from doubly labeled water and co-twin assessments. Int J Obes (Lond). 2010;34:437–445 [DOI] [PubMed] [Google Scholar]
- 29. Scagliusi FB, Ferriolli E, Pfrimer K, et al. Underreporting of energy intake in Brazilian women varies according to dietary assessment: a cross-sectional study using doubly labeled water. J Am Diet Assoc. 2008;108:2031–2040 [DOI] [PubMed] [Google Scholar]
- 30. Trost SG, Way R, Okely AD. Predictive validity of three ActiGraph energy expenditure equations for children. Med Sci Sports Exerc. 2006;38:380–387 [DOI] [PubMed] [Google Scholar]