Abstract
Context:
The magnitude of sleep-related gonadotropin rise required to activate pubertal feminization is not established.
Objective:
The objective of the study was to determine the normal relationship of pubertal hormone responses to sleep and to GnRH agonist (GnRHag) challenge across the female pubertal transition.
Design/Setting:
This was a prospective study in a General Clinical Research Center.
Participants:
Sixty-two healthy 6- to 13-year-old volunteer girls participated in the study.
Interventions:
Interventions included overnight blood sampling followed by GnRHag (leuprolide acetate) injection.
Primary Outcome Variables:
The primary outcome variables included LH, FSH, and estradiol.
Results:
LH levels rose steadily during sleep and after GnRHag throughout the prepubertal years. The LH response to sleep and GnRHag correlated well across groups (eg, r = 0.807, peak vs 4 h post-GnRHag value); however, this correlation was less robust than in boys (r = 0.964, P < .01). Sleep peak LH of 1.3 U/L or greater had 85% sensitivity and 2.1 U/L or greater 96% specificity for detecting puberty (thelarche). The LH 1-hour post-GnRHag value of 3.2 U/L or greater had 95% sensitivity and 5.5 U/L or greater 96% specificity for detecting puberty. Girls entered puberty at lower LH levels than boys. FSH levels rose day and night during the prepubertal years to reach 1.0 U/L or greater during puberty but discriminated puberty poorly. Estradiol of 34 pg/mL or greater at 20–24 hours after GnRHag was 95% sensitive and 60 pg/mL or greater was 95% specific for puberty. Thirty-six percent of overweight early pubertal girls had meager hormonal evidence of puberty.
Conclusions:
These data suggest that sleep-related pubertal hormone levels critical for puberty are normally reflected in the responses to GnRHag testing across the normal female pubertal transition. Inconsistencies between clinical and hormonal staging may arise from peripubertal cyclicity of neuroendocrine function and from excess adiposity.
The hallmark of early puberty is a sleep-related rise in gonadotropins, particularly LH (1, 2). Although this phenomenon was initially thought to be unique to the pubertal state, subsequent studies using sensitive and specific gonadotropin assays demonstrated that sleep-related gonadotropin secretion already exists in 5- to 7-year-old girls (3–5). Although these studies have shown a much greater nocturnal gonadotropin increase in pubertal than in prepubertal girls, none examined the magnitude of sleep-related gonadotropin rise required to activate ovarian function sufficiently to initiate feminization.
The hormonal responses to GnRH and a GnRH agonist (GnRHag) challenge are known to increase with pubertal maturation (6–10). Thus, GnRHag administration is commonly used as a diagnostic test for pubertal disorders (11, 12). It seems likely that the degree of responsiveness of the pituitary-ovarian axis to GnRHag reflects the degree of antecedent activation of the neuroendocrine axis (13, 14). This study was undertaken to systematically examine this concept to determine the normal relationship between pubertal hormones during sleep and pituitary-ovarian responsiveness to GnRHag across the pubertal transition.
Subjects and Methods
Subjects
Healthy volunteer 6- to 10-year-old prepubertal and 9- to 13-year-old pubertal premenarcheal girls were recruited by advertisement. Puberty was categorized according to absence (prepubertal, stage 1) or presence (pubertal) of breast development as determined by palpation by a physician trained in pediatric endocrinology; prepubertal girls lacked pubic hair, whereas pubertal girls' pubic hair stage was variable (15). Fifty percent were non-Hispanic black, 27% non-Hispanic white, 19% Hispanic, and 4% Asian.
The studies were conducted in 2 phases, 2000–2004 (n = 40), which have been reported in part (13), and 2007–2010 (n = 22). The University of Chicago Institutional Review Board, after initially granting approval, suspended the studies in 2004 and approved resumption of a streamlined version in 2007 after a review by a federal ethics panel, which determined that these procedures represented a minor increase over minimal risk and were approvable under federal regulations 45CFR46.407 and 21CFR50.54 because of their potential to further understanding of serious child health care problems (16). All studies were performed after obtaining assent of the girls and consent of the parents. Three pubertal girls had incomplete studies because of problems maintaining iv line placement. Subjects experienced no serious adverse events, and none reported menses in a survey 1 month after study completion.
Study protocol
All subjects were admitted to the University of Chicago General Clinical Research Center immediately after undergoing examination. The procedures have been reported in detail (13, 14). In summary (Table 1), a hormonal sleep test was started on day 1 at 7:00 pm, and sampling every 20 minutes lasted as tolerated until 2.7 or more hours of sleep, which was documented by observation. At 7:00 am on day 2, GnRHag test baseline sampling was begun. GnRHag (leuprolide acetate 10 μg/kg sc) was injected either at 6:00 pm (2000–2004; after 12:00 pm low dose dexamethasone) (13) or at 8:00 am without dexamethasone administration (2007–2010) (14), and blood sampling was carried out at intervals for 24 hours.
Table 1.
Study Design
| Study Phase 1: 2000–2004 | Study Phase 2: 2007–2010 |
|---|---|
| Day 1. Hormonal sleep test | Day 1. Hormonal sleep test |
| Admission: examination, BA | Admission: examination, BA |
| 7:00 pm to 7:00 am: overnight sampling Continuous pump blood withdrawal Sampling: LH at 20-min intervals, FSH and estradiol in 2-h pools | 7:00 pm to 7:00 am: overnight sampling Intermittent blood withdrawal Sampling: LH at 20-min intervals, FSH and estradiol in 2-h pools |
| Days 2–3. GnRHag test | Days 2–3. GnRHag test |
| 7:00-10:00 am: baseline sampling LH, FSH, and steroids sampled at 15-min intervals, then pooled for assay | 7:00-8:00 am: baseline sampling LH and FSH sampled at 20-min intervals, steroids at 8:00 am |
| 6:00 pm: post-GnRHag sampling × 24 h Dexamethasone 0.25 mg/m2, 12:00 pm every day Sampling: LH and FSH at 0.5, 1, 4, 20, and 24 h; estradiol at 20 and 24 h | 8:00 am: post-GnRHag sampling × 24 h Sampling: LH and FSH at 0.5, 1, 2, 3, 4, 8, 12, 16, 20, and 24 h; estradiol at 16, 20, and 24 h |
Differences between study phases are italicized.
Laboratory and procedural methods
The University of Chicago Hospital Laboratories measured serum LH and FSH by β-subunit-specific assays. Duplicate immunofluorometric assays were performed prior to August 2007 (Delfia; PerkinElmer, Waltham, Massachusetts), for which the LOD and functional sensitivity (precision 20%) were 0.15 U/L; thereafter singleton immunochemiluminometric assays (Immulite; Siemens Medical Solutions Diagnostics, Los Angeles, California), for which the LOD and functional sensitivity were 0.1 U/L, were performed; the results were highly correlated (r = 0.99) and are expressed in terms of Immulite (14). Plasma estradiol was measured by immunoassay kit (Pantex, Santa Monica, California) (17); LOD was 5 pg/mL; functional sensitivity 10 pg/mL (36 pM); and pubertal midrange precision 10%. Total testosterone was assayed by a RIA (Diagnostic Products Corp, Los Angeles, California) that has been validated against liquid chromatography/tandem mass spectrometry; LOD was 5 ng/dL, functional sensitivity 10 ng/dL (0.347 nM), and precision 11%; free testosterone and the SHBG binding capacity were calculated from a competitive protein-binding assay (18). Dehydroepiandrosterone sulfate (DHEAS) results are expressed in terms of Immulite (Siemens Medical Solutions Diagnostics) as recently reported (14).
Bone age (BA) was determined on a continuum by a Greulich-Pyle-based method (19). Body mass index (BMI) percentiles were determined from a national database (20).
Data analyses
The 2000–2004 and 2007–2010 data were pooled after preliminary analyses showed that continuous vs intermittent sampling did not affect sleep test outcomes, and time of day and dexamethasone administration did not significantly affect gonadotropin or estradiol responses to GnRHag (Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Secondary comparisons of overweight (OW; BMI ≥85th percentile) to normal-weight (NW; BMI 9th to 84th percentiles) pubertal girls were made because previous reports, 1 involving our 2000–2004 subset, indicated that the OW pubertal girls had subtle disturbances of hypothalamic-pituitary-gonadal function (13, 21) (Supplemental Analyses).
Hormonal outcome variables during sleep were compared within individuals or between groups by paired or unpaired Student or nonparametric t test, after log transformation as appropriate for data distribution normalization; P values were Bonferroni corrected for multiple comparisons as appropriate. Relationships between variables were assessed by linear regression analysis. Two-way, repeated-measures ANOVA was used to compare GnRHag test results between groups. Sensitivity and specificity of diagnostic cut points were determined by receiver-operating characteristic curve analysis. Data analyses were performed using Excel (Microsoft Corp, Richmond, California), Prism (GraphPad Software, Inc, San Diego, California), and STATA version 12 (StataCorp, College Station, Texas) programs. Results are expressed as mean ± SD. Two-tailed P < .05 was considered significant.
Significant pulses of LH were identified with the Chronobiologic Series Analyzer program (www.ibridgenetwork.org/uctech/chronobiological-series-analyzer-csa, courtesy of E. Van Cauter, PhD, University of Chicago, Chicago, Illinois). This program uses the ULTRA algorithm to eliminate all LH peaks for which the change did not exceed a threshold 3 times assay precision (22).
Results
Baseline characteristics of study groups
The groups differed in pubertal characteristics and hormonal markers much as expected (P < .01) (Table 2). One third of the pubertal girls were breast stage 2, 61% stage 3, and 6% stage 4. Although early-morning estradiol correlated with breast stage (r = 0.700, P < .001), it was below the functional sensitivity of the method in 30% of pubertal girls. Chronologic age and BA did not differ significantly, but outcomes more often correlated significantly with BA.
Table 2.
Baseline Characteristics of Study Groups
| Group | Age, Years | BA, Years | BMI, Percentile | Breast Stage | Pubic Hair Stage | Estradiol, pg/mL | Total T, ng/dL | Free T, pg/mL | DHEAS, μg/dL |
|---|---|---|---|---|---|---|---|---|---|
| Prepubertal (n = 27) | 8.3 ± 1.2 | 8.4 ± 1.4 | 72 ± 30 | 1.0 ± 0.0 | 1.0 ± 0.0 | 5.2 ± 2.0 | 10 ± 0.8 | 2.0 ± 0.2 | 39 ± 12 |
| Pubertal (n = 35) | 11.2 ± 1.1a | 11.4 ± 1.2a | 68 ± 27 | 2.7 ± 0.5a | 2.5 ± 1.1a | 20 ± 14a | 18 ± 11b | 4.0 ± 2.8b | 62 ± 36 |
Mean ± sd.
P < .0001, pubertal vs prepubertal.
P < .001, pubertal vs prepubertal.
Sleep tests in girls
Luteinizing hormone
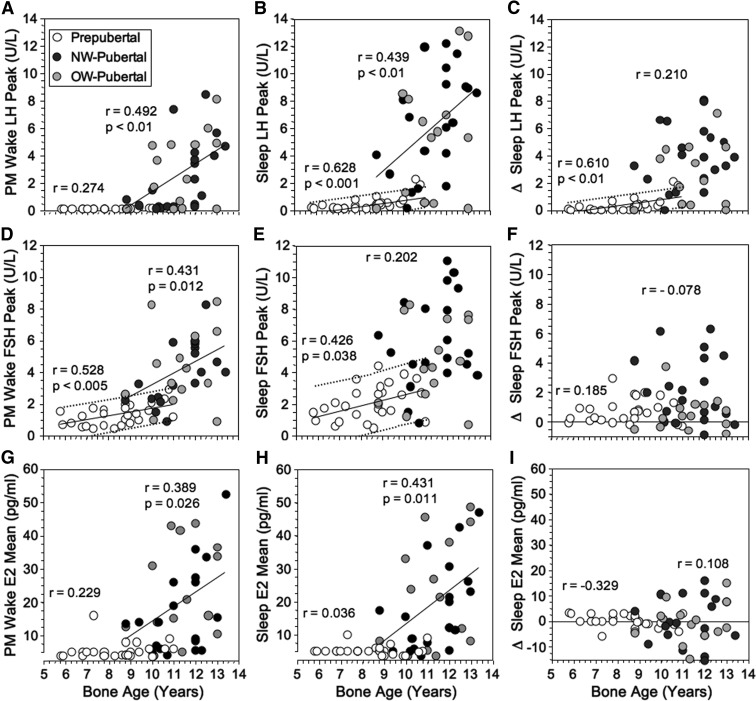
Prepubertal girls' wake LH levels were below the assay functional sensitivity (Figure 1A). Ninety-six percent experienced a significant sleep-related LH rise (sleep vs wake, P < .002). The magnitude of rise increased steadily throughout the prepubertal years (P < .04, Figure 1, B and C). Linear regression analysis showed that the peak sleep LH was 0.6 U/L or less at BA 6.0 yr and rose to an average 0.95 U/L (90% population limits 0.15–1.75 U/L) at BA 11.0 years. Supplemental Table 1 shows mean and peak ranges for the groups.
Figure 1.
Peak LH (A–C), FSH (D–F), and estradiol (E2) (G–I) levels during evening wake and nocturnal sleep periods in girls in relationship to BA. The sleep-related rise (Δ = peak sleep minus peak wake) is shown in the right-hand panel for each hormone. P values and regression lines are shown for significant correlations within the prepubertal and pubertal groups. Fine dotted lines designate 90% population limits for significant slopes in the prepubertal group.
Pubertal wake and sleep LH levels were significantly related to age (P < .02, Figure 1, A and B). A significant sleep-wake difference (rise) in mean LH occurred in 93% of girls (P = .0001), but its size was not significantly related to age (Figure 1C). The magnitude of this LH rise was less in OW- than NW-pubertal girls (see Supplemental Analyses). The peak wake LH level averaged 3.6 U/L (fifth to 95th percentiles, 0.15–8.0 U/L), and peak sleep LH averaged 6.0 (0.15–12.6) U/L (Supplemental Table 1). During puberty, the slopes of the wake and sleep LH-BA relationships increased significantly (P ≤ .03).
A peak sleep LH of 0.5 U/L or greater was 91% sensitive (64% specific) and 1.0 U/L or greater was 85% sensitive (89% specific) for detecting puberty, whereas 2.1 U/L or greater was 96% specific (74% sensitive). The only pubertal girls with peak sleep LH less than 1.0 U/L were either in very early stage 2 of breast development with tiny breast buds (0.5 cm diameter) or were obese with stage 3 breasts.
The sleep-related rises in LH levels were due primarily to significant increases in pulse amplitude (Supplemental Table 2). LH pulses were detectable more often in pubertal than prepubertal girls both awake (69% vs 4%) and asleep (91% vs 56%) (P < .005), and the sleep-related rise was significant in both groups (P < .03).
Follicle-stimulating hormone
Prepubertal girls' wake FSH increased significantly in relationship to BA (unlike LH), as did sleep FSH (P < .05) (Figure 1, D–F): linear regression analysis indicated that peak wake FSH increased from 0.75 (90% population limits ≤ 0.15–1.80) U/L at BA 6 years to 2.0 (0.9–3.0) U/L at BA 11 years, and peak sleep FSH increased from 1.2 U/L (≤0.15–3.2 U/L) at BA 6 years to 2.9 U/L (0.9–4.9) at BA 11 years. However, although the sleep-related FSH rise was significant (P < .001), it was not age related.
FSH levels during puberty loosely paralleled LH, increasing significantly in relation to BA awake and (for mean FSH in relation to age, r = 0.449, P < .05) asleep. Although FSH rose significantly during sleep (P < .01), a sleep-related rise was inconsistent and not significantly related to age (Figure 1, D–F). Pubertal FSH overlapped prepubertal values considerably: peak wake and sleep FSH of 1.0 U/L or greater was 94% sensitive for puberty (specificity 41% awake, 26% asleep); a peak sleep FSH of 4.0 U/L or greater was 95% specific (75% sensitive) for puberty.
Estradiol
Wake and sleep estradiol levels approximated the detection limit in more than 95% of prepubertal girls, the exception being an otherwise unremarkable 7.3-year-old with pubertal wake and sleep levels (Figure 1, G–I and Supplemental Table 1). During puberty, they increased significantly in relation to age (P < .05) (Figure 1, G–I). There was no significant sleep-related change. A mean sleep estradiol level of 10 pg/mL or greater was 96% specific for puberty but was only 76% sensitive.
GnRH agonist tests in girls
Baseline
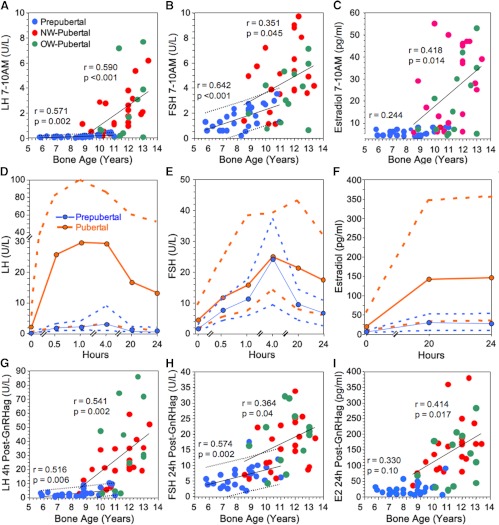
Prepubertal early-morning baseline hormone levels correlated with mean sleep levels (P ≤ .02), but morning LH averaged 49 ± 33% lower (P < .0001) (Supplemental Table 3). Throughout prepuberty, gonadotropins rose slightly but significantly with BA, but estradiol remained less than 10 pg/mL (Figure 2, A–C). According to linear regression analysis, prepubertal mean LH rose from less than 0.175 U/L at BA 6.0 years to average 0.22 U/L at 11.0 years (90% population limits ≤ 0.4 U/L), and FSH rose from 2.1 U/L or less at BA 6.0 years to average 2.65 U/L at BA 11.0 years (1.3–4.0).
Figure 2.
LH, FSH, and estradiol during GnRHag tests in normal girls. A–C, BA-related changes in baseline early morning LH, FSH, and estradiol levels; 90% population limits are shown for significant slopes in the prepubertal girls. D–F, LH, FSH, and estradiol levels at 7:00–10:00 am (time 0) and thereafter (on a discontinuous time line) in response to GnRHag. Mean (solid line) and 90% population limits (dotted lines) are shown for pooled NW and OW children (blue, prepubertal; orange, pubertal). G–I, BA-related changes in LH, FSH, and estradiol (E2) responses to GnRHag. Fine dotted lines designate 90% population limits for significant slopes in the prepubertal group.
During puberty, mean LH, FSH, and estradiol increased significantly in relation to age, and the LH and estradiol rate of increase with advancing BA accelerated significantly (P ≤ .04). LH averaged 74 ± 85%, FSH 86 ± 39%, and estradiol 155 ± 93% of their respective mean sleep levels (all P ≤ .003) (Supplemental Table 3). LH of 0.4 U/L or greater was 96% specific (76% sensitive) for puberty. FSH of 1.0 U/L or greater was 94% sensitive (30% specific) and FSH of 3.0 or greater was 96% specific (70% sensitive) for puberty. Estradiol of 10 pg/mL or greater was 100% specific and 70% sensitive.
Post-GnRHag values
Prepubertal girls all experienced significant gonadotropin responses to GnRHag (Figure 2, D and E, and Supplemental Table 3). The LH peak (average 3.4 U/L, fifth to 95th percentiles 1.2–8.9) occurred at 1 hour in one third, at 4 hours in two thirds. LH 4 hours after GnRHag, unlike LH 1 hour after GnRHag, correlated significantly with age and BA (r = 0.471–0.516, P ≤ .01): according to linear regression analysis, it rose from 0.75 U/L (90% population limits < 0.15–5.25 U/L) at BA 6.0 years to 5.6 U/L (1.2–10.0 U/L) at BA 11.0 years (Figure 2G). Prepubertal FSH peak responses consistently occurred 4 hours after GnRHag and were not related to age or BA, but 20- to 24-hour responses were (P = .03). FSH was 9.3–37 U/L (fifth to 95th percentiles) 4 hours and 2.5–11 U/L 24 hours after GnRHag (Figure 2H). Prepubertal estradiol rose significantly after GnRHag (P < .0001); the 20-hour and peak responses correlated with BA (r = 0.435–0.402, respectively, P ≤ .04): 73% of prepubertal girls had a significant response (to ≥ 15 pg/mL); peak responses occurred at 24 hours in half and averaged 29 pg/mL (fifth to 95th percentiles 9–56 pg/mL) (Figure 2, F and I).
Pubertal LH and estradiol responses to GnRHag exceeded prepubertal responses (group by time interactions, P < .0001) at all post-GnRHag time points (P < .01) (Figure 2, D and F and Supplemental Table 3). The pubertal LH peak (average 36 U/L, fifth to 95th percentiles 2.8–99) occurred at 1 hour in 34% and 4 hours in 60% and was related to age (Figure 2G). High sensitivity (95%) for distinguishing puberty from prepuberty was provided by LH of 3.2 U/L or greater 1 hour after GnRHag or LH of 4.5 U/L or greater 4 hours after GnRHag (specificity 81%–85%). High specificity (96%) was afforded by LH of 5.5 U/L or greater 1 hour after GnRHag or 9.9 U/L or greater 4 hours after GnRHag (sensitivity 76%–79%); the pubertal girls below these cutoffs were those who lacked specifically pubertal peak sleep LH (Table 3).
Table 3.
Peripubertal Volunteer Pituitary-Gonadal Function Statusa
| Subject | Breast Stage | Age, Years | Bone Age, Years | BMI, Percentile | Sleep Peak |
7–10 AM |
Post-GnRHag |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LH, U/L | FSH, U/L | Estradiol, pg/mL | LH, U/L | FSH, U/L | Estradiol, pg/mL | LH 1 h, U/L | LH 4 h, U/L | FSH 24 h, U/L | Estradiol 24 h, pg/mL | |||||
| A | 1 | 8.4 | 8.8 | 92 | 1.0 | 1.6 | 5 | 0.13 | 2.3 | 5 | 8.4 | 8.7 | 17.0 | 34 |
| B | 1 | 8.8 | 10.8 | 72 | 1.9 | 2.7 | 6 | 0.45 | 3.5 | 8 | 2.7 | 9.5 | 12.0 | 90 |
| C | 1 | 9.8 | 10.5 | 90 | 2.3 | 3.6 | 5 | 0.40 | 3.0 | 7 | 3.9 | 10.2 | — | — |
| D | 2 | 10.1 | 10.5 | 45 | 1.3 | 4.5 | 10 | 0.28 | 3.6 | 8 | 4.5 | 5.5 | 18.0 | 91 |
| E | 2 | 10.2 | 10.5 | 68 | 1.6 | 0.8 | <5 | 0.30 | 1.1 | 6 | 6.4 | 7.6 | 5.8 | 80 |
| F | 2 | 10.3 | 10.0 | 49 | 0.15 | 1.5 | 5 | 0.15 | 1.1 | 6 | 3.3 | 5.2 | 11.0 | 86 |
| G | 2 | 10.7 | 8.5 | 65 | — | — | — | <0.10 | 1.4 | 9 | 1.2 | 2.5 | 7.0 | 36 |
| H | 2 | 10.8 | 11.4 | 90 | 0.5 | 3.3 | <5 | 0.20 | 3.0 | 7 | 2.0 | 2.8 | 7.0 | 33 |
| I | 3 | 9.3 | 10.1 | 96 | 1.3 | 1.2 | <5 | 0.60 | 0.7 | 7 | 3.5 | 2.9 | 4.3 | 30 |
| J | 3 | 10.6 | 11.0 | 99 | 0.6 | 2.6 | 7 | 0.10 | 1.2 | 5 | 3.2 | 4.5 | 11.6 | 21 |
| K | 3 | 10.7 | 8.8 | 88 | 0.14 | 2.1 | 8 | 0.10 | 1.9 | 8 | 1.5 | — | 16.3 | 68 |
| L | 3 | 11.0 | 13.0 | 99 | 0.14 | 0.7 | 11 | 0.10 | 0.6 | 10 | 68.9 | 30.2 | 22.1 | 44 |
| ≥90% sensitive for puberty (specificity)a | ≥0.5 (63%) | ≥1.0 (26%) | ≥5 (15%) | ≥0.1 (50%) | ≥1.0 (30%) | ≥6 (52%) | ≥3.2 (85%) | ≥4.5 (81%) | ≥5.7 (42%) | ≥34 (77%) | ||||
| ≥95% specific for puberty: (sensitivity) | ≥2.1 (74%) | ≥4.0 (75%) | ≥10 (76%) | ≥0.4 (76%) | ≥3.0 (70%) | ≥10 (70%) | ≥5.5 (79%) | ≥9.9 (76%) | ≥13 (67%) | ≥60 (85%) | ||||
Dashes indicate missing data.
Bold indicates an experimentally determined 95% or greater-specific pubertal value.
Pubertal FSH peaked 4 hours after GnRHag in all but did not differ significantly from the prepubertal response until 20–24 hours after GnRHag (P < .0001); at the latter time, it increased with BA (Figure 2, E and H). FSH of 5.7 U/L or greater 24 hours after GnRHag was 95% sensitive and FSH of 13 U/L or greater was 96% specific for puberty.
Pubertal estradiol responses to GnRHag peaked in half the girls at 20 hours and half at 24 hours (Figure 2F); they were significantly related to age (Figure 2I). Estradiol of 34 pg/mL or greater was 95% (20 hours) to 91% (24 hours) sensitive (specificity 74%–77%, respectively) and estradiol of 60 pg/mL or greater was 96% specific (sensitivity 95%–85%, respectively) for puberty (Figure 2I).
Relationships among sleep and GnRHag test outcomes and pubertal stage
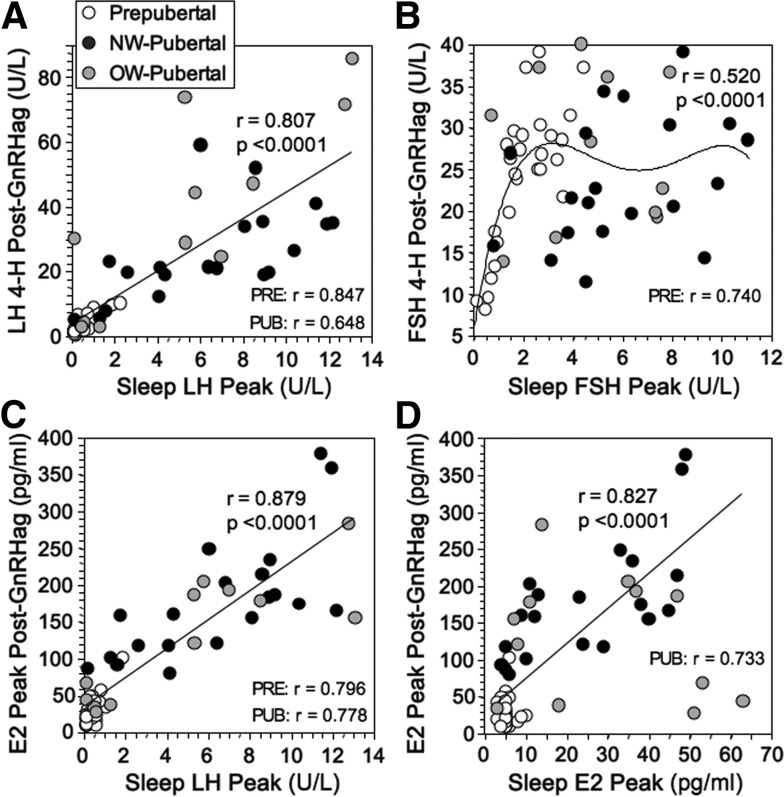
Sleep hormone levels correlated with those in response to GnRHag. Most notably, sleep LH correlated well with LH after GnRHag across groups, the peak sleep LH accounting for 65% of variance in the 4-hour response (Figure 3A). The correlation between sleep FSH and FSH 4 hours after GnRHag (Figure 3B) was primarily attributable to the prepubertal group because the correlation during puberty was nonsignificant (r = 0.164). Peak sleep FSH correlated better linearly across groups with FSH 24 hours after GnRHag (r = 0.662, P < .0001, data not shown). The estradiol response to GnRHag correlated well with sleep LH both across and within groups (Figure 3C). The correlation between sleep estradiol and after GnRHag across groups was primarily attributable to the pubertal group (Figure 3D) because most prepubertal girls' estradiol levels responded to GnRHag despite approximating LOD during sleep.
Figure 3.
Relationships of GnRHag and sleep test outcomes. A, Relationship of peak sleep and post-GnRHag LH levels. Correlation of mean sleep LH level with 4-hour post-GnRHag level was similar (r = 0.719) and correlations of mean and peak sleep LH levels with LH 1-hour post-GnRHag were slightly less robust (r = 0.578–0.628, P ≤ .003). Correlations of peaks were significant across and within the prepubertal and pubertal groups, as shown (P ≤ .005). B, Relationship between peak sleep FSH and FSH peak (4 hours) after GnRHag. A linear relationship was present only in prepubertal girls (P < .0001). C, Relationship between peak sleep LH and estradiol (E2) peak response to GnRHag. These correlations also pertained within both the prepubertal and pubertal groups (P < .0001). Mean sleep LH was similarly related to the estradiol peak response to GnRHag (r = 0.851, P < .0001). D, Relationship between peak sleep estradiol and estradiol peak response to GnRHag. The correlation across groups was primarily attributable to the pubertal group (P < .001) because the correlation in prepubertal girls was nonsignificant (r = 0.032). Regression lines with accompanying statistics are shown for significant across-group correlations; linear regression and polynomial fits yielded similar correlations except for FSH, in which a fourth-order polynomial equation provided the best fit because FSH levels plateau in pubertal girls (see text). Significant within-group correlation coefficients for linear regression are also displayed.
Fourteen percent of girls had disparities among their pubertal stage and 95%-specific sleep or GnRHag test outcomes; we considered them to be peripubertal (Table 3). Two of the 3 clinically prepubertal girls were overweight. One (subject A) had nonspecifically pubertal sleep gonadotropins (≥1.0 U/L) but had specifically pubertal gonadotropin responses to GnRHag that generated a nonspecific estradiol response. Another overweight girl (subject C) had a specifically pubertal sleep LH level. A lean prepubertal girl (subject B) had nonspecifically pubertal LH levels during sleep and in response to GnRHag but a specifically pubertal estradiol response to GnRHag. Two pubertal girls with very early breast budding (subjects D and E) had robust estradiol responses to GnRHag, 1 after isolated specifically pubertal FSH responses to sleep and GnRHag and the other after a specifically pubertal LH response to GnRHag but not to sleep. Yet another (subject F) had a pulsatile sleep LH at assay LOD and nonspecific post-GnRHag gonadotropins yet a specifically pubertal estradiol response to GnRHag. Subject G, who had 2-cm breast buds, was an outlier: the only lean, clinically pubertal girl without any specifically pubertal response to GnRHag. Five OW-pubertal girls with stage 2–3 breasts (subjects H-L), 36% of the OW-pubertal group, lacked specifically pubertal sleep LH and accounted for most of the pubertal girls with peak sleep LH less than 1.0 U/L (Figure 1B), yet 80% had 1 or more specifically pubertal values at baseline or in response to GnRHag.
Comparison of overweight with normal-weight pubertal girls
Sleep-related rises in LH and FSH were significantly blunted in OW-pubertal compared with NW-pubertal, although wake and sleep levels were similar (Supplemental Analyses).
LH and FSH responses to GnRHag tended to be higher and estradiol responses were significantly lower in OW-pubertal than NW-pubertal (Supplemental Analyses). This resulted in lower ratios of peak estradiol to peak FSH in OW-pubertal than NW-pubertal girls (P ≤ .05).
Comparison of girls with boys during the pubertal transition
Prepubertal girls had lower spontaneous and GnRHag-stimulated LH levels, but higher FSH levels, than contemporaneously studied prepubertal boys (14) (Supplemental Analyses). GnRHag-stimulated gonadotropins elicited pubertal sex steroid levels in prepubertal girls but not in prepubertal boys. The peak sleep LH and post-GnRHag levels that were greater than 90% sensitive for detecting puberty were about 3-fold lower in girls than boys. The correlation between the LH responses to sleep and GnRHag was significantly lower in girls than boys (r = 0.807 vs r = 0.964, P < .01). This contributed to our inability to identify a single sleep or post-GnRHag LH level that provided both 95% or greater sensitivity and specificity for the detection of puberty in girls. This contrasts to boys, in whom sleep peak LH of 3.7 U/L or greater and the LH 4 hours after GnRHag of 14.8 U/L or greater each met this criterion.
Discussion
This study demonstrates that girls' pubertal hormone responses to GnRHag testing indicate the degree to which their pituitary-gonadal axis has been activated during sleep and provides normative data on the maturation of girls' pituitary-gonadal axis and its response to GnRHag. In contrast to boys (14), however, no parameter provided both 95% sensitivity and specificity (Table 3). Peak sleep LH of 0.5 U/L or greater was 91% sensitive and peak sleep LH of 2.1 U/L or greater was 96% specific for detecting puberty. Post-GnRHag LH of 3.2 U/L or greater at 1 hour (or ≥ 4.5 U/L at 4 h) had 95% sensitivity for detecting puberty, and an LH of 5.5 U/L or greater at 1 hour (≥9.9 U/L at 4 h) had 96% specificity. Estradiol of 34 pg/mL or greater 20–24 hours after GnRHag was 91% sensitive and estradiol of 60 pg/mL or greater was 96% specific for puberty.
During the late prepubertal years, both girls and boys experience a steady and substantial increase in sleep-related LH levels and an even greater FSH increase that occurs both awake and asleep (5, 14). Girls' immunoreactive gonadotropins are bioactive, judging from an ultrasensitive bioassay for estrogen (23): the active formation of antral follicles greater than 2 mm diameter (13, 24), a size that indicates FSH responsiveness (25–29); and, in a primate model, gonadotropin sensitivity to estradiol negative feedback (30).
Prepubertal girls differ from boys, however, in their lower spontaneous and GnRHag-stimulated LH levels and higher FSH levels. Prepubertal girls' gonads are also functionally more sensitive to gonadotropin stimulation than boys': GnRHag-stimulated gonadotropins elicited pubertal sex steroid levels in prepubertal girls but not in prepubertal boys. Thus, girls entered puberty at lower LH levels than boys.
FSH levels rose only about 3-fold during the premenarcheal pubertal years, which contrasts to the greater than 10-fold LH rise. It seems likely that rising inhibin-B elaborated by increasing ovarian follicular development plays a key negative-feedback role in attenuating further FSH increase during puberty (31, 32). Although FSH levels were poorly discriminatory of pubertal status, 94% of pubertal girls attained levels similar to pubertal boys, 1.0 U/L or greater, which approximated the fifth percentile for 11.0-year-old prepubertal girls. These data suggest that after a critical level of FSH is achieved in the late prepubertal years, LH stimulation of thecal steroidogenesis (26) is the major factor in pubertal progression. Once puberty becomes clinically apparent, the tempo of LH rise accelerates because of GnRH self-priming, and ovarian gonadotropin-sensitivity increases (33–35).
We demonstrate that sleep LH correlates well with LH responsiveness to GnRHag across the pubertal transition. However, this relationship is significantly more variable than that in boys, which may contribute to our inability to identify a single sleep or post-GnRHag LH level in girls that provided both 95% or greater sensitivity and specificity for the detection of puberty, although we could in boys.
Cyclic gonadotropin secretion, which becomes apparent during the late prepubertal years (36, 37), may account for the excess variability in the relationship between girls' LH responses to sleep and GnRHag. Our peripubertal data seem informative in this regard, being compatible with the following interpretations, which are hypothetical in the absence of longitudinal data. Shortly after a cycle of gonadotropin secretion in late prepubertal girls, gonadotropin responsiveness to GnRHag is enhanced but not sufficiently to generate much estradiol secretion (Table 3, subject A). After more such cycles, pubertal pituitary-ovarian responsiveness to GnRHag increases before breasts bud (subject B). On the other hand, during phases of the cycle when the GnRH-primed pubertal pituitary-ovarian axis is quiescent, variable pubertal degrees of pituitary-estradiol GnRHag responsiveness exist (subjects D–G and L). Overweight/obese girls may develop breasts with little if any specific evidence of pubertal pituitary-ovarian activation (subjects H–K), suggesting a role of aromatized adrenal androgens (38, 39). The estradiol response to GnRHag appears to be the most consistent evidence of puberty: an estradiol peak of 34–60 pg/mL seemed to indicate peripuberty and an estradiol peak of greater than 60 pg/mL was 95% specific for the appearance of puberty. These considerations suggest that hormonal responses to GnRHag testing in girls may be more indicative of the state of pubertal pituitary-ovarian activation than those to sleep.
These data seem relevant to the diagnosis of disorders of puberty. Our data suggest that a baseline FSH of 1.0 U/L or greater, 1-hour post-GnRHag LH of 3.2 U/L or greater, and post-GnRHag estradiol of 34 pg/mL or greater are each 90% or greater sensitive for the onset of puberty, although higher levels are necessary for 95% specificity. These data may be useful in differentiating gonadotropin deficiency from constitutionally delayed puberty in girls, for whom very few data exist, especially with current-generation gonadotropin assays (11, 34, 40). Our data are consistent with the consensus opinion that a 1-hour post-GnRHag LH peak greater than 3.3–5.0 U/L suggests a diagnosis of central precocious puberty (41). Definitive data have been lacking, however, because of differences in study design, test regimens, and assays (42–46). An extreme variation of the apparently normal intermittent ovarian activation may account for the entity of premature menarche in prepubertal girls (47), but GnRHag test data are unavailable in this condition.
Thirty-six percent of our overweight early pubertal girls had meager hormonal evidence of puberty. As a group, overweight premenarcheal pubertal girls had normal average pubertal hormone levels, but blunted sleep-related gonadotropin rises, as previously reported (13, 21). This expanded data set also suggests that excess adiposity alters responses to GnRHag: gonadotropins tended to be higher and estradiol lower, resulting in a lower estradiol to FSH ratio in overweight pubertal girls. These data are consistent with obesity reducing gonadotropin biopotency by accelerating gonadotropin clearance (48).
The limitations of the study include its cross-sectional nature, the lack of polysomnographic staging of sleep and ultrasonographic documentation of internal genital development, the relative infrequency of nocturnal LH sampling, the known nonspecificity of estradiol assays in the prepubertal range (23), and the procedural changes that took place over the long course of the study. Many of these limitations are related to the practical and ethical issues that attend clinical investigation in normal children.
In summary, this study provides normative data for the responses to GnRHag testing across the normal female pubertal transition and demonstrates their relationship to sleep-related pubertal activation of the pituitary-ovarian axis.
Supplementary Material
Acknowledgments
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. We thank Kristen Wroblewski for biostatistical support and Eve Van Cauter and Rachel Leproult for pulse analysis consultation. We also thank Nancy Devine, RN, and our pediatric endocrine colleagues for their help in accomplishing this project. The critical review and helpful suggestions of Drs Yee-Ming Chan, David Cooke, Melvin Grumbach, and Dorit Koren are appreciated.
This work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreement U54-041859 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and Grant HD-39267 (to R.L.R.); Grant FD-R-001021; Grant 5T32DK064582 and a Lawson Wilkins Pediatric Endocrine Society Research Fellowship (to B.B.); and Grants RR-00055 and UL1RR024999 from the National Center for Research Resources.
Current address for B.B.: Academic Endocrine, Metabolism, and Nutrition, Inc, 2001 Gary Avenue, Suite 240, Wheaton, Illinois 60187.
Disclosure Summary: The authors have no conflicts of interest.
Footnotes
- BA
- bone age
- BMI
- body mass index
- DHEAS
- dehydroepiandrosterone sulfate
- GnRHag
- GnRH agonist
- NW
- normal weight
- OW
- overweight.
References
- 1. Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med. 1972;287:582–586 [DOI] [PubMed] [Google Scholar]
- 2. Shaw ND, Butler JP, McKinney SM, Nelson SA, Ellenbogen JM, Hall JE. Insights into puberty: the relationship between sleep stages and pulsatile LH secretion. J Clin Endocrinol Metab. 2012;97:E2055–E2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu FC, Butler GE, Kelnar CJ, Stirling HF, Huhtaniemi I. Patterns of pulsatile luteinizing hormone and follicle-stimulating hormone secretion in prepubertal (midchildhood) boys and girls and patients with idiopathic hypogonadotropic hypogonadism (Kallmann's syndrome): a study using an ultrasensitive time-resolved immunofluorometric assay. J Clin Endocrinol Metab. 1991;72:1229–1237 [DOI] [PubMed] [Google Scholar]
- 4. Apter D, Butzow TL, Laughlin GA, Yen SS. Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab. 1993;76:940–949 [DOI] [PubMed] [Google Scholar]
- 5. Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol secretion before the onset of female puberty in short children. J Clin Endocrinol Metab. 2000;85:1074–1080 [DOI] [PubMed] [Google Scholar]
- 6. Grumbach M, Roth J, Kaplan S, Kelch R. Hypothalamic-pituitary regulation of puberty in man: evidence and concepts derived from clinical research. In: Grumbach M, Grave C, Mayer F, eds. The Control of the Onset of Puberty. New York: John Wiley, Sons; 1974;115–207 [Google Scholar]
- 7. Brito VN, Batista MC, Borges MF, et al. Diagnostic value of fluorometric assays in the evaluation of precocious puberty. J Clin Endocrinol Metab. 1999;84:3539–3544 [DOI] [PubMed] [Google Scholar]
- 8. Resende EA, Lara BH, Reis JD, Ferreira BP, Pereira GA, Borges MF. Assessment of basal and gonadotropin-releasing hormone-stimulated gonadotropins by immunochemiluminometric and immunofluorometric assays in normal children. J Clin Endocrinol Metab. 2007;92:1424–1429 [DOI] [PubMed] [Google Scholar]
- 9. Rosenfield RL, Burstein S, Cuttler L, et al. Use of nafarelin for testing pituitary-ovarian function. J Reprod Med. 1989;34:1044–1050 [PubMed] [Google Scholar]
- 10. Potau N, Ibanez L, Sentis M, Carrascosa A. Sexual dimorphism in the maturation of the pituitary-gonadal axis, assessed by GnRH agonist challenge. Eur J Endocrinol. 1999;141:27–34 [DOI] [PubMed] [Google Scholar]
- 11. Goodpasture J, Ghai K, Cara J, Rosenfield R. Potential of gonadotropin-releasing hormone agonists in the diagnosis of pubertal disorders in girls. Clin Obstet Gynecol. 1993;36:773–785 [DOI] [PubMed] [Google Scholar]
- 12. Ibañez L, Potau N, Zampolli M, et al. Use of leuprolide acetate response patterns in the early diagnosis of pubertal disorders: comparison with the gonadotropin-releasing hormone test. J Clin Endocrinol Metab. 1994;78:30–35 [DOI] [PubMed] [Google Scholar]
- 13. Bordini BD, Littlejohn EE, Rosenfeld RL. Blunted sleep-related LH rise in healthy premenarcheal pubertal girls with elevated body mass index. J Clin Endocrinol Metab. 2009;94:1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenfield RL, Bordini B, Yu C. Comparison of detection of normal puberty in boys by a hormonal sleep test and a gonadotropin-releasing hormone agonist test. J Clin Endocrinol Metab. 2012;97:4596–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanner JM. Growth at adolescence. London: Blackwell; 1962 [Google Scholar]
- 16. Office for Human Research Protections, US Department of Health and Human Services Gonadotropin-releasing hormone (GnRH) agonist test in disorders of puberty. 2006. http://www.hhs.gov/ohrp/archive/children/gnrh.html Accessed February 22, 2013
- 17. Rosenfield RL, Perovic N, Ehrmann DA, Barnes RB. Acute hormonal responses to the gonadotropin releasing hormone agonist leuprolide: dose-response studies and comparison to nafarelin. J Clin Endocrinol Metab. 1996;81:3408–3411 [DOI] [PubMed] [Google Scholar]
- 18. Rosenfield RL, Mortensen M, Wroblewski K, Littlejohn E, Ehrmann DA. Determination of the source of androgen excess in functionally atypical polycystic ovary syndrome by a short dexamethasone androgen-suppression test and a low-dose ACTH test. Hum Reprod. 2011;26:3138–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roche AF, Eyman SL, Davila GH. Skeletal age prediction. J Pediatr. 1971;78:997–1003 [DOI] [PubMed] [Google Scholar]
- 20. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;1–27 [PubMed] [Google Scholar]
- 21. McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Cauter E. Estimating false-positive and false-negative errors in analyses of hormonal pulsatility. Am J Physiol. 1988;254:E786–E794 [DOI] [PubMed] [Google Scholar]
- 23. Klein KO, Baron J, Colli MJ, McDonnell DP, Cutler GB., Jr Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J Clin Invest. 1994;94:2475–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peters H, Byskov A, Grinsted J. Follicular growth in fetal and prepubertal ovaries of humans and other primates. Clin Endocrinol Metab. 1978;7:469–485 [DOI] [PubMed] [Google Scholar]
- 25. Ross C. Gonadotropins and preantral follicular maturation in women. Fertil Steril. 1974;25:52. [DOI] [PubMed] [Google Scholar]
- 26. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocrinol Rev. 1996;17:121–155 [DOI] [PubMed] [Google Scholar]
- 27. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204 [DOI] [PubMed] [Google Scholar]
- 28. Meduri G, Touraine P, Beau I, Lahuna O, et al. Delayed puberty and primary amenorrhea associated with a novel mutation of the human follicle-stimulating hormone receptor: clinical, histological, and molecular studies. J Clin Endocrinol Metab. 2003;88:3491–3498 [DOI] [PubMed] [Google Scholar]
- 29. McNatty KP, Makris A, Reinhold VN, De Grazia C, Osathanondh R, Ryan KJ. Metabolism of androstenedione by human ovarian tissues in vitro with particular reference to reductase and aromatase activity. Steroids. 1979;34:429–443 [DOI] [PubMed] [Google Scholar]
- 30. Wilson ME, Fisher J, Chikazawa K. Estradiol negative feedback regulates nocturnal luteinizing hormone and follicle-stimulating hormone secretion in prepubertal female rhesus monkeys. J Clin Endocrinol Metab. 2004;89:3973–3978 [DOI] [PubMed] [Google Scholar]
- 31. Crofton PM, Illingworth PJ, Groome NP, et al. Changes in dimeric inhibin A and B during normal early puberty in boys and girls. Clin Endocrinol (Oxf). 1997;46:109–114 [DOI] [PubMed] [Google Scholar]
- 32. Elsholz DD, Padmanabhan V, Rosenfield RL, Olton PR, Phillips DJ, Foster CM. GnRH agonist stimulation of the pituitary-gonadal axis in children: age and sex differences in circulating inhibin-B and activin-A. Hum Reprod. 2004;19:2748–2758 [DOI] [PubMed] [Google Scholar]
- 33. Moll GW, Jr, Rosenfield RL. Direct inhibitory effect of estradiol on pituitary luteinizing hormone responsiveness to luteinizing hormone releasing hormone is specific and of rapid onset. Biol Reprod. 1984;30:59. [DOI] [PubMed] [Google Scholar]
- 34. Zimmer CA, Ehrmann DA, Rosenfield RL. Potential diagnostic utility of intermittent short-acting GnRH agonist administration in gonadotropin deficiency Fertil Steril. 2010;94:2697–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenfield RL, Cooke DW, Radovick S. The ovary and female maturation. In: Sperling M, ed. Pediatric Endocrinology. 3rd ed Philadelphia, PA: Elsevier; 2008;530–609 [Google Scholar]
- 36. Hansen JW, Hoffman HJ, Ross GT. Monthly gonadotropin cycles in premenarcheal girls. Science. 1975;190:161–163 [DOI] [PubMed] [Google Scholar]
- 37. Maesaka H, Tachibana K, Adachi M, Okada T. Monthly urinary gonadotropin and ovarian hormone excretory patterns in normal girls and female patients with idiopathic precocious puberty. Pediatr Res. 1996;40:853–860 [DOI] [PubMed] [Google Scholar]
- 38. Edman CD, MacDonald PC. Effect of obesity on conversion of plasma androstenedione to estrone in ovulatory and anovulatory young women. Am J Obstet Gynecol. 1978;130:456–461 [DOI] [PubMed] [Google Scholar]
- 39. Shaw ND, Seminara SB, Welt CK, et al. Expanding the phenotype and genotype of female GnRH deficiency. J Clin Endocrinol Metab. 2011;96:E566–E576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Couzinet B, Young J, Brailly S, Le Bouc Y, Chanson P, Schaison G. Functional hypothalamic amenorrhoea: a partial and reversible gonadotrophin deficiency of nutritional origin. Clin Endocrinol (Oxf). 1999;50:229–235 [DOI] [PubMed] [Google Scholar]
- 41. Carel JC, Eugster EA, Rogol A, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009;123:e752–e762 [DOI] [PubMed] [Google Scholar]
- 42. Houk CP, Kunselman AR, Lee PA. The diagnostic value of a brief GnRH analogue stimulation test in girls with central precocious puberty: a single 30-minute post-stimulation LH sample is adequate. J Pediatr Endocrinol Metab. 2008;21:1113–1118 [DOI] [PubMed] [Google Scholar]
- 43. Poomthavorn P, Khlairit P, Mahachoklertwattana P. Subcutaneous gonadotropin-releasing hormone agonist (triptorelin) test for diagnosing precocious puberty. Horm Res. 2009;72:114–119 [DOI] [PubMed] [Google Scholar]
- 44. Bordini BD, Littlejohn EE, Rosenfield RL. LH dynamics in overweight girls with premature adrenarche and slowly progressive sexual precocity. Intl J Pediatr Endocrinol. 2010:2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sathasivam A, Garibaldi L, Shapiro S, Godbold J, Rapaport R. Leuprolide stimulation testing for the evaluation of early female sexual maturation. Clin Endocrinol (Oxf). 2010;73:375–381 [DOI] [PubMed] [Google Scholar]
- 46. Predieri B, Luisi S, Casarosa E, et al. Allopregnanolone levels decrease after gonadotropin-releasing hormone analog stimulation test in girls with central precocious puberty. J Endocrinol Invest. 2011;34:38–44 [DOI] [PubMed] [Google Scholar]
- 47. Saggese G, Ghirri P, Del Vecchio A, Papini A, Pardi D. Gonadotropin pulsatile secretion in girls with premature menarche. Horm Res. 1990;33:5–10 [DOI] [PubMed] [Google Scholar]
- 48. Rosenfield RL, Bordini B. Evidence that obesity and androgens have independent and opposing effects on gonadotropin production from puberty to maturity. Brain Res. 2010;1364:186–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.