Abstract
Context:
Cardiovascular risk is increased in individuals with impaired glucose tolerance (IGT) and impaired fasting glucose (IFG); however, those with IGT appear to be at greater risk. Lipoprotein abnormalities occur also in the prediabetic state.
Objective:
The authors examined lipoprotein composition in IGT and IFG.
Design and Setting:
Cross-sectional analysis of a large epidemiological study was done.
Participants:
The Insulin Resistance Atherosclerosis Study had a total of 1107 participants.
Main measures:
Lipoproteins and apolipoproteins were measured by conventional methods and lipoprotein composition by nuclear magnetic resonance spectroscopy.
Results:
Compared with normal glucose tolerance, apolipoprotein B (105.2 vs 99.8 mg/dL, P < .05) was high in isolated IFG, triglyceride (1.48 vs 1.16 mmol/L, P < .001) was high in isolated IGT, and high-density lipoprotein cholesterol was low in combined IFG/IGT (1.12 vs 1.26 mmol/L, P < .001). Nuclear magnetic resonance spectroscopy revealed additional changes: increased total low-density lipoprotein (LDL) particles (1190 vs 1096 nmol/L, P < .01) in isolated IFG; increased large very-low-density lipoprotein (3.61 vs 2.47 nmol/L, P < .01) and small LDL subclass particles (665 vs 541 nmol/L, P < .05) and decreased large LDL subclass particles (447 vs 513 nmol/L, P < .01) in isolated IGT; and decreased large high-density lipoprotein subclass particles in combined IFG/IGT (4.24 vs 5.39 μmol/L, P < .001).
Conclusions:
Isolated IFG is characterized by increased apolipoprotein B and total LDL particles, whereas isolated IGT is associated with increased triglycerides, large very-low-density lipoprotein subclass particles, and structural remodeling of LDL particles. These results may help to explain differences in cardiovascular disease risk in the prediabetic state.
Cardiovascular disease risk and mortality are increased in individuals in the prediabetic state who have either impaired glucose tolerance (IGT) or impaired fasting glucose (IFG); however, those with IGT appear to be at greater risk (1–6). Information on the differences in cardiovascular risk factors in these states of glucose tolerance is limited (1). Abnormalities in lipoprotein composition occur before the onset of type 2 diabetes and have been related to insulin resistance (7, 8). Increased insulin resistance is a characteristic of both IGT and IFG, but the site of insulin resistance appears to be different (9). Therefore, we hypothesized that these states of glucose tolerance differ in terms of lipoprotein composition.
The conventional lipid panel may be insufficient to demonstrate the complete atherogenic state of the diabetic and prediabetic states (7, 8). More sophisticated methods, such as nuclear magnetic resonance (NMR) spectroscopy (10), have revealed changes in lipoprotein composition (7, 8, 11). These changes have been shown to predict incidence of coronary heart disease (12, 13). To better understand the cardiovascular disease risk in the prediabetic state, we aimed to study differences in lipoproteins, apolipoproteins, and lipoprotein heterogeneity by glucose tolerance status.
Materials and Methods
Study population
Population data were obtained from the Insulin Resistance Atherosclerosis Study (IRAS), which includes data from 1625 participants, men and women aged 40 to 69 years (14). The IRAS was designed as a large epidemiologic study to investigate relationships between insulin resistance and cardiovascular disease among a diverse population. Participants in the IRAS were men and women from three ethnic groups: Hispanics, African Americans, and non-Hispanic whites. The study was conducted at four clinical centers, enrolling patients from Los Angeles, California; Oakland, California; San Antonio, Texas; and the San Luis Valley of Colorado, from 1992 through 1994. A full description of the study has been published previously (14). This report includes data on 1107 participants from the IRAS who were not taking lipid-lowering and glucose-lowering medications, had no previous diagnosis of diabetes, and had lipoprotein levels that were measured by conventional methods and NMR spectroscopy. All participants provided written informed consent as approved by their respective center's institutional review board.
Research methods
The IRAS protocol required two visits, 1 week apart, of approximately 4 hours each at baseline and a 5-year follow-up examination. Protocols were identical at the baseline and follow-up examinations. Age, sex, and race/ethnicity were assessed by self-report. Anthropometric variables were measured using standard protocols.
A 75-g oral glucose tolerance test was administered and blood was drawn 2 hours later for repeat measurements of glucose and insulin concentrations. Subjects were asked prior to each visit to fast for 12 hours, to abstain from heavy exercise and alcohol for 24 hours, and to refrain from smoking the morning of the examination. During the second baseline visit, insulin sensitivity and insulin secretion were determined using the frequently sampled iv glucose tolerance test (FSIGTT) (14); some modifications were made to the original test. An injection of regular insulin, rather than tolbutamide, was used to ensure adequate plasma insulin levels for the accurate computation of insulin sensitivity across a broad range of glucose tolerance. Glucose in the form of 50% solution (0.3 g/kg) and regular human insulin (0.03 U/kg) were injected through an iv line at 0 and 20 minutes, respectively. Blood was collected at −5, 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 minutes for measurement of plasma glucose and insulin. Insulin sensitivity, expressed as the insulin sensitivity index (SI), was calculated using mathematical modeling methods (MINMOD version 3.0 [1994]; Los Angeles, California, courtesy of Richard Bergman, PhD).
Levels of plasma glucose and insulin were measured by standard techniques (14). Plasma lipids and lipoproteins were obtained from fasting single fresh-plasma samples using Lipid Research Clinic methods and were measured at the central IRAS laboratory at Medlantic Research Institute, Washington, DC. Lipoprotein subclass particle concentrations and average very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) particle diameters were measured by NMR spectroscopy (LipoScience Inc, Raleigh, North Carolina) (8, 15). Particle concentrations were given by the measured amplitudes of the characteristic lipid methyl group NMR signals they emit. Nine subclasses were investigated: large VLDL (including chylomicrons if present) (>60 nm), medium VLDL (35–60 nm), small VLDL (27–35 nm), intermediate-density lipoprotein (IDL, 23–27 nm), large LDL (21.2–23 nm), small LDL (18–21.2 nm), large HDL (8.8–13 nm), medium HDL (8.2–8.8 nm), and small HDL (7.3–8.2 nm). VLDL and LDL subclass particle concentrations are given in units of nanomoles per liter and HDL in micromoles per liter. The sum of each particle's subclasses provides total VLDL, LDL, and HDL particle concentrations. Weighted-average VLDL, LDL, and HDL particle sizes (in nanometers) were calculated as the sum of the diameter of each subclass multiplied by its relative mass percentage as estimated from the amplitude of its methyl NMR signal. NMR spectroscopy and conventional methods have a high degree of agreement in quantifying lipoprotein subclass concentrations.
Participants who had an existing diagnosis of diabetes or were taking glucose- or cholesterol-lowering medications were excluded from this study. Diabetes was defined as fasting glucose concentration ≥ 7.0 mmol/L and/or 2-hour glucose concentration ≥ 11.1 mmol/L (16). In the absence of diabetes, IFG was defined as fasting glucose concentration between 5.6 and <7.0 mmol/L and IGT as 2-hour plasma glucose concentration between 7.8 and <11.1 mmol/L. Therefore, this study includes data on 1107 participants (306 African Americans, 373 Hispanics, and 428 non-Hispanic whites).
Statistical analysis was carried out using the SAS statistical software (version 9.2; SAS Institute Inc., Cary, North Carolina). We evaluated the relationships between lipoprotein levels (our outcome of interest) and our two exposures, impaired fasting glucose and impaired glucose tolerance. One-way analysis of covariance was used to compare demographic and metabolic variables between glucose tolerance categories to account for the effect of age, sex, race/ethnicity, and clinic. The strength of the relationship between measures of plasma glucose was estimated by Pearson correlation coefficients. We used multiple linear regression analysis to examine the relation of fasting glucose and 2-hour glucose (independent variables) to each of the lipoproteins and apolipoproteins (dependent variable). Collinearity diagnostics (variation inflation factor and condition index) were applied to demonstrate the absence of multicollinearity effects in models that had the two measures of plasma glucose. Variable skew was corrected using log-transformed values. A probability value of < .05 was considered statistically significant.
Results
Compared with the normal range category, isolated IGT was found to be more common in women and Hispanics but relatively rare in African Americans (Table 1). Isolated IFG was more common in men. Body mass index and waist circumference were higher and SI lower in isolated IFG and isolated IGT compared with normal glucose tolerance. Isolated IFG was associated with higher apolipoprotein B (apoB), total LDL particles, IDL subclass particles, and lower HDL size. In contrast, isolated IGT was associated with higher triglycerides and triglycerides-to-HDL cholesterol ratio, larger VLDL particle size with higher large VLDL subclass particles, smaller LDL particle size with lower large LDL subclass particles and higher small LDL subclass particles, and smaller HDL particle size with higher total HDL particles and medium HDL subclass particles. In head-to-head comparisons of isolated IFG and isolated IGT, isolated IGT had lower SI (P = .003), LDL cholesterol (P = .028), and large LDL subclass particles (P = .005), as well as higher triglycerides (P < .001), triglycerides-to-HDL cholesterol ratio (P = .033), larger VLDL particle size (P = .003) with higher large VLDL subclass particles (P = .008), and higher medium HDL cholesterol subclass particles (P = .001).
Table 1.
Lipoproteins, Apolipoproteins, and Other Metabolic Variables by Glucose Tolerance Status
| Variable | Normal | Isolated IFG | Isolated IGT | IFG/IGT | Newly Diagnosed Diabetes |
|---|---|---|---|---|---|
| n | 446 | 185 | 91 | 196 | 189 |
| Age, ya | 53.1 ± 0.4 | 54.4 ± 0.6 | 56.2 ± 0.9b | 56.8 ± 0.6c | 56.9 ± 0.6c |
| Female, %a | 60.8 (56.2–65.2) | 37.3 (30.6–44.5)c | 76.9 (67.2–84.4)b | 57.1 (50.1–63.9) | 55.0 (47.9–62.0) |
| Race/ethnicity, %a | |||||
| African Americans | 23.8 (20.0–27.9) | 27.0 (21.1–33.9) | 8.8 (4.5–16.6)b | 33.7 (27.4–40.6)b | 40.2 (33.5–47.7)c |
| Hispanics | 36.3 (32.0–40.9) | 30.8 (24.6–37.8) | 48.4 (38.3–58.5)d | 30.6 (24.6–37.4) | 26.5 (20.7–33.2)d |
| Non-Hispanic whites | 39.9 (35.5–44.5) | 42.2 (35.3–49.4) | 42.9 (33.1–53.2) | 35.7 (29.3–42.7) | 33.3 (27.0–40.4) |
| Fasting glucose (mmol/L) | 5.03 ± 0.05 | 5.86 ± 0.07c,e | 5.24 ± 0.11f | 6.07 ± 0.07c | 7.93 ± 0.07c,f |
| 2-h glucose, mmol/L | 5.71 ± 0.09 | 6.16 ± 0.14c,f | 8.88 ± 0.19c | 9.15 ± 0.13c | 14.4 ± 0.14c,f |
| Body mass index, kg/m2 | 26.9 ± 0.3 | 28.3 ± 0.4b,f | 28.3 ± 0.6d,f | 31.7 ± 0.4c | 32.3 ± 0.4c |
| Waist circumference, cm | 86.6 ± 0.6 | 90.2 ± 0.9c,f | 91.0 ± 1.2b,f | 98.5 ± 0.8c | 99.9 ± 0.9c |
| SI,h ×10−4 min−1 · μU−1 · mL−1 | 2.29 ± 0.07 | 1.80 ± 0.11c,f | 1.32 ± 0.12c,g | 0.95 ± 0.06c | 0.45 ± 0.06c,f |
| Lipoproteins, mmol/L | |||||
| Total cholesterol | 5.40 ± 0.05 | 5.44 ± 0.08 | 5.38 ± 0.12 | 5.59 ± 0.08 | 5.59 ± 0.08 |
| LDL cholesterol | 3.59 ± 0.04 | 3.72 ± 0.07 | 3.46 ± 0.10e | 3.75 ± 0.07d | 3.73 ± 0.07 |
| HDL cholesterol | 1.26 ± 0.02 | 1.23 ± 0.03g | 1.26 ± 0.04g | 1.12 ± 0.02c | 1.07 ± 0.03c |
| Non-HDL cholesterol | 4.14 ± 0.05 | 4.20 ± 0.08e | 4.12 ± 0.12e | 4.47 ± 0.08c | 4.51 ± 0.08c |
| Triglyceridesh | 1.16 ± 0.02 | 1.17 ± 0.05f | 1.48 ± 0.09c | 1.58 ± 0.06c | 1.67 ± 0.07c |
| Non-HDL-to-HDL cholesterol ratio | 3.69 ± 0.09 | 3.85 ± 0.14g | 3.68 ± 0.20g | 4.47 ± 0.23c | 4.56 ± 0.14c |
| Triglycerides-to-HDL cholesterol ratio | 1.31 ± 0.07 | 1.31 ± 0.11f | 1.73 ± 0.16d | 1.89 ± 0.11c | 2.14 ± 0.11c |
| Apolipoproteins, mg/dL | |||||
| ApoA-1 | 131.7 ± 1.3 | 133.1 ± 2.0g | 135.5 ± 2.8g | 125.4 ± 1.9b | 124.1 ± 2.0b |
| ApoB | 99.8 ± 1.2 | 105.2 ± 1.8d,e | 103.4 ± 2.6e | 111.6 ± 1.8c | 113.9 ± 1.8c |
| ApoB-to-ApoA-1 ratio | 0.79 ± 0.01 | 0.82 ± 0.02g | 0.83 ± 0.03g | 0.93 ± 0.02c | 0.96 ± 0.02c |
| Lipoprotein heterogeneity (by NMR) | |||||
| Total VLDL particles, nmol/L | 62.4 ± 1.4 | 65.3 ± 2.2 | 61.2 ± 3.2 | 68.7 ± 2.1d | 65.2 ± 2.2 |
| Large | 2.47 ± 0.14 | 2.57 ± 0.22g | 3.61 ± 0.32b | 3.49 ± 0.21c | 4.44 ± 0.22c,g |
| Medium | 17.2 ± 0.6 | 17.3 ± 0.9 | 17.1 ± 1.3 | 18.9 ± 0.9 | 17.4 ± 0.9 |
| Small | 42.7 ± 1.0 | 45.5 ± 1.5 | 40.5 ± 2.1e | 46.4 ± 1.4d | 43.4 ± 1.5 |
| Total LDL particles, nmol/L | 1096 ± 18 | 1190 ± 28b,g | 1152 ± 40g | 1302 ± 27c | 1317 ± 28c |
| IDL | 41.1 ± 1.2 | 45.7 ± 1.9d | 40.6 ± 2.7g | 49.1 ± 1.8c | 48.4 ± 1.9b |
| Large | 513 ± 10 | 526 ± 16g | 447 ± 23b | 468 ± 15d | 451 ± 16b |
| Small | 541 ± 21 | 618 ± 33f | 665 ± 47d,e | 786 ± 32c | 818 ± 33c |
| Total HDL particles, μmol/L | 31.3 ± 0.2 | 31.7 ± 0.4 | 32.6 ± 0.6d,e | 31.0 ± 0.4 | 31.2 ± 0.4 |
| Large | 5.39 ± 0.12 | 5.06 ± 0.18g | 4.93 ± 0.26e | 4.24 ± 0.17c | 3.84 ± 0.18c |
| Mediumh | 2.33 ± 0.17 | 2.55 ± 0.33 | 3.12 ± 0.47c | 2.33 ± 0.25 | 2.60 ± 0.30 |
| Small | 23.4 ± 0.2 | 24.2 ± 0.4 | 23.7 ± 0.5 | 24.1 ± 0.4 | 24.5 ± 0.4d |
| Particle size, nm | |||||
| VLDL | 47.2 ± 0.5 | 48.6 ± 0.8e | 52.5 ± 1.1c | 50.8 ± 0.7c | 56.0 ± 0.8c,f |
| LDL | 21.47 ± 0.04 | 21.37 ± 0.06f | 21.25 ± 0.08d | 21.07 ± 0.05c | 20.98 ± 0.06c |
| HDL | 9.04 ± 0.02 | 8.95 ± 0.03d,g | 8.94 ± 0.04d,g | 8.80 ± 0.03c | 8.74 ± 0.03c |
Data are n; rate, 95% confidence interval; and mean ± SE.
Results adjusted for age, sex, race/ethnicity, and clinic.
Nonadjusted results.
Normal glucose tolerance was the category of comparison to assess statistical significance: b P < .01; c P < .001; d P < .05.
The combined IFG/IGT category was also used as the category of comparison: e P < .01; f P < .001; g P < .05.
Log-transformed variable then back-transformed to its units for presentation in the table.
Adiposity was higher and SI lower in the combined IFG/IGT category compared with either isolated IFG or isolated IGT. The whole range of lipoprotein and apolipoprotein changes in isolated IFG and isolated IGT was demonstrated in the combined IFG/IGT category except for higher medium HDL subclass particles. Among the lipoprotein and apolipoprotein changes that were already demonstrated in isolated IFG, apoB and total LDL particles were further increased, and HDL particle size further decreased, in the combined IFG/IGT category. Similarly, among the changes present in isolated IGT, there were additional increases in small LDL subclass particles and decreases in HDL particle size in the combined IFG/IGT category. Furthermore, the combined IFG/IGT category was associated with higher non-HDL cholesterol, non-HDL-to-HDL cholesterol ratio, apoB-to-apolipoprotein A-1 (apoA-1) ratio, total VLDL particles, and small VLDL subclass particles, as well as lower HDL cholesterol, large HDL subclass particles, and apoA-1. The newly diagnosed diabetes category did not differ from the combined IFG/IGT category with regard to any lipoprotein or apolipoprotein except for higher large VLDL subclass particles and VLDL particle size.
Because the lipoprotein relationships with glucose tolerance might be explained by disproportional distribution of men and women in isolated IFG and IGT, we examined lipoprotein concentrations by gender (Table 2). Decreased HDL cholesterol and increased triglycerides were demonstrated in the combined IFG/IGT and newly diagnosed diabetes categories in both men and women. However, the analysis carried out in men and women separately markedly reduced statistical power. Race/ethnicity also could have contributed to the differences observed. To directly address these issues and have enough statistical power, we ascertained the heterogeneity effects of gender and race/ethnicity by regression analysis. We added appropriate interaction terms sex × fasting glucose (or 2-hour glucose) and race/ethnicity × fasting glucose (or 2-hour glucose) to linear regression models that had individual lipoprotein and apolipoproteins as the dependent variable. In addition, because many lipoprotein and apolipoprotein changes are demonstrated in the diabetic state, we analyzed the relationship between plasma glucose levels and lipoproteins and apolipoproteins in nondiabetic subjects.
Table 2.
Distribution of Glucose Tolerance Status and Lipoproteins by Gender
| Normal | Isolated IFG | Isolated IGT | IFG/IGT | Newly Diagnosed Diabetes | |
|---|---|---|---|---|---|
| Number, %a | |||||
| Men | 175 (36.4%) | 116 (24.1%) | 21 (4.4%) | 84 (17.5%) | 85 (17.7%) |
| Women | 271 (43.3%) | 69 (11.0%) | 70 (11.2%) | 112 (17.9%) | 104 (16.6%) |
| LDL cholesterol, mmol/L | |||||
| Men | 3.58 ± 0.07 | 3.64 ± 0.09 | 3.48 ± 0.20 | 3.74 ± 0.10 | 3.60 ± 0.10 |
| Women | 3.60 ± 0.06 | 3.82 ± 0.11 | 3.48 ± 0.11 | 3.75 ± 0.09b | 3.83 ± 0.09c |
| HDL cholesterol, mmol/L | |||||
| Men | 1.11 ± 0.02 | 1.07 ± 0.03 | 1.21 ± 0.07 | 0.98 ± 0.04d | 0.96 ± 0.04d |
| Women | 1.37 ± 0.02 | 1.38 ± 0.04 | 1.34 ± 0.04 | 1.22 ± 0.03d | 1.16 ± 0.04b |
| Triglycerides, mmol/L | |||||
| Men | 1.26 ± 0.05 | 1.28 ± 0.07 | 1.58 ± 0.20 | 1.84 ± 0.11b | 1.72 ± 0.11b |
| Women | 1.11 ± 0.03 | 1.08 ± 0.07 | 1.39 ± 0.09b | 1.39 ± 0.07b | 1.63 ± 0.08b |
Results adjusted for age, race/ethnicity, and clinic.
Nonadjusted results.
Normal glucose tolerance was the category of comparison to assess statistical significance: b P < .001; c P < .05; d P < .01.
After adjusting for age, sex, race/ethnicity, and clinic, both fasting and 2-hour glucose had statistically significant relationships with HDL cholesterol, non-HDL cholesterol, non-HDL-to-HDL cholesterol ratio, triglycerides, triglycerides-to-HDL cholesterol ratio, apoA-1, apoB, apoB-to-apoA-1 ratio, large VLDL subclass particles, total LDL particles, IDL subclass particles, small LDL subclass particles, large HDL particles, and VLDL, LDL, and HDL particle sizes (Table 3). Fasting glucose also had a significant relationship with LDL cholesterol, total VLDL particles, and small VLDL subclass particles; whereas 2-hour glucose had an additional relationship with large LDL and medium HDL subclass particles. In different regression models, we detected no P value reaching statistical significance for interaction terms sex × fasting glucose in any model that had a relevant association except for the model with small HDL subclass particles as the dependent variable. The interaction terms race/ethnicity × fasting glucose were significant in models with large or small HDL subclass particles or HDL particle size as the dependent variable. The interaction terms sex × 2-hour glucose were statistically significant in models that had HDL cholesterol, apoB, large HDL subclass particles, and VLDL and HDL particle sizes as the dependent variable. The interaction terms sex × race/ethnicity were not statistically significant in any model that had a relevant relationship. Interaction terms sex × fasting glucose (or 2-hour glucose) were statistically significant because of the weaker relationships in men than women; whereas interaction terms race/ethnicity × fasting glucose were significant as a result of the weaker relationships in Hispanics compared with other racial/ethnic groups (Online Supplemental Tables 1 and 2, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Table 3.
Linear Regression Analysis with Individual Lipoproteins and Apolipoproteins as the Dependent Variable and Fasting Glucose (or 2-h Glucose) as the Independent Variable in Nondiabetic Participants
| Dependent Variable | Fasting Glucose (Independent Variable)a |
2-h Glucose (Independent Variable)a |
||||
|---|---|---|---|---|---|---|
| β (SE)b | Interaction Sex × Fasting Glucose P Value | Interaction Race/Ethnicity × Fasting Glucose P Value | β (SE)b | Interaction Sex × 2-h Glucose P Value | Interaction Race/Ethnicity × 2-h Glucose P Value | |
| Lipoproteins, mmol/L | ||||||
| Total cholesterol | 0.73 ± 0.38 | .465 | .638 | 0.56 ± 0.37 | .735 | .741 |
| LDL cholesterol | 0.81 ± 0.30c | .387 | .777 | 0.13 ± 0.30 | .510 | .416 |
| HDL cholesterol | −0.62 ± 0.12d | .636 | .410 | −0.57 ± 0.12d | .032 | .624 |
| Triglyceridese | 0.09 ± 0.02d | .548 | .773 | 0.16 ± 0.02d | .461 | .440 |
| Non-HDL cholesterol | 1.34 ± 0.39d | .573 | .888 | 1.15 ± 0.38c | .718 | .991 |
| Non-HDL-to-HDL cholesterol ratio | 0.32 ± 0.06d | .671 | .737 | 0.29 ± 0.06d | .776 | .948 |
| Triglycerides-to-HDL cholesterol ratio | 0.19 ± 0.05d | .203 | .448 | 0.28 ± 0.05d | .240 | .379 |
| Apolipoproteins, mg/dL | ||||||
| ApoA-1 | −2.99 ± 0.91c | .406 | .763 | −1.83 ± 0.90f | .133 | .777 |
| ApoB | 4.19 ± 0.83d | .510 | .697 | 4.52 ± 0.81d | .025 | .843 |
| ApoB-to-apoA-1 ratio | 0.49 ± 0.09d | .528 | .900 | 0.51 ± 0.09d | .996 | .872 |
| Lipoprotein composition | ||||||
| Total VLDL particles, nmol/L | 3.56 ± 1.02d | .751 | .674 | 1.72 ± 1.00 | .051 | .507 |
| Large | 0.35 ± 0.10d | .536 | .485 | 0.65 ± 0.09d | .639 | .347 |
| Medium | 0.81 ± 0.43 | .318 | .384 | 0.45 ± 0.42 | .827 | .974 |
| Small | 2.40 ± 0.68d | .232 | .822 | 0.62 ± 0.67 | .007 | .170 |
| Total LDL particles, nmol/L | 83.5 ± 12.8d | .308 | .997 | 79.7 ± 12.5d | .096 | .488 |
| IDL | 2.79 ± 0.87c | .184 | .387 | 1.82 ± 0.85f | .061 | .170 |
| Large | −10.7 ± 7.1 | .237 | .321 | −27.8 ± 6.9d | .790 | .913 |
| Small | 91.5 ± 15.2d | .136 | .833 | 105.6 ± 14.7d | .237 | .734 |
| Total HDL particles, μmol/L | 0.04 ± 0.18 | .032 | .239 | 0.16 ± 0.18 | .002 | .700 |
| Large | −0.50 ± 0.09d | .884 | .025 | −0.49 ± 0.08d | .032 | .920 |
| Mediume | 0.00 ± 0.03 | .448 | .485 | 0.08 ± 0.03c | .933 | .430 |
| Small | 0.60 ± 0.17d | .006 | .004 | 0.28 ± 0.17 | <.001 | .045 |
| Particle size, nm | ||||||
| VLDL | 0.83 ± 0.34f | .189 | .789 | 2.13 ± 0.33d | .046 | .172 |
| LDL | −0.15 ± 0.03d | .308 | .577 | −0.18 ± 0.03d | .226 | .887 |
| HDL | −0.11 ± 0.01d | .300 | .021 | −0.10 ± 0.01d | .001 | .475 |
Results adjusted for age, sex, race/ethnicity, and clinic.
Results expressed per 1 SD unit increase.
P < .01.
P < .001.
Log-transformed variable.
P < .05.
In another set of linear regression models, we included both measures of plasma glucose in each of the models to account for the moderate relationship between fasting glucose and 2-hour glucose (r = 0.45) (Table 4). The relation that fasting glucose had with triglycerides, triglycerides-to-HDL cholesterol ratio, large VLDL subclass particles, and VLDL particle size was largely explained by 2-hour glucose; whereas the relation of 2-hour glucose with non-HDL cholesterol, apoA-1, and IDL subclass particles was explained by fasting glucose. There was not significant collinearity effect between fasting and 2-hour plasma glucose in any of the regression models (variation inflation factor < 1.5 and condition index < 3.9 for all regression models). After the effect of the central adiposity and SI was accounted for, fasting glucose remained associated with LDL cholesterol, apoB-to-apoA-1 ratio, total VLDL particles, small VLDL subclass particles, total LDL particles, small HDL subclass particles, and HDL particle size; whereas 2-hour glucose had an independent relationship with triglycerides, large VLDL subclass particles, large and small LDL subclass particles, and VLDL and LDL particle sizes.
Table 4.
Multiple Linear Regression Analysis with Individual Lipoproteins and Apolipoproteins as the Dependent Variable in Nondiabetic Subjects
| Dependent Variable | Model Adjustment 1a Fasting Glucose β (SE) | Model Adjustment 1a 2-h Glucose β (SE) | Model Adjustment 2b |
Model Adjustment 3c |
||
|---|---|---|---|---|---|---|
| Fasting Glucose β (SE) | 2-h Glucose β (SE) | Fasting Glucose β (SE) | 2-h Glucose β (SE) | |||
| Lipoproteins, mmol/L | ||||||
| Total cholesterol | 0.73 ± 0.38 | 0.56 ± 0.37 | 0.59 ± 0.42 | 0.30 ± 0.41 | 0.53 ± 0.45 | 0.17 ± 0.46 |
| LDL cholesterol | 0.81 ± 0.30d | 0.13 ± 0.30 | 0.95 ± 0.34d | −0.30 ± 0.34 | 0.78 ± 0.36e | −0.47 ± 0.38 |
| HDL cholesterol | −0.62 ± 0.12f | −0.57 ± 0.12f | −0.44 ± 0.14d | −0.37 ± 0.13d | −0.18 ± 0.14 | 0.13 ± 0.14 |
| Triglyceridesg | 0.09 ± 0.02f | 0.16 ± 0.02f | 0.03 ± 0.02 | 0.14 ± 0.02f | −0.01 ± 0.02 | 0.08 ± 0.02f |
| Non-HDL cholesterol | 1.34 ± 0.39f | 1.15 ± 0.38d | 1.02 ± 0.44e | 0.69 ± 0.43 | 0.70 ± 0.46 | 0.06 ± 0.47 |
| Non-HDL-to-HDL cholesterol ratio | 0.32 ± 0.06f | 0.29 ± 0.06f | 0.23 ± 0.07d | 0.18 ± 0.07e | 0.14 ± 0.08 | −0.03 ± 0.08 |
| Triglycerides-to-HDL cholesterol ratio | 0.19 ± 0.05f | 0.28 ± 0.05f | 0.07 ± 0.05 | 0.25 ± 0.05f | 0.01 ± 0.06 | 0.10 ± 0.06 |
| Apolipoproteins, mg/dL | ||||||
| ApoA-1 | −2.99 ± 0.91d | −1.83 ± 0.90e | −2.70 ± 1.03d | −0.60 ± 1.01 | −1.73 ± 1.09 | 0.59 ± 1.12 |
| ApoB | 4.19 ± 0.83f | 4.52 ± 0.81f | 2.62 ± 0.93d | 3.34 ± 0.91f | 1.80 ± 0.97 | 1.63 ± 1.00 |
| ApoB-to-apoA-1 ratio | 0.49 ± 0.09f | 0.51 ± 0.09f | 0.32 ± 0.11d | 0.37 ± 0.10f | 0.22 ± 0.11e | 0.18 ± 0.11 |
| Lipoprotein heterogeneity | ||||||
| Total VLDL particles, nmol/L | 3.56 ± 1.02f | 1.72 ± 1.00 | 3.49 ± 1.15d | 0.15 ± 1.12 | 2.63 ± 1.21e | −1.52 ± 1.24 |
| Large | 0.35 ± 0.10f | 0.65 ± 0.09f | 0.06 ± 0.11 | 0.63 ± 0.10f | −0.05 ± 0.11 | 0.39 ± 0.12f |
| Medium | 0.81 ± 0.43 | 0.45 ± 0.42 | 0.76 ± 0.49 | 0.11 ± 0.48 | 0.71 ± 0.52 | −0.19 ± 0.53 |
| Small | 2.40 ± 0.68f | 0.62 ± 0.67 | 2.67 ± 0.77f | −0.58 ± 0.75 | 1.96 ± 0.80e | −1.72 ± 0.82e |
| Total LDL particles, nmol/L | 83.5 ± 12.8f | 79.7 ± 12.5f | 58.3 ± 14.3f | 53.5 ± 14.0f | 40.9 ± 14.9d | 14.8 ± 15.3 |
| IDL | 2.79 ± 0.87d | 1.82 ± 0.85e | 2.46 ± 0.98e | 0.71 ± 0.95 | 1.36 ± 1.03 | −0.52 ± 1.06 |
| Large | −10.7 ± 7.1 | −27.8 ± 6.9f | 3.02 ± 7.96 | −29.1 ± 7.8f | 5.83 ± 8.48 | −20.2 ± 8.7e |
| Small | 91.5 ± 15.2f | 105.6 ± 14.7f | 52.8 ± 16.9d | 81.9 ± 16.5f | 33.7 ± 17.6 | 35.5 ± 18.1e |
| Total HDL particles, μmol/L | 0.04 ± 0.18 | 0.16 ± 0.18 | −0.04 ± 0.20 | 0.17 ± 0.20 | 0.09 ± 0.22 | 0.41 ± 0.22 |
| Large | −0.50 ± 0.09f | −0.49 ± 0.08f | −0.35 ± 0.10f | −0.33 ± 0.09f | −0.16 ± 0.10 | −0.04 ± 0.10 |
| Mediumg | 0.00 ± 0.03 | 0.08 ± 0.03d | −0.05 ± 0.03 | 0.10 ± 0.03d | −0.04 ± 0.03 | 0.12 ± 0.03f |
| Small | 0.60 ± 0.17f | 0.28 ± 0.17 | 0.59 ± 0.20d | −0.01 ± 0.19 | 0.47 ± 0.21e | −0.13 ± 0.21 |
| Particle size, nm | ||||||
| VLDL | 0.83 ± 0.34e | 2.13 ± 0.33f | −0.22 ± 0.38 | 2.23 ± 0.37f | −0.55 ± 0.40 | 1.33 ± 0.41d |
| LDL | −0.15 ± 0.03f | −0.18 ± 0.03f | −0.08 ± 0.03d | −0.15 ± 0.03f | −0.04 ± 0.03 | −0.07 ± 0.03e |
| HDL | −0.11 ± 0.01f | −0.10 ± 0.01f | −0.08 ± 0.02f | −0.06 ± 0.02f | −0.05 ± 0.02d | −0.02 ± 0.02 |
Results expressed per 1 SD unit increase.
Model adjustment 1: results adjusted for age, sex, race/ethnicity, and clinic.
Model adjustment 2: results adjusted for variables in model adjustment 1, but fasting glucose and 2-h glucose were both included in all models as independent variables.
Model adjustment 3: similar to model adjustment 2, but results were also adjusted for waist circumference and SI.
P < .01.
P < .05.
P < .001.
Log-transformed variable.
Discussion
Our study reveals distinct lipid profiles in IFG and IGT. Isolated IFG is associated with higher apoB, whereas isolated IGT is associated with higher triglycerides and the triglycerides-to-HDL cholesterol ratio. A more pronounced derangement of lipoproteins and apolipoproteins is demonstrated in the IFG/IGT and newly diagnosed diabetes categories, including lower HDL cholesterol and apoA-1 and higher non-HDL cholesterol, the non-HDL-to-HDL cholesterol ratio, and the apoB-to-apoA-1 ratio. NMR spectroscopy uncovers changes in lipoprotein composition: 1) high total LDL particle and IDL subclass particle concentrations and small HDL particle size in isolated IFG; 2) large VLDL particle size with high large VLDL subclass particles, small LDL particle size with high small LDL subclass particles and low large LDL subclass particles, and small HDL particle size in isolated IGT; and 3) low large HDL particles in the IFG/IGT state.
Garvey et al (7) have described lipoprotein changes associated with directly measured insulin resistance by the hyperinsulinemic-euglycemic clamp. These changes include high triglycerides and low HDL cholesterol, larger VLDL size with greater concentration of large VLDL particles, smaller LDL size with greater concentration of small LDL particles and lower concentration of large LDL particles and an overall increase in LDL particles, and smaller HDL size with lower concentration of large HDL particles and a modest increase of small HDL particles. We previously have reported similar results using SI as a direct measure of insulin sensitivity (11). Garvey et al and others have suggested the following sequence of lipoprotein changes in insulin resistance. Triglyceride concentration is increased as a consequence of both overproduction of triglyceride-rich VLDL particles and impairment of VLDL clearance (partially due to the reduced activity of plasma lipoprotein lipase) (17, 18). Triglyceride concentration may have a major role on LDL heterogeneity (19). Large VLDL particles affect cholesterol ester-triglyceride exchange between VLDL and LDL particles. These LDL particles tend to have more triglycerides and fewer cholesterol esters (20). Under the action of hepatic lipase, triglycerides are hydrolyzed, resulting in small dense LDL particles. Triglyceride-rich large VLDL and small LDL particles influence triglyceride exchange with HDL particles via lecithin-cholesterol acyltransferase and cholesterol ester transfer protein. The result is a reduction in the amount of large HDL particles (21).
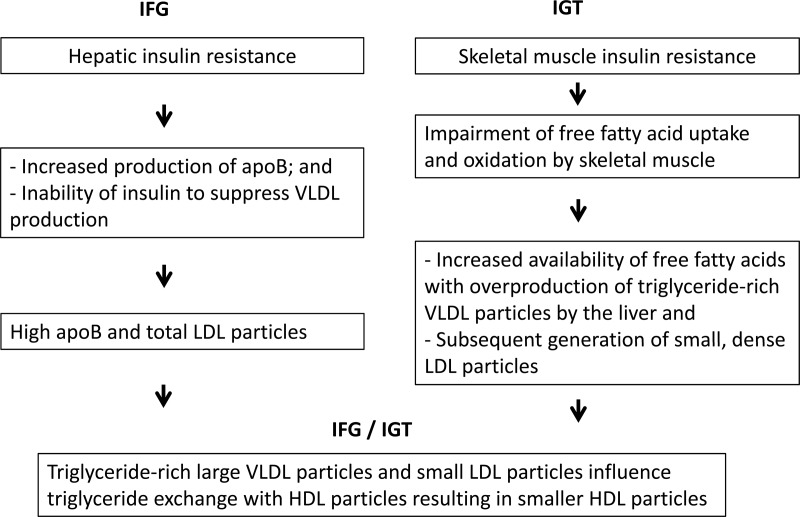
Our study suggests that the site of insulin resistance influences the array of lipoprotein changes (Figure 1). Hepatic insulin resistance is high and muscle insulin sensitivity is normal in isolated IFG, whereas hepatic insulin sensitivity is slightly decreased and muscle insulin resistance is high in isolated IGT (9). Both hepatic and muscle insulin resistance are high in individuals who have both IFG and IGT. Because fasting glucose closely reflects hepatic glucose production (22), lipoprotein heterogeneity in IFG may reflect hepatic insulin resistance. Individuals with IFG may have the following sequence of events. Liver fat has been associated with increased production rates of VLDL particles and apoB in multiple regression models (23) and with the inability of insulin to suppress VLDL production (24). Overproduction of VLDL particles results in the generation of more LDL subclass particles (12), but the proportion of large and small LDL subclass particles is not disturbed. This suggests that the triglyceride concentration in VLDL particles in individuals with isolated IFG is similar to that in those with normal glucose tolerance. Isolated IFG is characterized by a slight increase in apoB and total LDL particles and decrease in HDL particle size. Fasting glucose does not have a significant relationship with triglycerides, large VLDL particles, and large LDL particles after taking into account the effect of 2-hour glucose. The declines in apoA-1 and large HDL particles are more distinctive traits of a later stage in the diabetes disease process.
Figure 1.
Lipids, glucose tolerance, and insulin resistance.
Lipoprotein changes in isolated IGT are similar to previously described changes in insulin resistance (7, 11). Skeletal muscle free fatty acid uptake and oxidation are impaired in individuals with IGT (25). An imbalance between fatty acid uptake and oxidation may enhance the accumulation of triglycerides and other lipid intermediaries in skeletal muscle and induce insulin resistance (26). Increased availability of free fatty acids may be the driving force for the overproduction of VLDL particles (27) and the availability of triglycerides for VLDL assembly (28). Triglyceride concentration may have a major role on LDL heterogeneity (19). Large VLDL particles affect cholesterol ester-triglyceride exchange between VLDL and LDL particles. These LDL particles tend to have more triglycerides and fewer cholesterol esters (20). Under the action of hepatic lipase, triglycerides are hydrolyzed, resulting in small, dense LDL particles. Individuals with isolated IGT have high triglycerides, larger VLDL size with greater concentration of large VLDL subclass particles, smaller LDL size with greater concentration of small LDL subclass particles and lower concentration of large LDL subclass particles, and smaller HDL size. Therefore, IGT may be characterized by an increase in triglyceride-rich VLDL particles and structural remodeling of LDL particles. The reduction in large HDL particles occurs later in the disease process. The distinctive lipid profile in isolated IFG and isolated IGT may help to explain the often greater cardiovascular disease risk in individuals with IGT (1–6). The full array of lipoprotein and apolipoprotein changes in newly diagnosed diabetes is already demonstrated prior to the onset of diabetes (in individuals who have both IFG and IGT).
ApoB reflects the total number of atherogenic particles (VLDL, IDL, and LDL subclass particles), but LDL particles usually contribute to more than 90% of the total amount of apoB (29). Some authors have questioned the reliability of NMR spectrometry for defining the distribution of lipoproteins. Specifically, there is a discrepancy when apoB concentration (molecular weight of 550 000) is calculated from the lipoprotein particle number (total LDL particles plus VLDL particles) assuming one apoB per particle. When apoB and NMR LDL particles (or LDL + VLDL particles) are expressed in the same concentration units, the NMR values are about 35% lower. We attribute this systematic difference to the lack of standardization/harmonization of the NMR assay and apoB immunoassay. LDL particles and apoB immunoassay results are generally highly correlated (10), and in outcome studies the two measures provide closely comparable prediction of cardiovascular risk (30). The key observation in our study is that relative differences in apoB and LDL particle levels as a function of glucose tolerance status are virtually identical. Whether the observed bias in absolute concentrations of apoB and LDL particles is attributable to apoB inaccuracy, LDL particle inaccuracy, or inaccuracies in both methods is difficult to determine and must await a formal standardization effort. As reported by the International Federation of Clinical Chemistry, the quantification of apoB is difficult because lipid-free apoB cannot be used as a primary standard due to irreversible aggregation (31).
In conclusion, our results indicate that IFG and IGT are associated with different patterns of lipid changes. These changes are similar in men and women and different race/ethnic groups. However, gender and racial/ethnic differences may be relevant for HDL cholesterol and HDL subclass particles. Differences in lipoprotein composition in IFG and IGT may be the result of distinct pathophysiologic mechanisms, which may be related to the site of insulin resistance (hepatic or skeletal muscle). The cross-sectional nature of this study and lack of time ordering (eg, what comes first, lipoprotein changes or glucose metabolism abnormalities) limit our ability to make causal inferences. Future research regarding the natural history of lipoprotein changes as subjects progress across the spectrum of glucose tolerance would be of value. Lipoprotein heterogeneity may help to explain differences in cardiovascular risk between individuals with IFG and IGT.
Supplementary Material
Acknowledgments
This work was supported by Grants HL-47887, HL-47889, HL-47890, HL-47892, and HL-47902 from the National Heart, Lung, and Blood Institute; and Grants M01 RR431 and M01 RR01346 from the General Clinical Research Centers Program (National Center for Research Resources), National Institutes of Health.
Disclosure Summary: The authors declare that there is no duality of interest associated with this manuscript.
Footnotes
- apoA-1
- apolipoprotein A-1
- apoB
- apolipoprotein B
- HDL
- high-density lipoprotein
- IDL
- intermediate-density lipoprotein
- IFG
- impaired fasting glucose
- IGT
- impaired glucose tolerance
- IRAS
- Insulin Resistance Atherosclerosis Study
- LDL
- low-density lipoprotein
- NMR
- nuclear magnetic resonance
- SI
- insulin sensitivity index
- VLDL
- very-low-density lipoprotein.
References
- 1. Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 2. DECODE study group Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354:617–621 [PubMed] [Google Scholar]
- 3. Stern MP, Fatehi P, Williams K, Haffner SM. Predicting future cardiovascular disease: do we need the oral glucose tolerance test? Diabetes Care. 2002;25:1851–1856 [DOI] [PubMed] [Google Scholar]
- 4. Barr EL, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia. 2009;52:415–424 [DOI] [PubMed] [Google Scholar]
- 5. de Vegt F, Dekker JM, Ruhe HG, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 1999;42:926–931 [DOI] [PubMed] [Google Scholar]
- 6. Qiao Q, Dekker JM, de Vegt F, et al. Two prospective studies found that elevated 2-hr glucose predicted male mortality independent of fasting glucose and HbA1c. J Clin Epidemiol. 2004;57:590–596 [DOI] [PubMed] [Google Scholar]
- 7. Garvey WT, Kwon S, Zheng D, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462 [DOI] [PubMed] [Google Scholar]
- 8. Festa A, Williams K, Hanley AJ, et al. Nuclear magnetic resonance lipoprotein abnormalities in pre-diabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–3472 [DOI] [PubMed] [Google Scholar]
- 9. Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 10. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870 [DOI] [PubMed] [Google Scholar]
- 11. Goff DC, D'Agostino RB, Jr, Haffner SM, Otvos JD. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the Insulin Resistance Atherosclerosis Study. Metabolism. 2005;54:264–270 [DOI] [PubMed] [Google Scholar]
- 12. Kuller L, Arnold A, Tracy R, et al. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2002;22:1175–1180 [DOI] [PubMed] [Google Scholar]
- 13. Arsenault BJ, Lemieux I, Després JP, et al. Comparison between gradient gel electrophoresis and nuclear magnetic resonance spectroscopy in estimating coronary heart disease risk associated with LDL and HDL particle size. Clin Chem. 2010;56:789–798 [DOI] [PubMed] [Google Scholar]
- 14. Wagenknecht LE, Mayer EJ, Rewers M, et al. The Insulin Resistance Atherosclerosis Study (IRAS) objectives, design, and recruitment results. Ann Epidemiol. 1995;5:464–472 [DOI] [PubMed] [Google Scholar]
- 15. Goff DC, D'Agostino RB, Haffner SM, Saad MF, Wagenknecht LE. Lipoprotein concentrations and carotid atherosclerosis by diabetes status: results from the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2000;23:1006–1011 [DOI] [PubMed] [Google Scholar]
- 16. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 17. Knudsen P, Eriksson J, Lahdenpera S, Kahri J, Groop L, Taskinen M-R. Changes of lipolytic enzymes cluster with insulin resistance syndrome. Diabetologia. 1995;38:48–53 [DOI] [PubMed] [Google Scholar]
- 18. Chen Y-D, Facchini F, Landau C, Hollenbeck CB, Reaven GM. Plasma post-heparin lipoprotein lipase activity is decreased in normal individuals who are resistant to insulin-mediated glucose uptake. Endocrinol Metab. 1994;1:153–158 [Google Scholar]
- 19. Lahdenpera S, Syvanne M, Kahri J, Taskinen M-R. Regulation of low density particle size distribution in NIDDM and coronary disease: importance of serum triglycerides. Diabetologia. 1996;39:453–461 [DOI] [PubMed] [Google Scholar]
- 20. Malmstrom R, Packard CJ, Caslake M, et al. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 1997;40:454–462 [DOI] [PubMed] [Google Scholar]
- 21. Tilley-Kiesi M, Knudsen P, Groop L, Taskinen M-R. Hyperinsulinemia and insulin resistance are associated with multiple abnormalities of lipoprotein subclasses in glucose tolerant relatives of NIDDM patients. J Lipid Res. 1996;37:1569–1578 [PubMed] [Google Scholar]
- 22. Bavenholm PN, Pigon J, Ostenson CG, Efendic S. Insulin sensitivity of suppression of endogenous glucose production is the single most important determinant of glucose tolerance. Diabetes. 2001;50:1449–1454 [DOI] [PubMed] [Google Scholar]
- 23. Adiels M, Taskinen MR, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765 [DOI] [PubMed] [Google Scholar]
- 24. Adiels M, Westerbacka J, Soro-Paavonen A, et al. Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia. 2007;50:2356–2365 [DOI] [PubMed] [Google Scholar]
- 25. Corpeleijn E, Mensink M, Kooi ME, Roekaerts PM, Saris WH, Blaak EE. Impaired skeletal muscle substrate oxidation in glucose-intolerant men improves after weight loss. Obesity. 2008;16:1025–1032 [DOI] [PubMed] [Google Scholar]
- 26. Boden G, Laakso M. Lipids and glucose in type 2 diabetes: what is the cause and effect? Diabetes Care. 2004;27:2253–2259 [DOI] [PubMed] [Google Scholar]
- 27. Adiels M, Packard C, Caslake MJ, et al. A new combined multicompartmental model for apolipoprotein B-100 and triglyceride metabolism in VLDL subfractions. J Lipid Res. 2005;46:58–67 [DOI] [PubMed] [Google Scholar]
- 28. Zhang YL, Hernandez-Ono A, Ko C, Yasunaga K, Huang LS, Ginsberg HN. Regulation of hepatic apolipoprotein B-lipoprotein assembly and secretion by the availability of fatty acids. I. Differential response to the delivery of fatty acids via albumin or remnant-like emulsion particles. J Biol Chem. 2004;279:19362–19374 [DOI] [PubMed] [Google Scholar]
- 29. Walldius G, Jungner I. Rationale for using apolipoprotein B and apolipoprotein A-I as indicators of cardiac risk and as targets for lipid-lowering therapy. Eur Heart J. 2005;26:210–212 [DOI] [PubMed] [Google Scholar]
- 30. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marcovina SM, Albers JJ, Kennedy H, Mei JV, Henderson LO, Hannon WH. International Federation of Clinical Chemistry standardization project for measurements of apolipoproteins A-I and B. IV. Comparability of apolipoprotein B values by use of International Reference Material. Clin Chem. 1994;40:586–592 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.