Abstract
Context:
Most current knowledge of pancreatic islet pathophysiology in diabetes mellitus has come from animal models. Even though islets from humans are readily available, only a few come from diabetic donors. We had the uncommon opportunity to acquire islets from humans with type 2 diabetes and used it to perform a study not previously done with human or animal islets.
Objectives:
Oxidative stress has been proposed as a mechanism for impaired β-cell function in type 2 diabetes. Lipid peroxides caused by reactive oxygen species are damaging to body tissues. The objective was to determine whether lipid peroxide-protein adducts occur in pancreatic islets of humans with type 2 diabetes.
Design:
Immunoblots with two antibodies to hydroxynonenal and 2 other antibodies we generated against reactive small aliphatic compounds were used to detect lipid peroxide-protein adducts in islets of patients with type 2 diabetes and controls.
Results:
The antibodies reacted strongly to ≥5 islet proteins. The major hydroxynonenal adduct in the islets of type 2 diabetes patients was a 52-kDa protein seen with all 4 antibodies that was also seen in islets of nondiabetic humans, rat islets, and insulinoma cells and in mitochondria of various rat tissues. Nano-LC-MS/MS (liquid chromatography-tandem mass spectrometry) and MALDI-TOF (matrix-assisted laser desorption/ionization-time of flight) analysis identified the protein as the β-chain of the mitochondrial F-ATP synthase, an enzyme responsible for 95% of ATP formed in tissues.
Conclusions:
Lipid peroxide-protein adducts occur in β-cells in the nondiabetic state and in diabetes. Lipid peroxidation is thought to be damaging to tissues. Analogous to various other unhealthy characteristics, the presence in nondiabetic individuals of lipid peroxide-protein adducts does not necessarily indicate they are not detrimental.
A great deal of information has been learned from studying pancreatic islets isolated from animal models of type 2 diabetes that were usually rodent models. Although animal models are an excellent resource, islets from normal humans are known to differ from rodent islets in several morphologic (1, 2) and metabolic (3, 4) aspects, suggesting it is important to study islets from diabetic humans. Although human islets are now readily available, the number of human islet donors who have type 2 diabetes is still small. Thus, a chance to study the effect of diabetes on the human pancreatic islet presents an uncommon opportunity. We had this opportunity and used it to perform a study not previously performed with human or animal islets.
According to the lipotoxicity hypothesis, the hyperlipidemia often present in type 2 diabetes combines with hyperglycemia to impair insulin secretion by interfering with signal transduction pathways (5–10). One of the postulated mechanisms of this impairment is that hyperglycemia increases reactive oxygen species that generate lipid peroxides in the β-cell (11). Lipid peroxides are strong electrophiles that can covalently bond to nucleophilic groups on cellular proteins. In the current study, pancreatic islets of deceased patients with type 2 diabetes were examined to study the hypothesis that lipid peroxides might be generated from oxidation of cellular lipids and could modify islet proteins. We used immunoblot analysis with antibodies to the lipid peroxide hydroxynonenal and 2 other antibodies we generated against other small reactive aliphatic compounds to look for bonding of aliphatic peroxides to proteins in the islets of type 2 diabetic donors and nondiabetic controls. We used nano-LC-MS/MS (liquid chromatography-tandem mass spectrometry) and MALDI-TOF (matrix-assisted laser desorption/ionization-time of flight) fingerprint analysis to successfully identify a major lipid peroxide-protein adduct in mitochondria.
Materials and Methods
Materials
Pancreatic islets of two human donors with type 2 diabetes and two nondiabetic human donors were from the Karolinska Institutet in Sweden in 2007. Human islets from the United States were from 3 donors with type 2 diabetes and nondiabetic donors from the Juvenile Diabetes Research Foundation Islet Program or the Integrated Islet Distribution Program in the United States in 2010–2011. Rat islets were from female 350-g Sprague-Dawley rats isolated, as previously described (3, 4). The INS-1 832/13 cell line was from Chris Newgard and was maintained in culture in INS-1 medium (RPMI 1640 tissue culture medium supplemented with 10% fetal calf serum, 50μM β-mercaptoethanol, and 1mM pyruvate) as previously described (3). Clinical characteristics of the human pancreas donors are shown in Supplemental Tables 1 and 2 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Islets of type 2 diabetes patients supplied by the Karolinska Institutet showed a 28% decrease in insulin content relative to the control islets, and the protein content was similar in the two types of islets (12). Polyclonal antisera to 4-hydroxynonenal were from L.I.S. or from Chemicon (Temecula, California). The antiactin antibody was from Sigma (St Louis, Missouri) (catalog number A5060).
Antisera to aliphatic compounds
The antigens were conjugated to keyhole limpet hemocyanin (KLH) by mixing 0.5 ml of a 10mM solution of 3-hydroxyoctanoic acid (10mM) plus nanoic acid (10mM) (antibody 627) or 4-hydroxynonenal (10mM) plus nanoic acid (10mM) (antibody 628) in 0.9M NaCl and 0.1M 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 4.7) with 0.15 ml of 65mM 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) (Pierce Chemical Co, Rockford, Illinois). After 10 minutes, the activated antigen was added to 0.2 ml of KLH (10 mg/ml) and the mixture allowed to set at room temperature. After 2 hours, the unconjugated antigen was removed from the antigen-KLH conjugate by gel filtration as described in the Pierce immunogen EDC conjugation kit instructions. The conjugate was mixed with Freund's adjuvant, and 0.5 to 1.0 mg of hapten-protein was injected into rabbits at 6-week intervals 3 times and again a fourth time after 6 months. Antisera were harvested 3 weeks after the second, third, and fourth injections of hapten, as previously described (13).
Subcellular fractionation
Organs were homogenized in 220mM mannitol, 70mM sucrose, and 5mM potassium HEPES buffer (pH 7.5) and centrifuged at 600g for 10 min to obtain a pellet of nuclei and cell debris. The resulting supernatant fraction was centrifuged at 5500g (islets) or 15 000g (other tissues) for 10 min to obtain the mitochondrial pellet. Mitochondrial pellets from large tissues were resuspended in the mannitol, sucrose, and HEPES buffer and washed 3 times by centrifugation. Islet mitochondrial pellets were washed once. The postmitochondrial supernatant fraction was centrifuged at 21 000g for 10 minutes to obtain an additional supernatant fraction called cytosol (14).
Immunoblotting
Whole-cell homogenates were boiled in sample buffer [1% sodium dodecyl sulfate, 5% glycerol, 65mM Tris-chloride (pH 6.8), 0.01% bromphenol blue, and 2.5% β-mercaptoethanol]. Proteins were separated by electrophoresis on 7.5% SDS-PAGE gels and electrotransferred to nitrocellulose membranes. After transfer, the membrane was blocked with buffer containing 10mM Tris buffer (pH 8.0) with 150mM NaCl (Tris-buffered saline) and 5% nonfat powdered dry milk. The membrane was incubated 18 h with the primary antisera at 4°C. The membrane was rinsed with Tris-buffered saline and incubated for 40 minutes at room temperature with a horseradish peroxidase-conjugated goat antirabbit IgG (Thermo Scientific, Waltham, Massachusetts). The signal was detected using the Immobilon Western chemiluminescent horseradish peroxidase developer (Millipore, Billerica, Massachusetts). The blot was stripped with Restore Western blot stripping buffer (Thermo Scientific) before reprobing with a polyclonal antibody to actin with detection as above and as previously described (3).
Identification of the major hydroxynonenal-protein adduct
To identify the major hydroxynonenal protein adduct, extracts of cellular proteins (prepared as described in the Supplemental Data) were run on a pair of 2-dimensional electrophoresis gels in parallel. One gel was analyzed by immunoblot analysis with antihydroxynonenal antibody; the other gel was stained for protein, and the spot corresponding to the location of the hydroxynonenal-containing protein was excised and digested with trypsin. The identity of the peptides in the spot was determined by nano-LC-MS/MS and MALDI-TOF fingerprint analysis.
Results
Immunoblots of lipid peroxide-protein adducts
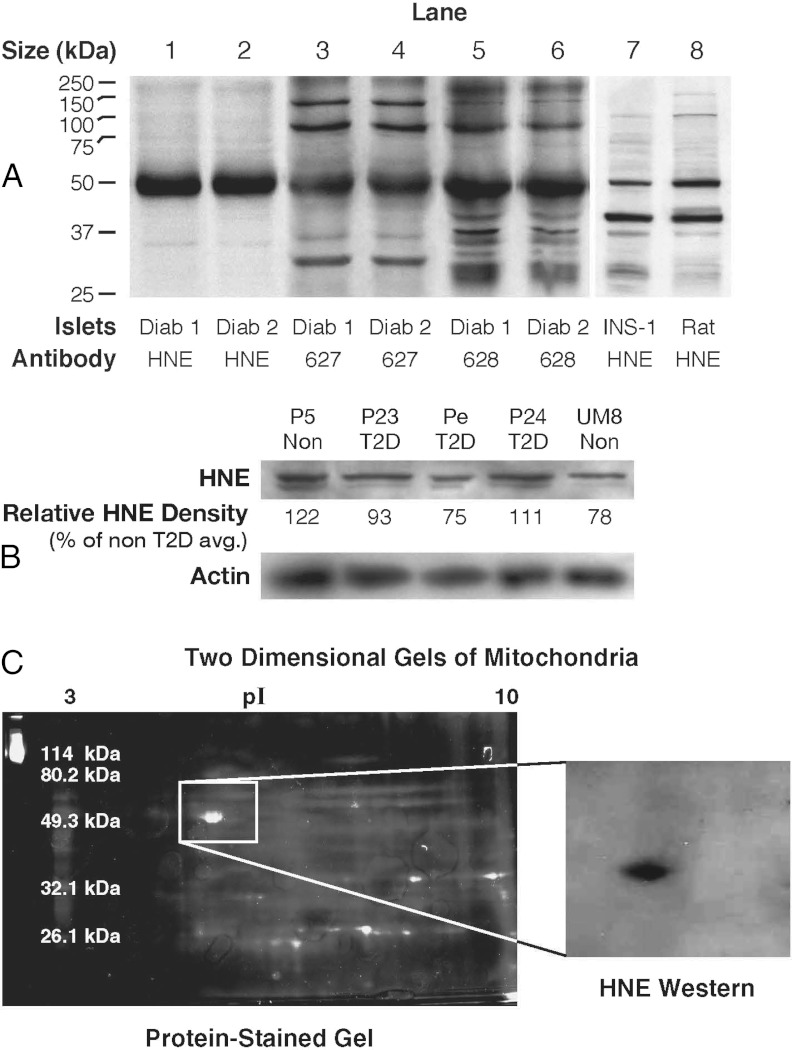
Immunoblot analysis using antihydroxynonenal antiserum (from L.I.S.) (15, 16) showed that a protein with a relative size of 52 kDa was modified in islets from the diabetic humans from the Karolinska Institutet, in islets from rats, and in the INS-1 832/13 insulinoma cell line (Figure 1A, lanes 1, 2, 7, and 8). The hydroxynonenal-modified 52-kDa protein band was also seen in immunoblots of islet proteins from nondiabetic humans from the Karolinska Institutet (Supplemental Figure 1). With the two additional antisera we generated against 3-hydroxyoctanoic acid plus nanoic acid (antibody 627) or against hydroxynonenal plus nanoic acid lactone (antibody 628), a protein of the same size, as well as proteins of other sizes, were detected in the islets of the human type 2 diabetic subjects (Figure 1A, lanes 3–6). It could not be clearly established whether the level of the 52-kDa hydroxynonenal-protein adduct was different between the diabetic islets and the nondiabetic islets from the Karolinska Institutet. Therefore, additional islets from 3 human donors with type 2 diabetes and various donors without diabetes from the United States were studied using a different antihydroxynonenal antibody (catalog number AB5605 from Chemicon, Temecula, California). Four blots using islet proteins from the donors with type 2 diabetes and from various different nondiabetic donors were studied. An example of one of these blots is shown in Figure 1B. To confirm the relative densities of the hydroxynonenal-adduct 52-kDa band, the density of the band was measured in multiple replicate lanes in the 4 immunoblots using the same 3 samples from the type 2 diabetes donors and islet preparations from various nondiabetic donors (n = 6). These measurements showed that there was no difference between the relative densities of the bands in islet donor samples from the diabetic donors and the nondiabetic islet donors [100 ± 8 (12) vs 98 ± 8 (9) (mean relative density ± SE (n)]. Two-dimensional gel electrophoresis and immunoblot analysis of subcellular fractions from various rat tissues showed that a protein adduct band with the same size (52 kDa) and isoelectric point (pI 4.95) was present in mitochondria (where the protein was ultimately found to be localized) of the various rat tissues, includingheart, liver, and kidney (Figure 1C, right panel). A partially purified preparation of the protein adduct from rat kidney mitochondria was used to provide an amount sufficient for identification (see Supplemental Data). The protein was extracted from the electrophoresis gel (Figure 1C, left panel) and identified as the β-subunit of the mitochondrial F-ATP synthase with nano-LC-MS/MS and MALDI-TOF fingerprint analysis. The 52-kDa protein was the only protein or the major protein in human islets (Figure 1, A and B) and rat kidney mitochondria (Figure 1C) detected with the antihydroxynonenal antibody.
Figure 1.
Hydroxynonenal-protein adducts and other adducts in pancreatic islets from humans with type 2 diabetes and without diabetes and in rat islets and INS-1 832/13 cells. A, Immunoblots of proteins (10 μg protein per lane) from islets of humans with type 2 diabetes (Diab) from Sweden or from rats or INS-1 832/13 cells were probed with antibodies raised against 4-hydroxynonenal (HNE) by L.I.S. or 3-hydroxyoctanoic acid plus nanoic acid (627) and 4-hydroxynonenal plus nanoic acid lactone (628) raised by M.J.M. B, A different antihydroxynonenal antibody (from Chemicon) was used to analyze islets from human donors with type 2 diabetes (T2D) and nondiabetic donors (Non) from the United States. This blot (15 μg protein per lane) is representative of 4 similar blots in which islets from the same human donors with type 2 diabetes and different human donors without diabetes were analyzed. The relative density of the HNE bands are expressed as the average density of the protein bands of the islets from 2 nondiabetic donors set at 100%. The density of the 52-kDa band was not consistently darker in islets from either type of donor as judged from densitometry of the HNE band (as shown in B; also see Results). A blot of β-actin is shown as a control for loading of protein. C, Two-dimensional gel electrophoresis of the 52-kDa HNE-bonded protein from rat kidney mitochondria. The protein was partially purified from rat kidney mitochondrial proteins and then applied to 2 companion 2-dimensional electrophoresis gels that were run in parallel. The gel shown on the right was analyzed by immunoblotting with the anti-HNE antibody; the gel shown on the left was stained for protein, and the protein spot in this gel that corresponded to the anti-HNE–stained protein in the immunoblot was excised and subjected to nano-LC-MS/MS and MALDI-TOF fingerprint analysis to identify the HNE protein adduct.
Discussion
Hydroxynonenal is one of the most abundant and reactive lipid peroxides formed from lipid peroxidation (17) and is known to be harmful to cells. Hydroxynonenal modification of nonislet proteins in nondiabetic animals has been reported to inhibit their function (15, 16, 18), and when applied at a high concentration to pancreatic islets, hydroxynonenal impairs glucose-stimulated insulin secretion (11, 19). Hyperglycemia in type 2 diabetes generates oxidative stress that in turn generates lipid peroxides. In addition, cytokines known to be damaging to islets in type 1 diabetes increase the concentration of lipid peroxides when applied to β-cells (20). The hydroxynonenal-modified 52-kDa protein adduct found in the islets of diabetic humans was identified as the β-subunit of the mitochondrial ATP synthase that catalyzes more than 95% of cellular ATP production and suggests lipid peroxidation could be damaging to mitochondria. The presence of the ATP synthase adduct in islets of fairly healthy humans who did not have diabetes as well as islets from nondiabetic rats, the INS-1 832/13 insulinoma cell line, and mitochondria of various rat tissues does not necessarily suggest that this adduct is not damaging. Similar to other characteristics or conditions, such as overweight, seen in living nondiabetic individuals, the overt pathogenic effects could be subtle, gradual in onset, and dependent on individual genetic background. Although lipid peroxidation might not seriously interfere with mitochondrial function in the short run, over time, it might contribute to aging and prediabetes in the β-cell as well as in peripheral tissues. It is noteworthy that David Bernlohr (personal communication) independently detected a hydroxynonenal adduct of the β-subunit of ATP synthase in adipose tissue of mice with type 2 diabetes. The results of the two studies support the identity of the β-subunit of ATP synthase as a hydroxynonenal adduct.
Supplementary Material
Acknowledgments
We thank Michael J. Fallon and Meghan A. Johnson for excellent technical assistance.
This work was supported by National Institutes of Health Grant DK28348 and the Nowlin Family Trust administered by the Lutheran Community Foundation (to M.J.M.) and Swedish Research Council and Swedish Diabetes Association (to C.G.O.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- KLH
- keyhole limpet hemocyanin
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- MALDI-TOF
- matrix-assisted laser desorption/ionization-time of flight.
References
- 1. Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and comparison by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1097–1097 [DOI] [PubMed] [Google Scholar]
- 2. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacDonald MJ, Longacre MJ, Stoker SW, et al. Differences between human and rodent pancreatic islets: low pyruvate carboxylase, ATP citrate lyase and pyruvate carboxylation; high glucose-stimulated acetoacetate in human pancreatic islets. J Biol Chem. 2011;286:18383–18396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacDonald MJ. Differences between mouse and rat pancreatic islets: succinate responsiveness, malic enzyme, and anaplerosis. Am J Physiol Endocrinol Metab. 2002;283:E302–E310 [DOI] [PubMed] [Google Scholar]
- 5. Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. β-Cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-β-cell relationships. Proc Natl Acad Sci U S A. 1994;91:10878–10882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sako Y, Grill V. A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology. 1990;127:1580–1589 [DOI] [PubMed] [Google Scholar]
- 7. Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–2474 [DOI] [PubMed] [Google Scholar]
- 8. Robertson RP, Harmon J, Tran PO, Poitout V. β-Cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53:S119–S124 [DOI] [PubMed] [Google Scholar]
- 9. Muoio DM, Newgard CB. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205 [DOI] [PubMed] [Google Scholar]
- 10. Prentki M, Madiraju SR. Glycerolipid metabolism and signaling in health and disease. Endocr Rev. 2008;29:647–676 [DOI] [PubMed] [Google Scholar]
- 11. Pi J, Bai Y, Zhang Q, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791 [DOI] [PubMed] [Google Scholar]
- 12. Ostenson CG, Gaisano H, Sheu L, Tibell A, Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006;55:435–440 [DOI] [PubMed] [Google Scholar]
- 13. MacDonald MJ, Husain RD, Hoffmann-Benning S, Baker TR. Immunochemical identification of coenzyme Q0-dihydrolipoamide adducts in the E2 components of the α-ketoglutarate and pyruvate dehydrogenase complexes partially explains the cellular toxicity of coenzym Q0. J Biol Chem. 2004;279:27278–27285 [DOI] [PubMed] [Google Scholar]
- 14. MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets: further implication of cytosolic NADPH in insulin secretion. J Biol Chem. 1995;270:20051–20058 [PubMed] [Google Scholar]
- 15. Cohn JA, Tsai L, Friguet B, Szweda LI. Chemical characterization of a protein-4-hydroxy-2-nonenal cross link: immunochemical detection in mitochondria exposed to oxidative stress. Arch Biochem Biophys. 1996;328:158–164 [DOI] [PubMed] [Google Scholar]
- 16. Humphries KM, Szweda LI. Selective inactivation of α-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–15841 [DOI] [PubMed] [Google Scholar]
- 17. Schneider C, Tallman KA, Porter NA, Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. J Biol Chem. 2001;276:20831–20838 [DOI] [PubMed] [Google Scholar]
- 18. Bennaars-Eiden A, Higgins L, Hertzel AV, Kapphahn RJ, Ferrington DA, Bernlohr DA. Covalent modification of epithelial fatty acid-binding protein by 4-hydroxynonenal in vitro and in vivo. Evidence for a role in antioxidant biology. J Biol Chem. 2002;277:50693–50702 [DOI] [PubMed] [Google Scholar]
- 19. Miwa I, Ichimura N, Sugiura M, Hamada Y, Taniguchi S. Inhibition of glucose-induced insulin secretion by 4-hydroxy-2-nonenal and other lipid peroxidation products. Endocrinology. 2000;141:2767–2772 [DOI] [PubMed] [Google Scholar]
- 20. Suarez-Pinzon WL, Strynadka K, Rabinovitch A. Destruction of rat pancreatic β-cells by cytokines involves the production of cytotoxic aldehydes. Endocrinology. 1996;137:5290–5296 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.