Abstract
Context:
In obesity, increases in free fatty acid (FFA) flux can predict development of insulin resistance. Adult women release more FFA relative to resting energy expenditure (REE) and have greater FFA clearance rates than men. In adolescents, it is unknown whether sex differences in FFA flux occur.
Objective:
Our objective was to determine the associations of sex, REE, and body composition with FFA kinetics in obese adolescents.
Participants:
Participants were from a convenience sample of 112 non-Hispanic white and black adolescents (31% male; age range, 12–18 years; body mass index SD score range, 1.6–3.1) studied before initiating obesity treatment.
Main Outcome Measures:
Glucose, insulin, and FFA were measured during insulin-modified frequently sampled iv glucose tolerance tests. Minimal models for glucose and FFA calculated insulin sensitivity index (SI) and FFA kinetics, including maximum (l0 + l2) and insulin-suppressed (l2) lipolysis rates, clearance rate constant (cf), and insulin concentration for 50% lipolysis suppression (ED50). Relationships of FFA measures to sex, REE, fat mass (FM), lean body mass (LBM) and visceral adipose tissue (VAT) were examined.
Results:
In models accounting for age, race, pubertal status, height, FM, and LBM, we found sex, pubertal status, age, and REE independently contributed to the prediction of l2 and l0 + l2 (P < .05). Sex and REE independently predicted ED50 (P < .05). Sex, FM/VAT, and LBM were independent predictors of cf. Girls had greater l2, l0 + l2 and ED50 (P < .05, adjusted for REE) and greater cf (P < .05, adjusted for FM or VAT) than boys.
Conclusion:
Independent of the effects of REE and FM, FFA kinetics differ significantly in obese adolescent girls and boys, suggesting greater FFA flux among girls.
In obesity, high circulating free fatty acids (FFAs) have been associated with increased risk for developing insulin resistance, type 2 diabetes, metabolic syndrome, and cardiovascular disease (1–5). In adults, heterogeneity in FFA flux is influenced by obesity, sex, and regional body fat distribution (6–10). Adult women have greater nonoxidative FFA disposal and recycling than men (11, 12), and the rate of FFA release into plasma is greater in women than men (13). However, there is little information about predictors of FFA flux in children and adolescents. Previous reports in adolescents compared lipolysis rates (14) and FFA kinetics (15) in obese and lean adolescents, finding that obesity is associated with lower basal glycerol release per unit fat mass (FM) and with diminished maximal lipolytic response to epinephrine (14). When FFA flux (palmitate kinetics) was measured in adolescents with and without nonalcoholic fatty liver disease (NAFLD), the rate of whole-body palmitate release into plasma was higher in adolescents with NAFLD when compared with those with normal intrahepatic triglyceride content (15). Others have reported that visceral fat accumulation was associated with greater FFAs in the portal circulation (16, 17) and increased risk of metabolic abnormalities (18) and insulin resistance (19). However, in adolescents, the factors that influence sex-associated differences in FFA flux have not been previously reported.
The purpose of this study was to examine sex-related differences in lipolysis rates and FFA clearance in a large cohort of obese adolescents by mathematically modeling data from insulin-modified frequently sampled iv glucose tolerance tests (IM-FSIGTs) (20, 21). Furthermore, we wanted to assess the impact of resting energy expenditure (REE) and body fat distribution on sex-associated differences in FFA kinetics of obese adolescents.
Subjects and Methods
Subjects
A convenience sample of obese non-Hispanic white and non-Hispanic black adolescents, age range 12 to 18 years, were recruited through newspaper advertisements and letters to local physicians for participation in a weight-loss study (22). Inclusion criteria were a body mass index (BMI) (kilograms per square meter) greater than the National Health and Nutrition Examination Survey I (1971–1974) 95th percentile for age, sex, and race (23) and the presence of one of the following obesity-related comorbidities: hypertension, impaired glucose homeostasis [fasting hyperinsulinemia (insulin ≥ 15 μU/L), impaired fasting glucose (fasting serum glucose 100–125 mg/dL), impaired glucose tolerance (2-h serum glucose during an oral glucose tolerance test 140–199 mg/dL), or type 2 diabetes (fasting serum glucose ≥ 126 mg/dL or 2-h glucose tolerance test value ≥ 200 mg/dL)] (24), dyslipidemia (total triglycerides ≥ 200 mg/dL, total cholesterol ≥ 200 mg/dL, or low-density lipoprotein cholesterol ≥ 130 mg/dL), NAFLD, or sleep apnea documented by a formal sleep study. NAFLD was defined as patients with otherwise unexplained ALT elevation and evidence of liver fat on imaging studies without another known explanation such as alcohol use, systemic illnesses known to cause fatty liver disease, recent use of drugs or supplements known to raise alanine aminotransferase, infection with hepatitis B virus or hepatitis C virus, Wilson's disease, or α1-antitrypsin deficiency.
Individuals were excluded if they had a major pulmonary, hepatic, cardiac, or musculoskeletal disorder affecting body weight; had a history of substance abuse or other psychiatric disorder that would impair compliance with the study protocol; had used an anorexiant in the past 6 months; or had lost weight in the past 2 months. Each adolescent, along with a parent, gave written consent for protocol participation. The protocol was approved by the Institutional Review Board of the Eunice Kennedy-Shriver National Institute of Child Health and Human Development.
Protocol
Subjects were studied at a baseline inpatient visit at the Clinical Center at the National Institutes of Health (NIH) before initiating weight loss treatment. Breast and testicular pubertal staging was determined with a physical examination by an endocrinologist or trained nurse practitioner. Height was obtained at baseline using a stadiometer (Holtain Ltd, Crymych, Wales, United Kingdom) calibrated before each height measurement to the nearest 1 mm. Weight was obtained to the nearest 0.1 kg using a calibrated digital scale (Scale-Tronix, Wheaton, Illinois). These measurements were made with subjects fasting and in minimal clothing and without shoes.
Subjects underwent dual-energy x-ray absorptiometry, as previously described (25). Magnetic resonance imaging was used to measure visceral and sc abdominal adipose tissue at L2–3 (GE Medical Systems, Milwaukee, Wisconsin) as previously described (25). Resting energy expenditure (REE) was assessed in the morning after an overnight fast using open-circuit indirect calorimetry with the use of a respiratory metabolic cart (Sensor-Medics 2900 or DeltaTrac II; SensorMedics Corp, Yorba Linda, Californis) as previously described (26).
Laboratory measurements
Blood specimens were collected for glucose, insulin, and nonesterified fatty acid (NEFA) (27) analyses. Serum glucose and insulin were analyzed by the NIH Clinical Pathology Department using standard methodologies as previously described (28). Glucose was measured on an Hitachi 917 analyzer using reagents from Roche Diagnostics (Indianapolis, Indiana). Insulin concentrations were determined using an immunochemiluminometric assay purchased from Diagnostic Products Corporation (Los Angeles, California) on an Immulite 2000 machine (Diagnostic Products). The cross-reactivity of the insulin assay with proinsulin was <8% and with C-peptide was <1%, sensitivity was 2 μU/mL, and the mean inter- and intra-assay coefficients of variation were 5.8% and 3.6%, respectively. Plasma FFA was measured using the Wako HR Series NEFA kit (Wako Chemicals USA, Inc, Richmond, Virginia). The intra- and interassay coefficients of variations were 3.1% and 7.8%, respectively. To assess insulin sensitivity, subjects underwent an IM-FSIGT. Dextrose, 0.3 g/kg, was administered iv as a smooth bolus over 2 minutes followed by insulin, 0.05 U/kg, given as a bolus iv at 20 minutes. Blood specimens were collected through a second iv line for glucose, insulin, and FFA determinations at −20, −10, 0, 2, 3, 4, 5, 6, 8, 10, 14, 19, 22, 24, 27, 30, 35, 40, 45, 50, 70, 90, 120, 150, 180, 210, 240, 270, and 300 minutes relative to dextrose injection. Insulin sensitivity index (SI) and acute insulin response to glucose (AIRG) were calculated from the serum glucose and insulin concentrations in the first 10 minutes of the IM-FSIGT using the minimal model for glucose disposal (SAAM II version 1.11; SAAM Institute, Inc, Seattle, Washington) (6). To examine FFA kinetics, plasma FFAs from the IM-FSIGT were modeled as previously described (21) assuming that insulin acts through 1 remote compartment (X model, where X is defined as the remote compartment) to increase glucose disposal and to suppress lipolysis and FFA concentrations from 10–120 minutes during the IM-FSIGT and obeys the equation: dFFA/dt = l0 + (l2/[1 + X/X2]A) − cf × FFA. We estimated the 5 parameters in the model – basal lipolysis rate (l0), insulin-suppressible lipolysis rate (l2), X2 (defined as the Hill constant), A (defined as the Hill coefficient), and the fatty acid clearance rate constant (cf), using maximum likelihood for a normal error model (21). Of these 5 model parameters, we examined 4 physiologically salient lipolysis parameters: the insulin-suppressible lipolysis rate (l2), the maximum lipolysis rate (l0 + l2), the concentration of insulin needed for 50% suppression of lipolysis: ED50 = X2 × (21/A − 1), similar to EC50 previously reported (29), and the fatty acid clearance rate constant (cf) (21).
Statistical analysis
To describe the demographics of our cohort, data were analyzed by 2-way ANOVA without adjustments for covariates. We tested the effects of sex and race as well as the interaction of sex with race (Table 1). To examine the effects of sex on serum insulin and plasma NEFA levels during the IM-FSIGT, serum insulin and plasma NEFAs were analyzed by a repeated-measures ANOVA. Means were compared with unpaired t tests when the sex effect tested in the model was significant (P < .05). To examine the effect of sex on the 4 lipolysis parameters, data were analyzed by analysis of covariance (ANCOVA) adjusting for height, age, FM (30), pubertal status, and race. Visceral abdominal adipose tissue (VAT) area and REE were also included in some models (as specified in Results). VAT area was log transformed before ANCOVA. Because few boys and no girls were prepubertal, for ANCOVA, adolescents in prepuberty (Tanner I breast stage for girls and testicular volume <4 mL for boys) and midpuberty (Tanner II and III breast stages for girls and testicular volume <12 mL for boys) were grouped together for analyses. When the P value for the model was significant (P < .05), parameter estimates examining the effects of sex, race, and pubertal status were reported and considered significant at P < .05. Means with SD or SEM are reported and adjusted for covariates as specified in the tables and figure legends. The effects of adipose tissue distribution, lean body mass (LBM), and REE were tested by ANCOVA. When LBM was included in the model, percent body fat was a covariate instead of FM to minimize issues related to multicollinearity among variables.
Table 1.
Subject Characteristics
| Boys (n = 35) |
Girls (n = 77) |
Two-way ANOVA (P) |
|||||
|---|---|---|---|---|---|---|---|
| NHW (n = 16) | NHB (n = 19) | NHW (n = 33) | NHB (n = 44) | Sex | Race | Sex by Race Interaction | |
| Race, % | 35.7 | 54.3 | 42.9 | 57.1 | |||
| Tanner stage, % | |||||||
| Prepubertal | 5.7 | 2.9 | 0.0 | 0.0 | |||
| Midpubertal | 20.0 | 28.6 | 2.6 | 7.9 | |||
| Late pubertal | 22.9 | 20.0 | 39.5 | 50.0 | |||
| Age, ya | 13.7 ± 1.3 | 14.6 ± 1.4 | 14.2 ± 1.3 | 14.6 ± 1.5 | .25 | .07 | .47 |
| Height, cma | 166.7 ± 7.2 | 166.3 ± 12.9 | 164.3 ± 5.9 | 162.3 ± 6.6 | .34 | .88 | .62 |
| Weight, kga | 101.2 ± 17.3 | 117.3 ± 15.2 | 96.5 ± 15.2 | 101.3 ± 15.1 | .32 | .00 | .08 |
| BMI (kg/m2)a | 36.4 ± 5.8 | 43.0 ± 8.9 | 35.6 ± 4.4 | 38.4 ± 5.1 | .65 | .00 | .11 |
| BMI-Za | 2.48 ± 0.28 | 2.71 ± 0.16 | 2.29 ± 0.28 | 2.40 ± 0.26 | .02 | .01 | .24 |
| Lean mass, kga | 56.7 ± 9.6 | 66.4 ± 9.7 | 53.0 ± 7.0 | 56.6 ± 7.4 | .14 | .00 | .07 |
| Body fat, %a | 42.3 ± 4.5 | 41.8 ± 5.0 | 42.9 ± 3.6 | 42.4 ± 3.5 | .62 | .69 | .99 |
| Fat mass, kga | 43.4 ± 10.0 | 49.6 ± 9.6 | 41.8 ± 8.3 | 43.7 ± 8.6 | .56 | .04 | .24 |
| REE, kcal/da | 2036 ± 253.0 | 2016 ± 274.4 | 1765 ± 221.2 | 1751 ± 249.4 | .00 | .81 | .95 |
| REE, kcal/d/LBM, kga | 36.4 ± 3.9 | 31.1 ± 4.9 | 33.7 ± 2.7 | 31.2 ± 4.3 | .87 | .00 | .09 |
| Fasting FFA, μMa | 448.9 ± 179.0 | 596.2 ± 256.2 | 493.9 ± 149.1 | 473.4 ± 184.5 | .43 | .02 | .03 |
| Fasting glucose, mg/dLa | 95.6 ± 19.2 | 88.9 ± 5.1 | 83.1 ± 9.3 | 86.6 ± 12.9 | .00 | .11 | .04 |
| Fasting insulin, μU/mLa | 21.3 ± 12.5 | 23.1 ± 13.4 | 19.8 ± 15.0 | 22.7 ± 14.8 | .73 | .71 | .85 |
| SI, μU/mL/min × 10−4a,b | 1.30 ± 0.62 | 1.01 ± 0.57 | 1.90 ± 1.28 | 1.18 ± 0.78 | .04 | .37 | .26 |
| AIRG, pmol/La,b | 1292 ± 83.0 | 1353 ± 95.8 | 1312 ± 90.2 | 1304 ± 84.1 | .47 | .05 | .07 |
| Disposition indexa,c | 0.12 ± 1.1 | 0.11 ± 1.2 | 0.20 ± 1.1 | 0.15 ± 1.2 | .57 | .21 | .49 |
| Fasting TGs, mg/dLa | 183 ± 147.0 | 113 ± 66.4 | 139 ± 66.3 | 118 ± 106.2 | .14 | .04 | .22 |
Abbreviation: TG, triglyceride.
Unadjusted means reported with SD.
SI was calculated using Bergman's minimal model (n = 34 boys and 76 girls) and AIRG by trapezoidal rule.
Disposition index was calculated by multiplying SI by AIRG and log transformed for 2-way ANOVA (n = 33 boys and 76 girls).
Results
A total of 112 obese adolescents, 35 boys and 77 girls, were studied (Table 1). In this cohort, BMI SD score (BMI-Z) for girls was significantly lower than for boys (Table 1; P < .02). Furthermore, BMI-Z for non-Hispanic blacks (NHBs) was significantly higher compared with non-Hispanic whites (NHWs) (Table 1; P < .01). Fasting serum triglycerides were significantly lower in NHBs when compared with NHWs (Table 1; P < .04). Only 3 children had fasting glucose >100 mg/dL. Girls had significantly lower LBM than boys (55.0 ± 6.7 vs 62.1 ± 6.6 kg, P < .01, adjusted for race, puberty, age, and height). Girls had a nonsignificant trend toward lower REE than boys (1812.7 ± 202.6 vs 1900.2 ± 224.1, P < .08, after adjustment for race, puberty, age, height, percent body fat, and LBM). However, when REE (not adjusted for covariates) was divided by LBM, there was no difference (P = .87) in girls and boys, whereas among NHWs, REE/LBM was significantly higher (P < .00) than among NHBs (Table 1). Percent body fat and fasting serum insulin were not significantly different by sex or race (Table 1). NHB boys had higher fasting plasma FFAs compared with NHW boys, whereas NHB girls had lower fasting FFAs than NHW girls (P < .03 for interaction, Table 1). Fasting serum glucose was higher in NHW boys when compared with NHB boys, whereas NHW girls had lower fasting glucose than NHB girls (P < .04 for interaction, Table 1). Subjects generally had high mean fasting insulin and marked insulin resistance, as measured by SI (Table 1), but fasting glucose was within the normal range. AIRG was significantly higher in NHBs compared with NHWs (P< 0.05, Table 1). The difference in AIRG in NHBs vs NHWs is most likely driven by the large difference observed in the boys because the girls' mean AIRG values are very similar.
By repeated-measures analysis, there was a significant interaction (P < .01) between sex and serum insulin over time (Figure 1A). The serum insulin during the first 10 minutes of the IM-FSIGT was lower for the girls than for the boys but did not result in greater plasma FFA suppression (Figure 1B). There was no significant difference in plasma FFA concentration in girls vs boys during IM-FSIGT (P = .08, Figure 1B). We did not estimate a time factor needed for achieving ED50, because the response to insulin was so rapid in most of the subjects that the ED50 value of X was attained very quickly, before the bolus of insulin given at 20 minutes had mixed completely. The model tries to avoid mixing complications by starting the modeling at time = 10 minutes. Thus, these data cannot address how much time is taken to reach ED50.
Figure 1.
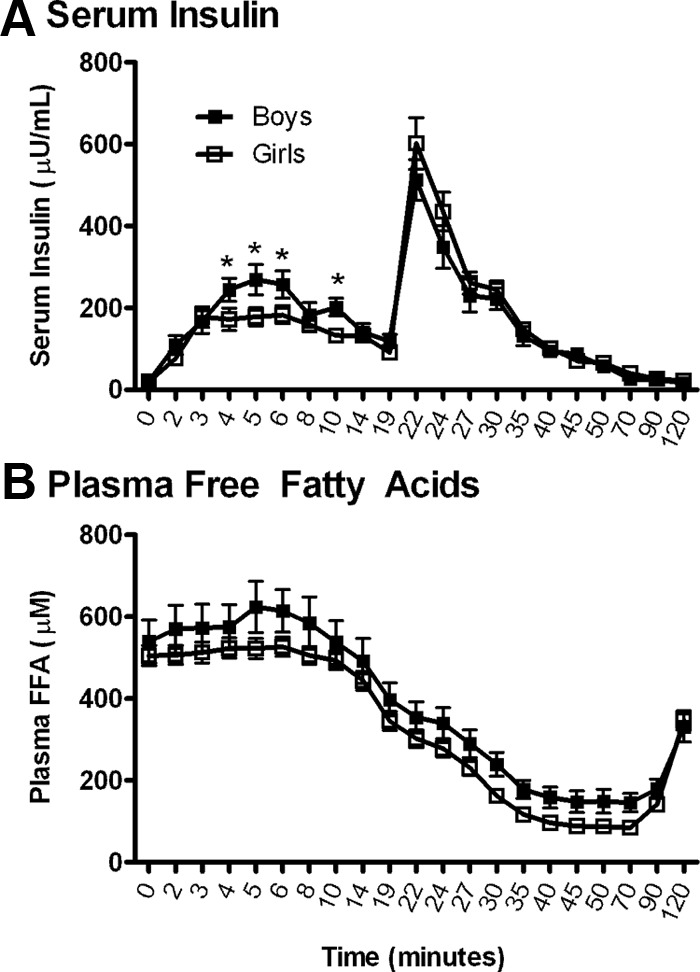
Serum insulin and plasma NEFA in obese adolescent boys and girls during the IM-FSIGT. A, Mean serum insulin (microunits per milliliter). B, Mean plasma NEFA (micromolar) (40). Repeated-measures ANOVA was performed as described in Subjects and Methods, and unadjusted means are reported with SEM (for NEFA, n = 28 for boys and 68 for girls; for insulin, n = 28 for boys and 61 for girls). *P < .05 for boys vs girls from unpaired t test.
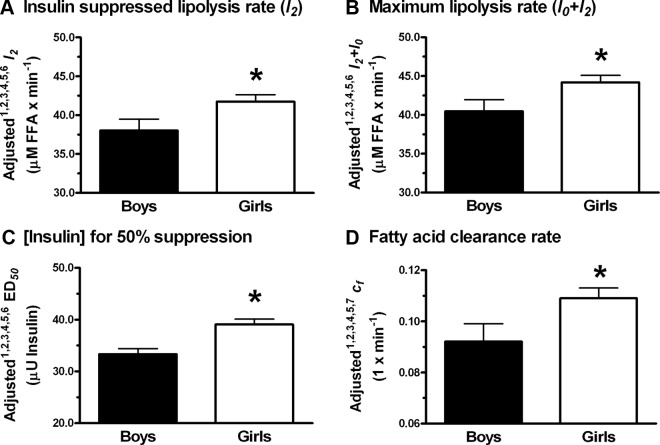
We found no independent statistically significant effects of race on insulin-suppressible lipolysis rate (l2), maximum lipolysis rate (l0 + l2), the concentration of insulin needed for 50% suppression of lipolysis (ED50), or fatty acid clearance rate constant (cf) in any of the models considered (Table 2). Pubertal status and REE were significant predictors of l2 and l0 + l2 (P = .02 and .01, respectively, Table 2), and pubertal status was a strong predictor of l2 and l0 + l2 independent of changes in body composition or REE (Table 2; models with FM, LBM, and REE; P values < .03). With race, height, age, FM, and REE as the modeled covariates, l2, l0 + l2, and ED50 were significantly higher in girls than boys (P values < .05, Figure 2, A–C). When considering baseline FFA concentration as a covariate instead of FM (other model covariates were sex, race, pubertal status, height, and age) to determine whether baseline FFA concentration might significantly contribute to l2, l0 + l2, and ED50, we found that baseline FFA concentration was not a significant predictor of l2 (P = .09), l0 + l2 (P = .12), and ED50 (P = .23). With height, age, FM, and VAT as the modeled covariates, cf in girls was significantly greater than boys (Figure 2D). When LBM was included in the model, cf was not different between girls and boys. In late puberty, l2 and l0 + l2 were increased when compared with early puberty (Figure 3). When we modeled SI as a covariate instead of FM (covariates sex, race, pubertal status, height, and age) to test whether SI was a significant predictor of l2, l0 + l2, ED50, and cf, we found that SI was not a significant predictor of l2 (P = .11) and l0 + l2 (P = .07) but did predict ED50 (P < .001) and cf (P = .05). In addition, pubertal status was no longer significant for l2 (P = .054) or l0 + l2 (P = .061) when SI was accounted for. In summary, sex, REE, pubertal status, and age were independent predictors of l2, l0 + l2, and ED50. SI was an independent predictor of ED50. FM, LBM, VAT, and SI, but not REE, were independent predictors of cf.
Table 2.
Univariate ANCOVA Examining the Effects of Sex, Race, and Pubertal Status on Parameters of Lipolysis Estimated From the IM-FSIGT (n = 35 Boys and 77 Girls)a
| Variable entered | Insulin-Suppressed Lipolysis Rate (l2) |
Maximum Lipolysis Rate (l0 + l2) |
[Insulin] for 50% Suppression of Lipolysis (ED50) |
Fatty Acid Clearance Rate Constant (cf) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| Model with FM | ||||||||||||
| Sex | 1.32 | 1.79 | .46 | 1.30 | 1.80 | .47 | 1.12 | 2.77 | .68 | 0.02 | 0.01 | .02 |
| Race | −1.06 | 1.47 | .47 | −1.10 | 1.48 | .46 | 0.89 | 2.27 | .69 | −0.40c | 0.70c | .57 |
| Pubertal status | 4.82 | 2.04 | .02 | 4.72 | 2.04 | .02 | 2.23 | 3.14 | .48 | −0.01 | 0.01 | .41 |
| Height | 0.07 | 0.09 | .44 | 0.07 | 0.09 | .43 | 0.18 | 0.14 | .20 | 0.02c | 0.04c | .64 |
| Age | −1.51 | 0.56 | .01 | −1.51 | 0.56 | .01 | −1.00 | 0.86 | .25 | −0.22c | 0.27c | .43 |
| FM | 0.10 | 0.08 | .24 | 0.09 | 0.08 | .26 | 0.21 | 0.13 | .10 | −0.08c | 0.04c | .04 |
| P value for model with FM | .03 | .03 | .35 | .03 | ||||||||
| Model with LBM | ||||||||||||
| Sex | 1.52 | 1.99 | .44 | 1.46 | 2.00 | .47 | 0.02 | 0.04 | .54 | 0.40c | 0.88c | .65 |
| Race | −1.15 | 1.57 | .46 | −1.16 | 1.57 | .46 | 0.01 | 0.03 | .72 | 0.63c | 0.70c | .36 |
| Pubertal status | 4.74 | 2.11 | .03 | 4.67 | 2.12 | .03 | 0.02 | 0.04 | .63 | 0.31c | 0.93c | .74 |
| Height | 0.64 | 0.11 | .57 | 0.07 | 0.11 | .55 | 0.14c | 0.21c | .50 | 6.01d | 4.92d | .00 |
| Age | −1.55 | 0.58 | .01 | −1.54 | 0.58 | .01 | −0.02 | 0.01 | .13 | 4.88d | 0.25c | .85 |
| % FM | 0.20 | 0.19 | .30 | 0.20 | 0.19 | .31 | 0.48c | 0.36c | .13 | 0.14c | 8.47d | .47 |
| LBM | 0.10 | 0.11 | .39 | 0.09 | 0.11 | .44 | 0.33c | 0.21c | .12 | −0.24c | 5.02d | .00 |
| P value for model with LBM | .04 | .05 | .29 | .00 | ||||||||
| Model with REE | ||||||||||||
| Sex | 3.72 | 1.88 | .05 | 3.71 | 1.89 | .05 | 0.68 | 0.03 | .05 | 0.02 | 0.01 | .09 |
| Race | −1.12 | 1.46 | .44 | −1.16 | 1.46 | .43 | 0.02 | 0.03 | .38 | −0.48c | 0.01 | .51 |
| Pubertal status | 4.54 | 1.97 | .02 | 4.43 | 1.98 | .03 | 0.01 | 0.04 | .73 | −0.55c | 0.01 | .58 |
| Height | 0.04 | 0.09 | .66 | 0.04 | 0.09 | .65 | 0.10c | 0.17c | .56 | 3.59d | 4.65d | .44 |
| Age | −1.12 | 0.55 | .05 | −1.11 | 0.55 | .05 | −0.56 | 0.01 | .58 | −0.38c | 0.27c | .17 |
| FM | −0.04 | 0.09 | .64 | −0.05 | 0.09 | .61 | −8.16d | 0.17c | .64 | −2.65d | 4.70d | .57 |
| REE | 0.01 | 0.30c | .01 | 0.01 | 0.30c | .00 | 2.34d | 0.65d | .00 | −0.28d | 0.17d | .11 |
| P value for model with REE | .00 | .00 | .02 | .02 | ||||||||
| Model with FM and VAT | ||||||||||||
| Sex | 1.77 | 1.81 | .33 | 1.79 | 1.81 | .32 | 1.54 | 2.81 | .58 | 0.02 | 0.01 | .04 |
| Race | −0.26 | 1.55 | .86 | −0.23 | 1.55 | .88 | 1.58 | 2.40 | .51 | −0.01 | 0.01 | .21 |
| Pubertal status | 5.74 | 2.11 | .01 | 5.72 | 2.11 | .01 | 3.08 | 3.29 | .35 | −0.01 | 0.01 | .16 |
| Height | 0.10 | 0.09 | .30 | 0.10 | 0.09 | .29 | 0.20 | 0.14 | .16 | 0.45d | 4.40d | .92 |
| Age | −1.58 | 0.56 | .01 | −1.58 | 0.56 | .00 | −1.06 | 0.86 | .22 | −0.17c | 0.26c | .53 |
| FM | 0.03 | 0.09 | .76 | 0.02 | 0.09 | .85 | 0.15 | 0.14 | .30 | −3.52c | 4.38d | .42 |
| VATb | 6.07 | 3.92 | .12 | 6.64 | 3.93 | .09 | 5.37 | 6.10 | .38 | −0.04 | 0.02 | .04 |
| P value for model with VAT | .02 | .02 | .38 | .01 | ||||||||
Abbreviations: β, parameter estimate; FFM, fat-free mass.
Positive β indicates higher for girls and higher for NHW; bold text indicates significant effects (P < .05). For ANCOVA, pubertal status was divided into early (Tanner 1–3 breast development for girls; testis volume <12 mL for boys) and late (Tanner 4–5 breast development for girls; testis volume ≥12 mL for boys) groups.
For ANCOVA, VAT was log transformed to meet the homogeneity of variance assumption.
× 10−2.
× 10−4.
Figure 2.
Differences in insulin-suppressed (l2) and maximum lipolysis (l0 + l2) rates, ED50, and fatty acid clearance rate constant (cf) between obese adolescent boys and girls. A–D, Insulin-suppressed lipolysis rate (A), maximum lipolysis rate (B), concentration of insulin for 50% suppression (C), and fatty acid clearance rate constant (D) with SEM. Means are adjusted for the following covariates: 1 race, 2 pubertal status, 3 height, 4 age, 5 FM, 6 REE, 7 VAT (n = 33 for boys and 74 for girls, except cf for which n = 35 for boys and 77 for girls). *P < .05 for boys vs girls.
Figure 3.
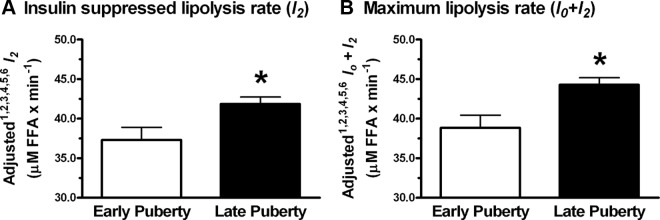
Differences in insulin-suppressed (l2) and maximum (l0 + l2) lipolysis rates according to pubertal status in obese adolescents. A and B, Insulin-suppressed lipolysis rate (A) and maximum lipolysis rate (B) with SEM. Means are adjusted for the following covariates: 1 race, 2 sex, 3 height, 4 age, 5 FM, and 6 REE (n = 30 for early; n = 77 for late). *P < .05 for late vs early puberty.
Discussion
The aim of this study was to examine the associations of sex, REE, and body fat distribution with FFA flux among obese, insulin-resistant adolescents. Our approach was to model FFA data from the IM-FSIGT using the minimal FFA model (21). We found that sex and REE were independent predictors of insulin-suppressed and maximum FFA appearance (l2 and l0 + l2) rates and the concentration of insulin needed to suppress lipolysis by 50% (ED50). Each of these 3 measures was greater in girls than boys after adjusting for REE and FM, suggesting that, independent of REE and body composition, sex impacted both insulin-induced suppression of FFA release and insulin-mediated FFA uptake into tissues. Our data are consistent with Koutsari et al (11), who reported that during IM-FSIGT, women had increased lipolysis rates compared with men even when expressed relative to REE. In insulin clamp studies in nondiabetic adult men and women, FFA flux was most strongly related to REE rather than body composition, and when flux was adjusted for REE, women had greater lipolysis rates than men (31). Nielsen et al (32) reported greater FFA release rates in adult women compared with men when adjusting for REE with no change in plasma FFA levels between women and men and proposed increased nonoxidative FFA clearance rate as an explanation for these findings. In this cohort, race did not influence lipolysis rates or FFA clearance rate constant (when adjusted for FM or other measures of body composition), which is consistent with previous reports comparing adult African-American women with white women (20). We show that in adolescents, as in adult men and women, REE is an important predictor of lipolysis rates in girls and boys and that when accounting for REE, lipolysis rates are greater in girls compared with boys.
Furthermore, we found that adolescents in late puberty had significantly higher insulin-suppressed and maximum FFA appearance (l2 and l0 + l2) rates than adolescents in early puberty. Although we were not able to study the transition phase from prepuberty to late-stage puberty in FFA appearance rates due to insufficient numbers of prepubertal subjects, these data suggest that adolescents in late puberty become less insulin sensitive with respect to fatty acid flux than those with less pubertal development. Indeed, when we accounted for differences in SI, pubertal status no longer significantly predicted lipolysis rates, suggesting that insulin sensitivity did partially explain the increased lipolysis rates we observed in late-stage puberty. These data are consistent with other reports of decreased insulin sensitivity toward glucose (SI) (30, 33–35) and insulin-stimulated glucose metabolism (36) as adolescent boys and girls enter the later stages of puberty, which may be related to changes in the hormonal milieu associated with puberty such as changes in GH. Indeed, Arslanian et al (37) suggested that insulin-mediated glucose disposal rate in adolescent girls was lower than boys during a hyperinsulinemic-euglycemic clamp due to increased GH levels in girls. These data confirm and extend observations by others that there is greater total body lipolysis (measured by glycerol rate of appearance) in pubertal than prepubertal children (35, 38). Although we show less insulin effectiveness in suppressing lipolysis measured by FFA appearance rates in later puberty compared with early puberty, Arslanian and Kalhan (38) found no differences in insulin-suppressed lipolysis rate when measuring glycerol appearance rates. Perhaps the difference in our findings can be explained by previous observations that adolescents entering puberty have increased FFA/glucose oxidation ratios requiring greater availability of FFA for fuel (38). It will be important to examine differences in FFA appearance rates during the transition from early to late puberty in future studies.
FFA clearance rate constant (cf) was increased in girls compared with boys when adjusting for either total body fat or VAT area. Our data are consistent with previously reported findings of adult women who had increased nonoxidative FFA disposal when compared with adult men (11). Furthermore, FFA storage in adult women when compared with men was increased and associated with increased acyl coenzyme A synthase activity, suggesting greater FFA recycling in women than men (10). We found that when cf was adjusted for percent body fat and LBM, cf was the same in girls and boys. Because boys had significantly greater LBM than girls and similar fasting FFA, we hypothesize that cf may be similar in boys and girls due to FFA being more rapidly taken up into boys' lean tissues including muscle. We would further hypothesize that both greater FFA appearance rates (l2 and l0 + l2) and disposal (cf) in girls than boys (when adjusted for FM) suggest that, as in adults, girls are more efficient in recycling FFA than boys.
There are several limitations of this study. We were not able to compare lipolysis and clearance rates between lean children and obese children to determine whether the sex-dependent differences we observed are independent of adiposity and total body mass. Very few children who were prepubertal were included in this cohort, and a study of differences in FFA flux in prepubertal children would be of interest. We were not able to include adolescents other than NHBs to compare with NHWs; studying subjects with more diverse race and ethnicity would be of interest in the future. Furthermore, without the use of tracers, we were unable to examine region-specific FFA flux or distinguish between changes in plasma FFA due to lipolysis, recycling, or oxidation. Rather, we have reported the net result of all 3 processes. Lastly, this mathematical model of FFA flux has not been validated against a gold standard such as tracer studies as of yet. However, our model has been compared with 23 different physiologically based mathematical models of FFA kinetics in response to changing glucose and insulin levels of which 3 have been published (21, 38, 39). The strength of our study is the use, in a relatively large and well characterized cohort, of a noninvasive method to assess sex-related changes in FFA flux in adolescents, resulting in findings that agree quite well with previously reported changes in adults.
In conclusion, our data suggest that obese girls have higher maximum and insulin-suppressible lipolysis rates, require a greater concentration of insulin to suppress lipolysis and have higher FFA clearance rates, than obese boys. In these obese and insulin-resistant adolescents, sex-associated differences in lipolysis rates were observed after adjusting for the significant contribution of resting energy expenditure, suggesting energy balance modulates lipolysis rates. Comparison of lipolysis rates in prepubertal and pubertal children may provide insight into the mechanisms involved. Furthermore, differences in the clearance rate constant were predicted by FM and LBM, consistent with an important role for body composition in modulating FFA clearance rate.
Acknowledgments
We thank the patients who participated in this study along with research assistants Erica Fallon, Samareh Ghorbani, Lisa Ranzenhofer, Jenna Checchi, Ayelet Spitzer, Abena Akomeah, and Elizabeth A. Stern for their contributions to this work.
This work was supported by the Intramural Research Program, NIH (Grant Z1AHD00641 to J.A.Y.), from the Eunice Kennedy Shriver National Institute of Child Health and Human Development with supplemental funding from National Institute on Minority Health and Health Disparities, NIH. J.A.Y. is a Commissioned Officer in the U.S. Public Health Service, Department of Health and Human Services.
Disclosure Summary: All of the authors (D.C.A.-W., V.P., A.H.A., S.M.B., J.R.M., G.I.U., M.T.K., C.G.S., V.S.H., J.C.R., C.C., A.E.S., and J.A.Y.) have nothing to declare.
Footnotes
- AIRG
- acute insulin response to glucose
- ANCOVA
- analysis of covariance
- BMI
- body mass index
- BMI-Z
- BMI SD score
- FFA
- free fatty acid
- FM
- fat mass
- IM-FSIGT
- insulin-modified frequently sampled iv glucose tolerance test
- LBM
- lean body mass
- NAFLD
- nonalcoholic fatty liver disease
- NEFA
- nonesterified fatty acid
- NHB
- non-Hispanic black
- NHW
- non-Hispanic white
- REE
- resting energy expenditure
- SI
- insulin sensitivity index
- VAT
- visceral abdominal adipose.
References
- 1. Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003;111:121–124 [DOI] [PubMed] [Google Scholar]
- 2. Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep. 2006;6:177–181 [DOI] [PubMed] [Google Scholar]
- 3. Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90:11G–18G [DOI] [PubMed] [Google Scholar]
- 4. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23 [DOI] [PubMed] [Google Scholar]
- 5. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229 [DOI] [PubMed] [Google Scholar]
- 6. Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–1527 [DOI] [PubMed] [Google Scholar]
- 7. Jensen MD. Lipolysis: contribution from regional fat. Annu Rev Nutr. 1997;17:127–139 [DOI] [PubMed] [Google Scholar]
- 8. Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989;83:1168–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein S. The case of visceral fat: argument for the defense. J Clin Invest. 2004;113:1530–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali AH, Koutsari C, Mundi M, et al. Free fatty acid storage in human visceral and subcutaneous adipose tissue: role of adipocyte proteins. Diabetes. 2011;60:2300–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koutsari C, Basu R, Rizza RA, Nair KS, Khosla S, Jensen MD. Nonoxidative free fatty acid disposal is greater in young women than men. J Clin Endocrinol Metab. 2011;96:541–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 2007;56:1369–1375 [DOI] [PubMed] [Google Scholar]
- 13. Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring). 2009;17:1872–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bougneres P, Stunff CL, Pecqueur C, Pinglier E, Adnot P, Ricquier D. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J Clin Invest. 1997;99:2568–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fabbrini E, deHaseth D, Deivanayagam S, Mohammed BS, Vitola BE, Klein S. Alterations in fatty acid kinetics in obese adolescents with increased intrahepatic triglyceride content. Obesity (Silver Spring). 2009;17:25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor beta-cell function, and increasing visceral fat. Diabetes. 2008;57:3007–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Syme C, Abrahamowicz M, Leonard GT, et al. Intra-abdominal adiposity and individual components of the metabolic syndrome in adolescence: sex differences and underlying mechanisms. Arch Pediatr Adolesc Med. 2008;162:453–461 [DOI] [PubMed] [Google Scholar]
- 18. Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367–371 [DOI] [PubMed] [Google Scholar]
- 19. Kursawe R, Eszlinger M, Narayan D, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes. 2010;59:2288–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chow CC, Periwal V, Csako G, et al. Higher acute insulin response to glucose may determine greater free fatty acid clearance in African-American women. J Clin Endocrinol Metab. 2011;96:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Periwal V, Chow CC, Bergman RN, Ricks M, Vega GL, Sumner AE. Evaluation of quantitative models of the effect of insulin on lipolysis and glucose disposal. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1089–R1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anonymous Safety and efficacy of Xenical in children and adolescents with obesity-related diseases. In: NIH Clinical Research Studies. http://wwwclinicaltrials.gov/ct/show/NCT00001723 Bethesda, MD: National Institutes of Health; 1998 [Google Scholar]
- 23. Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23(Suppl 2):S2–S11 [DOI] [PubMed] [Google Scholar]
- 24. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yanovski JA, Yanovski SZ, Filmer KM, et al. Differences in body composition of black and white girls. Am J Clin Nutr. 1996;64:833–839 [DOI] [PubMed] [Google Scholar]
- 26. McDuffie JR, Adler-Wailes DC, Elberg J, et al. Prediction equations for resting energy expenditure in overweight and normal-weight black and white children. Am J Clin Nutr. 2004;80:365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faulconnier Y, Roy A, Ferlay A, et al. Effect of dietary supply of butters rich either in trans-10--18:1 or in trans-11-18:1 plus cis-9, trans-11-18:2 on rabbit adipose tissue and liver lipogenic activities. Br J Nutr. 2006;96:461–468 [PubMed] [Google Scholar]
- 28. Shomaker LB, Tanofsky-Kraff M, Young-Hyman D, et al. Psychological symptoms and insulin sensitivity in adolescents. Pediatr Diabetes. 2010;11:417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism. 2007;56:68–76 [DOI] [PubMed] [Google Scholar]
- 30. Cook JS, Hoffman RP, Stene MA, Hansen JR. Effects of maturational stage on insulin sensitivity during puberty. J Clin Endocrinol Metab. 1993;77:725–730 [DOI] [PubMed] [Google Scholar]
- 31. Shadid S, Kanaley JA, Sheehan MT, Jensen MD. Basal and insulin-regulated free fatty acid and glucose metabolism in humans. Am J Physiol Endocrinol Metab. 2007;292:E1770–E1774 [DOI] [PubMed] [Google Scholar]
- 32. Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest. 2003;111:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Travers SH, Jeffers BW, Bloch CA, Hill JO, Eckel RH. Gender and Tanner stage differences in body composition and insulin sensitivity in early pubertal children. J Clin Endocrinol Metab. 1995;80:172–178 [DOI] [PubMed] [Google Scholar]
- 34. Bloch CA, Clemons P, Sperling MA. Puberty decreases insulin sensitivity. J Pediatr. 1987;110:481–487 [DOI] [PubMed] [Google Scholar]
- 35. Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60:759–763 [DOI] [PubMed] [Google Scholar]
- 36. Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–219 [DOI] [PubMed] [Google Scholar]
- 37. Arslanian SA, Heil BV, Becker DJ, Drash AL. Sexual dimorphism in insulin sensitivity in adolescents with insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72:920–926 [DOI] [PubMed] [Google Scholar]
- 38. Arslanian SA, Kalhan SC. Correlations between fatty acid and glucose metabolism. Potential explanation of insulin resistance of puberty. Diabetes. 1994;43:908–914 [DOI] [PubMed] [Google Scholar]
- 39. Thomaseth K, Pavan A. Model-based analysis of glucose and free fatty acid kinetics during glucose tolerance tests. In: Hargrove J, Berdanier C. eds. Mathematical Modeling in Nutrition and Toxicology. Athens, GA: Mathematical Biology Press; 2005:21–40 [Google Scholar]
- 40. Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the body. Prog Biophys Mol Biol. 2006;91:249–286 [DOI] [PubMed] [Google Scholar]