Abstract
HIV protease inhibitors (HIV-PI) are oral drugs for HIV treatment. HIV-PI have antitumor activity via induction of ER-stress, inhibition of phospho-AKT (p-AKT) and the proteasome, suggesting antimyeloma activity. We characterize the effects of all approved HIV-PI on myeloma cells. HIV-PI were compared regarding cytotoxicity, proteasome activity, ER-stress induction and AKT phosphorylation using myeloma cells in vitro. Nelfinavir is the HIV-PI with highest cytotoxic activity against primary myeloma cells and with an IC50 near therapeutic drug blood levels (8–14 μM), irrespective of bortezomib sensitivity. Only nelfinavir inhibited intracellular proteasome activity in situ at drug concentrations <40 μℳ. Ritonavir, saquinavir and lopinavir inhibited p-AKT comparable to nelfinavir, and showed similar synergistic cytotoxicity with bortezomib against bortezomib-sensitive cells. Nelfinavir had superior synergistic activity with bortezomib/carfilzomib in particular against bortezomib/carfilzomib-resistant myeloma cells. It inhibited not only the proteasomal β1/β5 active sites, similar to bortezomib/carfilzomib, but in addition the β2 proteasome activity not targeted by bortezomib/carfilzomib. Additional inhibition of β2 proteasome activity is known to sensitize cells for bortezomib and carfilzomib. Nelfinavir has unique proteasome inhibiting activity in particular on the bortezomib/carfilzomib-insensitive tryptic (β2) proteasome activity in intact myeloma cells, and is active against bortezomib/carfilzomib-resistant myeloma cells in vitro.
Keywords: proteasome, drug resistance, myeloma
Introduction
HIV protease inhibitors (HIV-PI) are standard components of highly active antiretroviral therapy for HIV-infected patients. They were developed to specifically inhibit the HIV protease, an aspartate protease that lacks mammalian close homologs. Meanwhile, nine HIV-PI have been approved (saquinavir, nelfinavir, lopinavir, amprenavir, atazanavir, darunavir, tipranavir, indinavir), most of which are structural homologs of the lead drug ritonavir, but have improved pharmacokinetics, tolerability or activity.1 In addition, HIV-PI emerge as a novel class of potential antineoplastic drugs.2 In particular ritonavir, indinavir, saquinavir, nelfinavir and lopinavir have demonstrated preclinical antineoplastic activity against several human tumors in mice, including liver, prostate, lung, breast thymoma, lymphoma, myeloma, Kaposi's sarkoma and leukemia.3, 4, 5, 6, 7, 8, 9, 10 The antineoplastic activity of HIV-PI is also supported by the decreased mortality from HIV-associated tumors as the advent of highly active antiretroviral therapy, which lacks correlation with either HIV load reduction or CD4 gain,11, 12 suggesting a direct antineoplastic effect of highly active antiretroviral therapy.13 The main molecular effects that presumably build the basis for the antineoplastic activity of HIV-PI are the inhibition of the PI3K/phospho-AKT (p-AKT) pathway, as demonstrated not only in vitro but also in patients receiving HIV-PI,14 as well as proteasome inhibition and the induction of endoplasmic reticulum stress that have been repeatedly shown in vitro.15 HIV-PI have synergistic antineoplastic activity with radiation therapy and several antineoplastic drugs, including bortezomib.10, 16
Novel inhibitors of the proteasome, ER-stress inducing agents and inhibitors of the PI3K/p-AKT axis are currently in preclinical and clinical development to overcome bortezomib resistance.17 Based on their molecular properties, but also on their availability, oral use and lack of hematological toxicity, HIV-PI are extremely interesting drugs for a potential repositioning as antimyeloma therapy.
A landmark paper compared the cytotoxic activity of all HIV-PI against lung cancer cell lines, and identified nelfinavir as the HIV-PI with the potentially highest antineoplastic activity.6 Myeloma cells have a unique protein biosynthesis machinery, response to ER-stress and sensitivity towards proteasome inhibition,18 and in this respect differ from all other types of malignant cells. ER-stress activates the unfolded protein response (UPR), a homeostatic system that balances protein biosynthesis, folding and destruction, that eliminates cells experiencing excessive ER-stress via UPR-induced apoptosis.19 Constitutive activation of the UPR is required for plasma cell differentiation,20 and patient serum levels of active XBP-1, which regulates UPR activity, correlate with the clinical response towards bortezomib,18 illustrating the unique association between UPR activity, and therapeutic efficacy of proteasome inhibition in myeloma. Owing to this extraordinary role of the UPR for myeloma cells, the molecular and biological effects of HIV-PI on solid tumor cell lines may not be representative for myeloma in particular. To select the most appropriate HIV-PI for clinical trials in myeloma, to estimate potentially therapeutic blood levels, to select the putatively most appropriate target population of myeloma patients, and to identify combination partners for this drug, a detailed analysis and comparison of the effects of HIV-PI on myeloma cells was performed.
Materials and methods
Cells and inhibitors
The human myeloma cell lines RPMI8226, U266, AMO-1, LP-1, as well as HL-60 leukemia cells were obtained from American Type Culture Collection and maintained in fetal calf serum-supplemented RPMI-1640 with penicillin/streptomycin. The bortezomib-adapted cells have been described elsewhere.21 Cells were treated with bortezomib (provided by Ortho Biotech, Neuss, Germany), 6 μg/ml tunicamycin, 100 nM thapsigargin (both Biomol, Neuss, Germany), 50 μM of the vinylsulfone-type proteasome inhibitor 4-Hydroxy-5-iodo-3-nitrophenylacetyl-Leu-Leu-Leu-vinylsulfone (NLVS)22, 23 and the other inhibitors for 16 h, if not stated otherwise. Amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir and tipranavir have been kindly provided by the NIH AIDS Reagent Program. Lenalidomide and Sorafenib were purchased from LC Laboratories (Woburn, MA, USA). Carfilzomib was provided by Onyx Pharmaceuticals, Inc. (South San Francisco, CA, USA).
Dimethylthiazol-diphenyltetrazole assay
The CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA) was used to determine the cytotoxicity of the used inhibitors, according to the manufacturer's instructions, and the absorbance of the formazan product was determined in 96-well microplates at 492 nm. Results represent mean values from quadruplicate wells in one of at least three independent experiments.
Western blot, antibodies
SDS-polyacrylamide gel electrophoresis and western blot was performed on precast NuPage Bis-Tris gels (Life Technologies, Carlsbad, CA, USA). Anti-CHOP (Gadd 153) antibody was purchased from Santa Cruz Biotechnology (Heidelberg, Germany), anti-BiP (Grp78) antibody from Becton Dickinson (Heidelberg, Germany), anti-p38, anti-pT180/pY182-p38, anti-ERK1/2, anti-pT202/pY204-ERK1/2, anti-JNK/SAPK, anti-pT183/pY185-JNK/SAPK from Transduction Laboratories (Becton Dickinson), anti-HSP70 antibody from Dianova (Hamburg, Germany), anti-IRE1α, anti-AKT, anti-eIF2α and anti-pS51eIF2α from New England Biolabs (Frankfurt, Germany), anti-caspase 4, anti-ATF6 from Biomol, anti-GAPDH and anti-β-actin from Sigma-Aldrich (St Louis, MO, USA) and anti-pS473-AKT1 from Epitomics (Burlingame, CA, USA). Antibodies against caspase 9 and caspase 8 were kindly provided by S. Wesselborg (University of Tübingen, Germany). The PARP-1 antibody detects the p85 spliced form (Promega). The anti-PDI rabbit antiserum was provided by H. Ploegh (MIT, Boston).
Determination of proteasome activity by active-site labeling
The proteasome-specific affinity probe Bodipy TMR-Ahx3L3VS (MV151) was synthesized as described.24 Both the constitutive (β1(Y), β2(Z) and β5(X)) and the immunoproteasome subunits (β1i (LMP2), β2i (MECL-1), β5i (LMP7)) were labeled by MV-151 in intact cells, washed and lysed. Where indicated, cells were challenged with proteasome inhibitors or HIV-PI for 16 h before labeling. Samples were adjusted for equal total protein after cell lysis, and SDS-polyacrylamide gel electrophoresis was performed on NuPage 12% precast gels (Life Technologies). Visualization of labeled species was performed by fluorescence detection with Fusion FX7 (Vilber Lourmat, Eberhardzell, Germany) and proteasome subunit-specific fluorescence signals (separately for β2/2i and β1/1i/5/5i) were quantified using Bio 1D software (Vilber Lourmat).
Human cell samples
All cell samples from humans were obtained after approval by the independent ethics review board and after written informed consent had been obtained, in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guideline for Good Clinical Practice and local regulations. Myeloma cells were retrieved from bone marrow or peripheral blood of patients and were enriched by Ficoll density centrifugation to a purity of >80%, where necessary. Purity was assessed by cytomorphology on stained samples. Monocytes were enriched from peripheral blood mononuclear cell (PBMC) to >80% purity using a percoll gradient and purity confirmed by flow cytometry.25 Normal CD138+ cells were isolated from the leukapheresis product of a healthy stem cell donor after stem cell mobilization with granulocyte colony stimulating factor. After successful stem cell collection for an familiar allogeneic transplant from this donor, the donor had given informed consent to undergo one additional leukapheresis collection procedure for research purposes. CD138-positive cells were collected from this apheresis product using magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany).
Statistical analysis
Unless stated otherwise, one representative experiment out of at least three independent experiments is shown; for dimethylthiazol-diphenyltetrazole assays mean values from quadruplicate samples are represented. Synergism between bortezomib and the different HIV-PI was calculated using combination index described in.26 A combination Index <1 indicates synergism, >1 indicates antagonism. Normalized isobolograms were produced by plotting the bortezomib ratio (monotherapy dose vs dose needed in combination to reach the same effect) on the x-axis vs the HIV-PI ratio on the y-axis.
Results
Cytotoxic activity of HIV-PI on myeloma cell lines and bortezomib-adapted cells
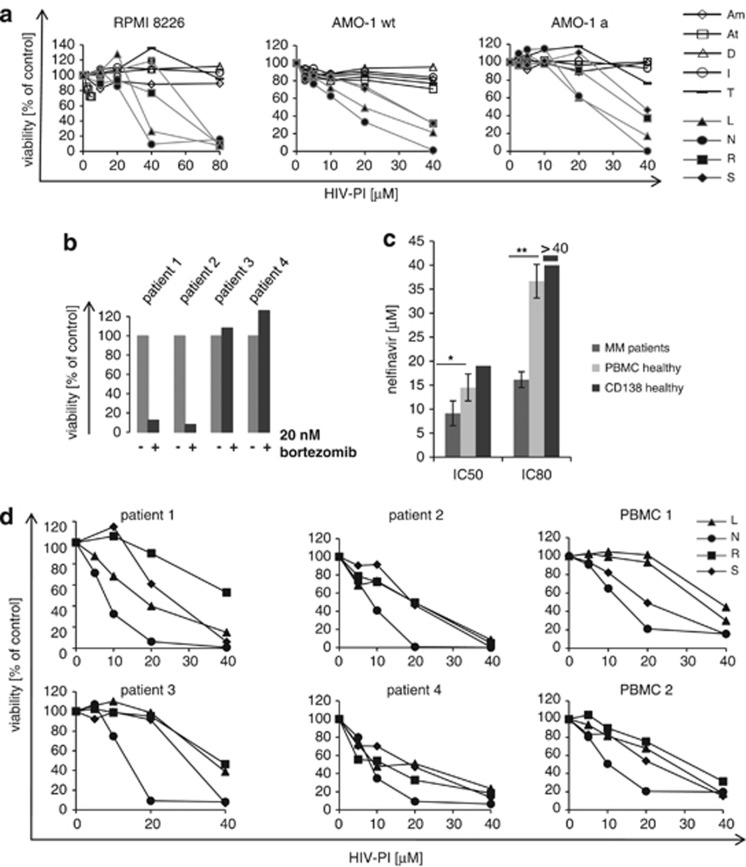
We first systematically compared the cytotoxic activity of all available HIV-PI on myeloma cells. Nelfinavir, lopinavir, ritonavir and saquinavir induced cytotoxicity in RPMI8226, U266, LP-1 and AMO-1 myeloma cells with IC50 below 80 μℳ. Only nelfinavir and lopinavir showed potentially clinically relevant IC50 values below 40 μℳ (Figure 1a and Supplementary Figure 1), however, these IC50 values were between 20 and 40 μℳ, and, therefore, considerably higher than the average nelfinavir concentrations of 5.2 μℳ reported to inhibit 50% growth in solid tumor cell lines.6 As proteasome inhibition has been postulated as the major mechanism for the cytotoxic activity of HIV-PI on myeloma cells,10 we also compared the effect of HIV-PI between bortezomib-resistant cells (AMO-1a myeloma cells and also HL-60a AML cells, adapted to be resistant against 80 nM bortezomib (HL-60), or 40 nM (AMO-1), respectively21) and their respective wild-type parental cell lines with normal bortezomib sensitivity (AMO-1, HL-60). All HIV-PI showed a very similar cytotoxic effect on bortezomib-resistant vs bortezomib-sensitive cells, suggesting that the mechanism that provides bortezomib resistance in bortezomib-adapted cell lines does not affect their sensitivity against HIV-PI. Thus lopinavir, nelfinavir, ritonavir and saquinavir have cytotoxic activity against myeloma cell lines, including bortezomib-resistant cells, at low to medium micromolar drug levels, suggesting that these HIV-PI may be useful to overcome bortezomib resistance of myeloma.
Figure 1.
Cytotoxic activity of different HIV-PI on myeloma cell lines and primary cells. (a) Myeloma cell lines (RPMI8226, AMO-1), as well as bortezomib-resistant myeloma cells (AMO-1a, in comparison with their bortezomib-sensitive wild-type (wt) parental cell lines AMO-1), were incubated with increasing concentrations of all nine approved HIV-PI (Am, amprenavir; At, atazanavir; D, darunavir; I, indinavir; T, tipranavir; L, lopinavir; N, nelfinavir; R, ritonavir; S, saquinavir) and cell viability determined by dimethylthiazol-diphenyltetrazole test. (b) Myeloma cells isolated from patients that had failed prior bortezomib-containing therapy were challenged with bortezomib 20 nℳ in vitro. Cell viability was assessed by dimethylthiazol-diphenyltetrazole test. (c) Primary myeloma cells from patients (n=4), as well as normal PBMC (n=4), as well as CD138 plasma cells from a healthy stem cell donor were incubated with increasing concentrations of nelfinavir and the IC50 and IC80 were assessed by dimethylthiazol-diphenyltetrazole test. * indicates statistically significant differences between the primary myeloma cells and PBMC from healthy donors, as assessed by student's t-test. (d) Primary myeloma cell samples characterized above and PBMC were incubated with increasing concentrations of lopinavir (L), nelfinavir (N), ritonavir (R) and saquinavir (S), and cell viability was measured by dimethylthiazol-diphenyltetrazole test.
Cytotoxic activity of HIV-PI against primary human myeloma cells
We next isolated primary myeloma cells from four individual patients that had progressed under prior bortezomib-containing therapy. To establish bortezomib-resistence of these primary cells, we first exposed them to clinically relevant bortezomib concentrations (20 nM) in vitro (Figure 1b). Two of the four patient samples showed bortezomib resistance in vitro, so that their cell viability was unaffected by 20 nM bortezomib. To assess a potential therapeutic window for a clinical treatment of myeloma with nelfinavir, we compared the IC50 and IC80 between the four myeloma cell samples, normal PBMC samples and normal CD138 plasma cells from a healthy stem cell donor (Figure 1c). The mean IC50 for nelfinavir was 9.1 μℳ for myeloma cells and thus slightly lower than for PBMC at 11.5 μℳ (difference statistically significant, P=0.042) or normal CD 138 (IC50 at 19 μℳ). The IC80 for nelfinavir was between 14 and 18 μℳ (mean 15.75 μℳ) for the primary myeloma cell samples, irrespective of their degree of bortezomib sensitivity, and 35 μℳ for normal PBMC (P=0.011), while an IC80>40 μℳ was found for normal CD138 cells.
When these primary myeloma cells were exposed to lopinavir, nelfinavir, ritonavir or saquinavir in vitro (Figure 1d), cytotoxic activity was observed in the 5–40 μℳ dose range, without a clearcut difference between bortezomib-resistant vs bortezomib-sensitive cell samples. However, nelfinavir was consistently the most effective HIV-PI to induce cytotoxicity in primary myeloma cells with IC50 in the 8–14 μℳ range, while the IC50 for ritonavir, saquinavir and lopinavir were between 10 and 40 μℳ.
Effect of nelfinavir on different proteasome active subunits in intact myeloma cells
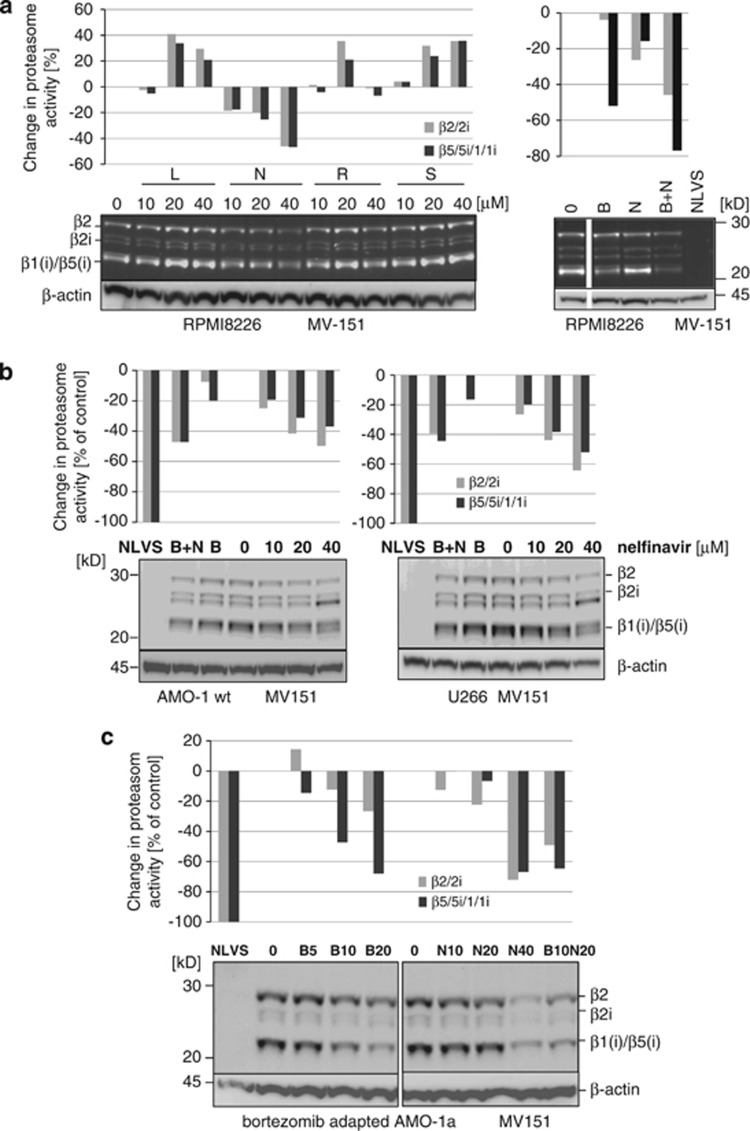
Inhibition of the proteasome's β2 (trypsin-like) activity in addition to β5 inhibition is required to achieve optimal cytotoxic activity of proteasome inhibitors.27 Except marizomib, which is a pan-proteasome inhibitor, all proteasome inhibitors in current clinical development are selective inhibitors of the β1/β5-type active sites. We, therefore, analyzed the subunit specificity of proteasome inhibition by nelfinavir, using the cell-permeable, proteasome specific, fluorescent affinity probe MV151.24 This tool allows to specifically label all active proteasome subunits in intact cells and can provide a reliable quantitative estimate of changes in the intracellular β1/β5 and β2 activities under vital conditions. Labeling of proteasome activity in intact RPMI8226 myeloma cells revealed that nelfinavir is a pan-proteasome inhibitor that inhibits not only the β1/β5-type of activity, but also the β2 type of activity with equal potency, in contrast to lopinavir, ritonavir and saquinavir (Figure 2a). Similarly, all remaining HIV-PI did not show meaningful proteasome inhibition in intact cells (Supplementary Figure 2). Proteasome inhibition by nelfinavir was already observed at 10 μℳ where it resulted in close to 20% intracellular inhibition of β1/β5 activity in RPMI8226 cells. While nelfinavir at low concentrations provided less efficient inhibition of the bortezomib-targeted subunits than bortezomib 20 nM (Figure 2a, right panel), it resulted in a more effective inhibition of β2 proteasome activity, compared with bortezomib. At nelfinavir concentrations of 40 μℳ, >40% β1/β5 inhibition was achieved in RPMI8226 cells, which made it almost as effective for β1/β5 inhibition as bortezomib 20 nM.
Figure 2.
Proteasome inhibition by nelfinavir alone and in combination with bortezomib. (a) RPMI8226 myeloma cells were incubated with lopinavir (L), nelfinavir (N), ritonavir (R) or saquinavir (S) at the indicated concentrations and active proteasome subunits in intact cells were compared quantitatively with the untreated control cell sample using the MV151 covalent affinity labeling procedure (left panel). Right panel: Untreated cells (0) were also compared with bortezomib (B) or nelfinavir (N)-treated cells or to cells treated with bortezomib and nelfinavir in combination (B+N, 20 nℳ and 20 μℳ, respectively; right panel). Cells treated with the pan-proteasome inhibitor 4-Hydroxy-5-iodo-3-nitrophenylacetyl-Leu-Leu-Leu-vinylsulfone (NLVS) 50 μℳ were used as comparison for complete inhibition of proteasome activity. The bar graphs illustrate the quantitative changes in specific fluorescence signals for β1/β5 activity or β2 activity, relative to the untreated control sample. (b, c) AMO-1 and U266 (2B), as well as bortezomib-resistant AMO-1A (2C) myeloma cells, were incubated with either bortezomib (B: 5 nM for AMO-1, 10 nℳ for U266, (c) 5 and 10 nℳ for AMO-1A), nelfinavir (N) up to 40 μℳ or the combination of nelfinavir 20mM+bortezomib (B+N), and intracellular proteasome activity was assessed using the affinity probe MV151. The bar graphs represent relative changes in proteasome subunit-specific (β1/β5 vs β2) fluorescence signals compared with untreated (0) controls.
Given the fact the PI are peptide-like protease substrate-mimetics that block the active-site of the HIV protease, it is conceivable that they may compete with bortezomib for active-site binding at the proteasome. However, this was not the case, because the combination of 20 nM bortezomib with 20 μℳ nelfinavir resulted in additive inhibition of the β1/β5 in (Figure 2a, right panel), suggesting independent and non-competing mechanisms for β1/β5 restricted proteasome inhibition between nelfinavir and bortezomib. The combination of both drugs significantly inhibited β2 proteasome activity, in addition to the β1/β5 inhibition provided by bortezomib. Similarly, nelfinavir induced pan-proteasome inhibition in AMO-1 and U266 myeloma cells, as well as in the bortezomib-resistant AMO-1A cells (Figures 2a and c). In contrast to non-adapted AMO-1 cells, bortezomib-adapted AMO-1a cells had a higher relative β2 activity, consistent with published data.21 Increasing concentrations of bortezomib led in particular to a decrease in β1/β5 activity, while β2 activity was considerably less affected by bortezomib also in bortezomib-resistant cells, suggesting that bortezomib has maintained its molecular on target activity also in adapted cells, and arguing against the presence of possible mutations in the β1/β5 active sites that provide bortezomib resistance via loss of bortezomib binding. By contrast, nelfinavir at 40 μℳ significantly inhibited all active proteasome subunits including the β2 activity also in bortezomib-adapted cells. The combination of bortezomib 10 nM and nelfinavir 20 μℳ resulted in highly efficient inhibition of β2 proteasome activity, as well as β1/β5 activity, and was clearly more effective than either drug alone at this dose.
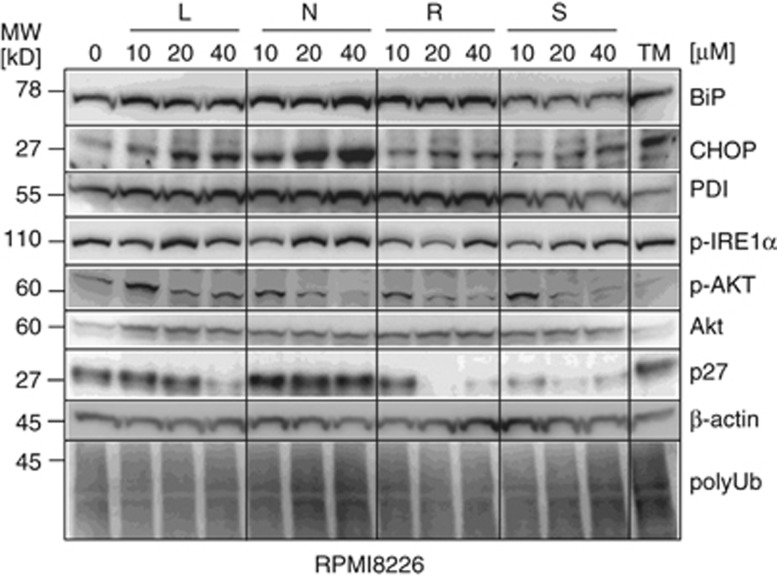
Compared with the other HIV-PI, nelfinavir showed the strongest dose-dependent UPR activation and UPR-dependent proapoptotic signaling, as revealed by upregulation of BIP and CHOP (Figure 3), as well as by the increase in p-IRE1α already at the intermediate 20 μℳ drug concentration. Consistent with also a functional relevance of proteasome inhibition by nelfinavir, we observed accumulation of the proteasome client protein p27, as well as a dose-dependent increase in polyubiquitinated protein in nelfinavir-treated myeloma cells. Of note, lopinavir, ritonavir and saquinavir decreased AKT phosphorylation with similar efficacy and dose response as nelfinavir, albeit in the absence of proteasome inhibition, demonstrating that p-AKT inhibition by these HIV-PI is independent from proteasome inhibition in myeloma cells.
Figure 3.
Molecular effects of nelfinavir on the UPR of myeloma cells. Myeloma cell samples from Figure 2a treated with either nelfinavir (N), ritonavir (R), saquinavir (S) or lopinavir (L) were assessed for expression of BIP, CHOP, p-IRE1α, Akt, p-AKT, p27 and polyubiquitinated protein by western blot.
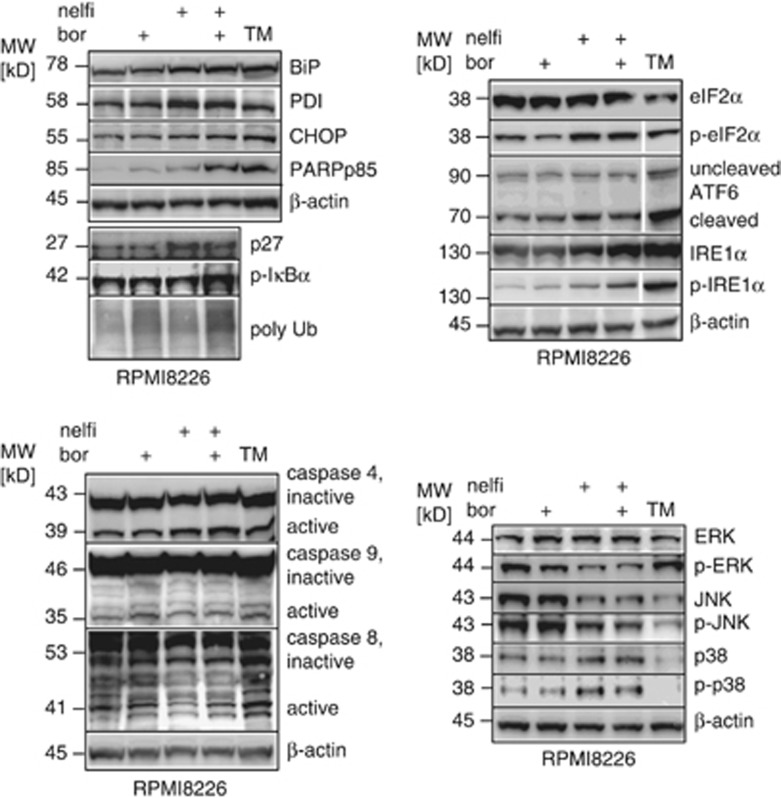
Bortezomib and nelfinavir induce synergistic cytotoxicity against myeloma cell lines in vitro,10 but the effects of both drugs in combination have neither been analyzed on a molecular level nor in primary myeloma cells. When compared with bortezomib alone in RPMI8226 cells (Figure 4), the combination bortezomib+nelfinavir resulted in increased expression of the ER chaperones PDI and BIP and increased triggering of UPR-induced apoptotic signaling, as revealed by CHOP upregulation, as well as in synergistically increased levels of cleaved PARP, consistent with synergistic UPR-triggered apoptosis induction by bortezomib and nelfinavir. Importantly, nelfinavir increased the accumulation of the proteasome client proteins P27, p-IκB, as well as polyubiquitinated protein, in bortezomib treated cells, demonstrating an increased biological effect of proteasome inhibition when both drugs are combined. With respect to the three different UPR-activating molecular pathways, we observed that nelfinavir triggered all three activating mechanisms of the UPR in myeloma, in contrast to bortezomib, as revealed by increased expression of p-elF2α, ATF6 cleavage and p-IRE1α. The addition of bortezomib to nelfinavir had little additional effect on p-elF2α or ATF6 cleavage, compared with the effect of nelfinavir alone, but it in particular increased the level of p-IRE1α in a synergistic fashion. Analysis of caspases 4, 8 and 9 showed increased caspase 4 cleavage after treatment with the nelfinavir-bortezomib combination, compared with untreated control or to either drug alone, but little effect on caspase 9 or 8 cleavage, consistent with UPR-induced apoptosis. Indeed, nelfinavir treatment resulted in a concentration dependent 91% increase of the activity of caspase 3/7, compared with control cells, consistent with apoptotic cell death (Supplementary Table 1). Accordingly, this, the fraction of cells with an early apoptotic phenotype by flow cytomertry (Annexin V-positive and 7-AAD negative) increased from 7.8 to 14% with nelfinavir exposure and further to 17% when nelfinavir was combined with bortezomib (Supplementary Figure 4).
Figure 4.
Molecular effects of nelfinavir in combination with bortezomib on myeloma cells. RPMI8226 myeloma cells were exposed to bortezomib (bor, 20 nℳ) and nelfinavir (nelfi, 20 μℳ) alone or in combination in vitro. Cells treated with tunicamycin (TM, 6 mg/ml) served as a positive control for UPR induction. Western blot was performed to compare the degree of UPR activation and its translation into apoptosis (BiP, PDI, CHOP, cleaved PARP, upper left panel), the cellular translation of proteasome inhibition (p27, p-IκBα, polyubiquitinated protein), the activation of the three major UPR-inducing signals (p-elF2α, cleaved active ATF6, p-IRE1α expression; upper right), as well as the UPR-dependent activation of caspases 4, 8 and 9 (lower left) and the effect of nelfinavir+bortezomib on the mitogen-activated protein kinase pathways (p-ERK, p-JNK, p-p38, p-c-JUN; lower right).
Inhibition of p-AKT by nelfinavir is well established in different cell types, and inhibition of the mitogen-activated protein kinase pathway by nelfinavir has been shown in adenoid cystic cancers.28 As activation of p-AKT (see Figure 3), as well as p-ERK are associated with bortezomib resistance of myeloma,29 we also investigated the effects of the combination of nelfinavir and bortezomib on the mitogen-activated protein kinase and the PI3K/p-AKT pathways. Nelfinavir inhibited activation of ERK, in contrast to bortezomib, and inhibition of ERK was likewise observed in myeloma cells treated with the combination of nelfinavir and bortezomib. Nor significant changes in the ratio between the native and the phosphorylated versions of JNK and p38 were induced by nelfinavir. This suggested that nelfinavir inhibits the prosurvival signaling provided by p-ERK, which may also contribute to the bortezomib-sensitizing effect of nelfinavir.
Synergistic cytotoxicity between proteasome inhibitors and different HIV-PI on myeloma cells
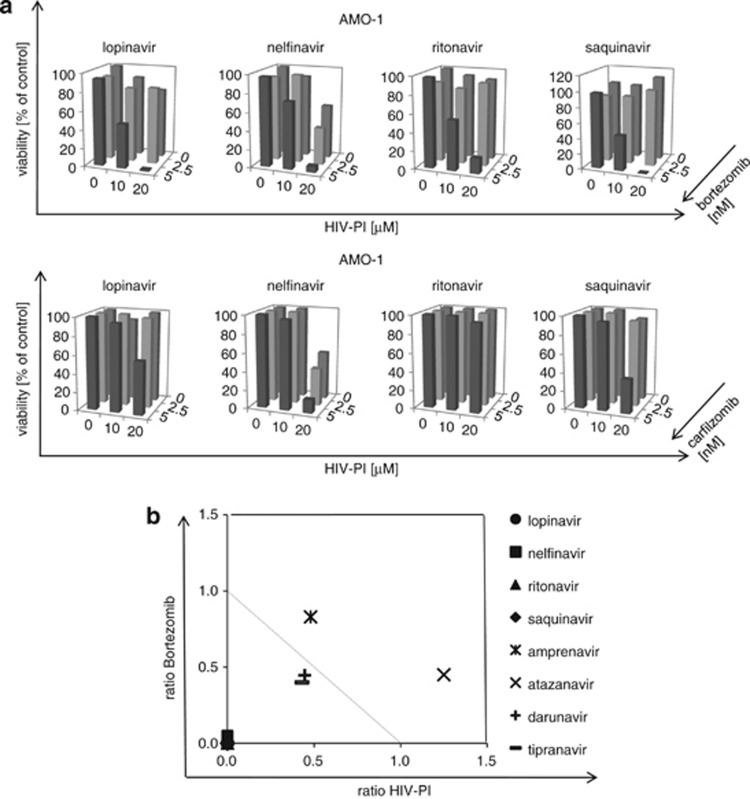
We next compared all HIV-PI with respect to their cytotoxic activity on myeloma cells in combination with bortezomib (Figure 5, Supplementary Figure 3). The synergistic nature of this effect was statistically confirmed by isolobogram analysis (Figure 5b). Besides nelfinavir, also lopinavir, saquinavir and ritonavir showed strong and quantitatively meaningful synergistic cytotoxicity with bortezomib against AMO-1 cells, in contrast to the remaining HIV-PI. Similarly, nelfinavir, lopinavir, and saquinavir, but not ritonavir, induced synergistic cytotoxicity with subeffective concentrations of carfilzomib (5nM), an alternative irreversible proteasome inhibitor with a different reactive group but with a β1/β5 inhibition preference similar to bortezomib. Nelfinavir was the most potent in combination with carfilzomib against myeloma cells, while meaningful synergistic cytotoxicity was not observed when PBMC were exposed to the combination of nelfinavir and bortezomib/carfilzomib (Supplementary Figure 3).
Figure 5.
Synergistic cytotoxicity of HIV-PI with bortezomib and carfilzomib. (a) AMO-1 cells were incubated with the respective HIV-PI (0–20 μℳ) in combination with bortezomib 0–5 nM (upper panel), or in combination with carfilzomib (0–5 nM, lower panel), and cell viability was assessed by dimethylthiazol-diphenyltetrazole test. (b) The synergistic cytotoxic effect of nelfinavir, lopinavir, saquinavir, ritonavir with bortezomib (a upper panel), in contrast to the other HIV-PI (Supplementary Figure 3), is statistically confirmed by Isolobograms (symbols below the dashed line statistically indicate synergism).
The synergy between proteasome inhibitors and HIV-PI implicated that subeffective, low concentrations of HIV-PI might resensitize bortezomib-resistant myeloma cells to proteasome inhibitor treatment. Thus nelfinavir increased proteasome inhibition by bortezomib and induces synergistic cytotoxicity also in bortezomib-resistant myeloma cells.
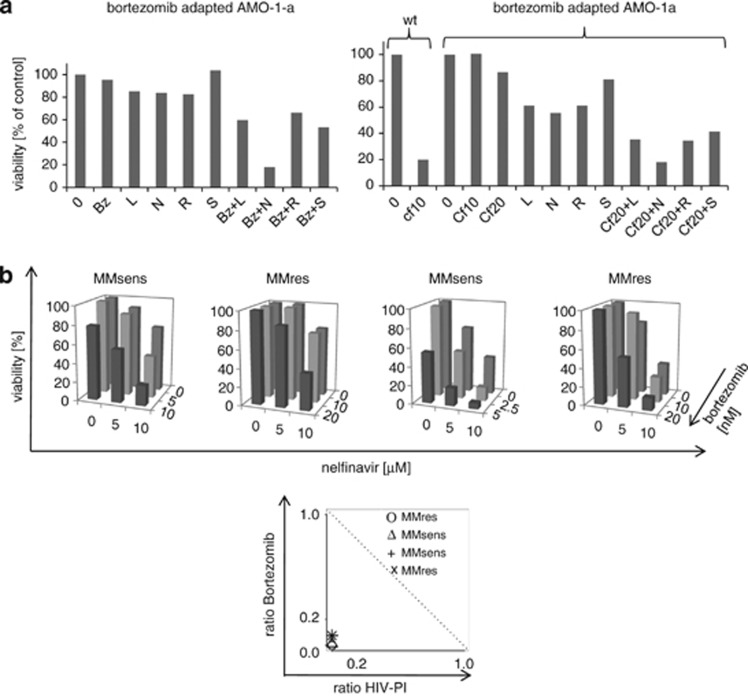
When we challenged bortezomib-adapted AMO-1a myeloma cells with bortezomib in combination with 20 μℳ HIV-PIs, we observed strong synergistic cytotoxicity with nelfinavir, while the synergistic activity with lopinavir, ritonavir or saquinavir on bortezomib-adapted cells was considerably weaker (Figure 6a left panel, and Supplemetary Table 2). To establish whether the addition of HIV-PI would also overcome resistance against carfilzomib, we investigated to what extent bortezomib-adapted AMO-1a cells would also show a decreased carfilzomib sensitivity (Figure 6a, right panel). Indeed, incubation of AMO-1a cells in comparison to the respective wild-type AMO-1 cell line showed that AMO-1a cells had also acquired carfilzomib resistance, as indicated by >80% cytotoxicity of AMO-1 cells at 10 nℳ carfilzomib, while AMO-1a cells were still >80% viable even at 20 nℳ carfilzomib. Lopinavir, ritonavir, saquinavir and nelfinavir at 20 μℳ sensitized AMO-1a cells also to carfilzomib. Also in combination with carfilzomib, nelvinavir appeared to be more active than lopinavir, saquinavir or ritonavir, to overcome acquired resistance against proteasome inhibitors. The superior synergistic activity between bortezomib/carfilzomib and nelfinavir, in comparison with lopinavir, ritonavir and saquinavir, was corroborated by the respective combination indices, which were consistently calculated at extremely low values <0.01 only for nelfinavir for both AMO-1 and AMO-1a cells (Supplementary Table 2).
Figure 6.
Synergistic activity of nelfinavir and bortezomib on bortezomib/carfilzomib-resistant myeloma cells. (a) right panel: Bortezomib-resistant AMO-1a cells were coincubated with bortezomib (Bz) 20 nℳ and the HIV-PI indicated (L. lopinavir; N: nelfinavir; R: ritonavir; S: saquinavir, 20 μℳ each) alone or in combination, and cytotoxicity was measured. Right panel: AMO-1 or AMO-1a cells were incubated with the indicated concentrations of carfilzomib (Cf, 10=10 nM, 20=20 nℳ) alone or in combination with nelfinavir (N), ritonavir (R), lopinavir (L) or saquinavir (S) (20 μℳ each) and the proportion of viable cells was assessed by dimethylthiazol-diphenyltetrazole test. (b) Upper panel: primary cell samples from bortezomib-resistant or -sensitive myeloma cell samples (MMsens, MMres) were coincubated with increasing concentrations of nelfinavir (0-10 μℳ) in combination with increasing concentrations of bortezomib (0- up to 20 nℳ, as indicated), and cell viability was assessed. Lower panel: The synergistic cytotoxic effect of nelfinavir and bortezomib on bortezomib-resistant primary myeloma cell samples is statistically confirmed by Isolobograms (symbols below the dashed line statistically indicate synergism).
Low concentrations of nelfinavir restore bortezomib sensitivity in bortezomib-resistant primary myeloma cell samples
We finally probed bortezomib-sensitive, as well as bortezomib-resistant primary myeloma cell samples obtained from myeloma patients with progressive disease under bortezomib-containing therapy with the combination of bortezomib and nelfinavir (Figure 6b). In both bortezomib-sensitive and resistant primary myeloma cells, low concentrations of nelfinavir (10 μℳ) had already a moderate intrinsic cytotoxic effect (20–60% cytotoxicity). The combination of these low nelfinavir concentrations with bortezomib resulted in up to 95% cell death, even with suboptimal bortezomib concentrations of 5–10 nℳ in bortezomib-sensitive samples. In bortezomib-resistant primary myeloma cells, where bortezomib up to 20 nℳ did not induce any cytotoxicity, as expected, nelfinavir 10 μℳ resensitized primary bortezomib-resistant myeloma cells to bortezomib, so that the combination of both drugs achieved a robust cytotoxic effect. The highly synergistic nature of this was statistically corroborated using isolobograms (bottom panel, for combination indices see Supplementary Table 2).
Discussion
HIV-PI are in particular attractive as potential treatment of multiple myeloma, given the availability of HIV-PI for clinical use, and the fact that proteasome inhibition, induction of ER-stress and inhibition of AKT phosporylation, the major molecular mechanisms identified for the antineoplastic activity of HIV-PI,2 are key targets for myeloma therapy.17 Surprisingly, only very little is known about the activity of HIV-PI against myeloma.30 Although nelfinavir has been shown to induce cytotoxicity in primary myeloma cells in vitro10 and shows antimyeloma activity in a murine xenograft model, it remains essentially unclear, which HIV-PI would be likely the most active to be moved forward into clinical trials in myeloma. In addition, the potential activity of HIV-PI against proteasome inhibitor-resistant myeloma remains to be elucidated.
We here identifiy nelfinavir as the most active antimyeloma drug of all nine available HIV-PI. This is supported by the low IC50 of 8–14 μℳ against primary myeloma cells, the superior cytotoxic activity of nelfinavir against bortezomib-resistant myeloma cells, as well as by its superior synergistic cytotoxicity in combination with carfilzomib. The Cmax for nelfinavir in HIV patients is 7–9 μℳ at the standard nelfinavir dose 2 × 1250 mg/day, p.o.6, 23 While a formal maximum tolerated dose for nelfinavir was never established in HIV patients, a dose escalation trial in patients with solid tumors reports that 2 × 4250 mg nelfinavir could safely be administered without reaching a maximum tolerated dose, and 10–15 μℳ (mean 12.5 μℳ) nelfinavir peak plasma level were measured at the 3000 mg bid dose level.31 Nelfinavir plasma levels that are presumably sufficient to induce reliable myeloma cell apoptosis (estimated to be>15 μℳ, based on our data with primary myeloma cells in vitro) are, therefore, difficult to reach in patients. For this reason, we believe that nelfinavir may best be exploited clinically as part of a combination therapy against myeloma. We here demonstrate that clinically achievable nelfinavir concentrations (5–10 μℳ) are sufficient to achieve synergistic cytotoxicity with bortezomib against primary human myeloma cells. Indeed, very recently the synergistic activity of bortezomib and nelfinavir was confirmed also in vivo in murine models of myeloma and non small cell lung cancer,32 and it was suggested that nelfinavir in combination with bortezomib may be useful to overcome bortezomib resistance.
While a growing number of active antimyeloma drugs becomes available, we still in particular are lacking drugs that are active in myeloma patients whose disease has become refractory to bortezomib, or to novel second generation proteasome inhibitors like carfilzomib. Given that proteasome inhibition is believed to be the most important mechanism for the antimyeloma activity of nelfinavir,10 it was unclear whether ‘proteasome inhibitor resistance' of myeloma would also extend to HIV-PI as antiproteasome agents. We here clearly demonstrate that this is not the case: not only nelfinavir, but also ritonavir, lopinavir and saquinavir had cytotoxic activity against bortezomib-refractory primary myeloma cells, as well as cell lines with acquired bortezomib- or carfilzomib insensitivity, and primary bortezomib-refractory myeloma cells were effectively killed when nelfinavir was added to bortezomib treatment. Of note, also carfilzomib showed synergy with nelfinavir, but also with lopinavir, saquinavir and ritonavir. However, the degree of synergy between carfilzomib and the respective HIV-PI is lower than observed for bortezomib, as shown by consistently lower combination indices for bortezomib (Supplementary Table 2), which may be a result of the inhibitory effect of bortezomib on β1 proteasome activity that is not shared to the same degree by carfilzomib. Such synergy may also extend to other peptide-borate or epoxyketone-type of proteasome inhibitors that share the active-site chemistry of bortezomib or carfilzomib. Such drugs in clinical development (MLN9708, ONX0912) with oral availability would match well with nelfinavir as orally available drug.
Our experiments for the first time assess the intracellular effect of HIV-PI on the proteasome in situ.24 The data in part contrasts with earlier work, where inhibition of proteasome activity by the majority of HIV-PI has been detected in cell lysates of various cell types, when the turnover of fluorogenic substrates was measured.2, 10 However, proteasome activity profiles obtained by performing measurements in cell extracts are known to be not necessarily representative of the in vivo activity patterns, stressing the need for live cell-based assays.24 In fact, we have repeated such types of experiments with fluorogenic substrates in cell lysates, and have observed >50% inhibition of the rate-limiting chymotryptic β5 proteasome activity by all HIV-PI, except nelfinavir, in such assays. Nelfinavir stood out, as it not only showed the most effective β5 inhibition in cell lysates (> 90%), but also in addition significant β2 inhibition (> 60%), while all other HIV-PI lacked β2 inhibiting activity in cell lysates (data not shown). However, this method is prone to postlysis artefacts, so that proteasome substrates may in cell lysates also be degraded by cathepsins or caspases whose activity cannot completely and specifically be ruled out. Importantly, we observed accumulation of the proteasome client proteins p27, as well as a sizable increase in polyubiquitinated protein exclusively in nelfinavir-treated cells, supporting that biologically relevant proteasome inhibition in intact myeloma cells is exclusively delivered by nelfinavir.
The molecular features of combining bortezomib and nelfinavir in myeloma cells are unknown. Ritonavir has been suggested to interact with a putative regulatory site at the 19S cap structure in isolated proteasomes, and not with the proteolytically active subunits.33 Our results are consistent with such a mechanism for nelfinavir.
Inhibition of the β2 proteasome subunit for therapeutic purposes has not been systematically explored, partly because cell-permeable β2 selective proteasome inhibitors are difficult to obtain. However, there is evidence that the inhibition of the proteasome β2 subunit could be of therapeutic value, especially in myeloma that has acquired resistance against β1/β5 restricted conventional proteasome inhibitors: low bortezomib sensitivity is correlated with low relative β2 proteasome activity,34 and bortezomib-resistant myeloma cells upregulate β2 proteasome activity, compared with non-resistant cells21 (compare also the ratio between β2 and β1/β5 in AMO-1 cells vs AMO-1a, Figure 2c), suggesting that high β2 activity may help myeloma cells to escape the effects of bortezomib-induced proteasome inhibition. In addition, selective β2 inhibition specifically sensitizes myeloma cells for bortezomib or carfilzomib treatment.27
Our study identifies nelfinavir as the most effective HIV-PI against myeloma. However, the activity of ritonavir, saquinavir and lopinavir against myeloma cells in the absence of intracellular proteasome inhibition remains a puzzling question. Our results suggest that proteasome inhibition and the inhibition of AKT phosphorylation are two independent molecular mechanisms of activity of HIV-PI in myeloma cells, in contrast to results from other cell types,35 because ritonavir, lopinavir and saquinavir decreased p-AKT in the absence of intracellular proteasome inhibition. The superior activity of nelfinavir especially against bortezomib-resistant myeloma cells is likely based on the synergy between proteasome inhibition and p-AKT inhibition triggered exclusively by nelfinavir.
In summary, our data provide a strong rationale to test nelfinavir in combination with proteasome inhibitors such as bortezomib, carfilzomib or novel oral β1/β5-targeting proteasome inhibitors in bortezomib-resistant myeloma in a clinical study.36, 37, 38, 39
Acknowledgments
This research was supported by the Swiss National Research Foundation SNF (31003A-120476), the Karl Danzer Stiftung and the Wilhelm Sander Stiftung für Krebsforschung. Carfilzomib was obtained from Onyx Pharmaceuticals, Inc.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Wlodawer A, Vondrasek J. Inhibitors of HIV-1 protease: a major success of structure-assisted drug design. Annu Rev Biophys Biomol Struct. 1998;27:249–284. doi: 10.1146/annurev.biophys.27.1.249. [DOI] [PubMed] [Google Scholar]
- Chow WA, Jiang C, Guan M. Anti-HIV drugs for cancer therapeutics: back to the future. Lancet Oncol. 2009;10:61–71. doi: 10.1016/S1470-2045(08)70334-6. [DOI] [PubMed] [Google Scholar]
- Esposito V, Palescandolo E, Spugnini EP, Montesarchio V, De LA, Cardillo I, et al. Evaluation of antitumoral properties of the protease inhibitor indinavir in a murine model of hepatocarcinoma. Clin Cancer Res. 2006;12:2634–2639. doi: 10.1158/1078-0432.CCR-05-2188. [DOI] [PubMed] [Google Scholar]
- Sgadari C, Barillari G, Toschi E, Carlei D, Bacigalupo I, Baccarini S, et al. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med. 2002;8:225–232. doi: 10.1038/nm0302-225. [DOI] [PubMed] [Google Scholar]
- Gaedicke S, Firat-Geier E, Constantiniu O, Lucchiari-Hartz M, Freudenberg M, Galanos C, et al. Antitumor effect of the human immunodeficiency virus protease inhibitor ritonavir: induction of tumor-cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer Res. 2002;62:6901–6908. [PubMed] [Google Scholar]
- Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, et al. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007;13:5183–5194. doi: 10.1158/1078-0432.CCR-07-0161. [DOI] [PubMed] [Google Scholar]
- Dewan MZ, Tomita M, Katano H, Yamamoto N, Ahmed S, Yamamoto M, et al. An HIV protease inhibitor, ritonavir targets the nuclear factor-kappaB and inhibits the tumor growth and infiltration of EBV-positive lymphoblastoid B cells. Int J Cancer. 2009;124:622–629. doi: 10.1002/ijc.23993. [DOI] [PubMed] [Google Scholar]
- Dewan MZ, Uchihara JN, Terashima K, Honda M, Sata T, Ito M, et al. Efficient intervention of growth and infiltration of primary adult T-cell leukemia cells by an HIV protease inhibitor, ritonavir. Blood. 2006;107:716–724. doi: 10.1182/blood-2005-02-0735. [DOI] [PubMed] [Google Scholar]
- Donia M, Maksimovic-Ivanic D, Mijatovic S, Mojic M, Miljkovic D, Timotijevic G, et al. In vitro and in vivo anticancer action of Saquinavir-NO, a novel nitric oxide-derivative of the protease inhibitor saquinavir, on hormone resistant prostate cancer cells. Cell Cycle. 2011;10:492–499. doi: 10.4161/cc.10.3.14727. [DOI] [PubMed] [Google Scholar]
- Bono C, Karlin L, Harel S, Mouly E, Labaume S, Galicier L, et al. The HIV-1 protease inhibitor nelfinavir impairs proteasome activity and inhibits the multiple myeloma cells proliferation in vitro and in vivo. Haematologica. 2012;97:1101–1109. doi: 10.3324/haematol.2011.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard I, Tassie JM, Kazatchkine MD, Orth G. Highly active antiretroviral therapy enhances regression of cervical intraepithelial neoplasia in HIV-seropositive women. AIDS. 2002;16:1799–1802. doi: 10.1097/00002030-200209060-00013. [DOI] [PubMed] [Google Scholar]
- Uberti-Foppa C, Ferrari D, Lodini S, Reina S, Ameglio F, Grasso MA, et al. Long-term effect of highly active antiretroviral therapy on cervical lesions in HIV-positive women. AIDS. 2003;17:2136–2138. doi: 10.1097/00002030-200309260-00021. [DOI] [PubMed] [Google Scholar]
- Monini P, Sgadari C, Toschi E, Barillari G, Ensoli B. Antitumour effects of antiretroviral therapy. Nat Rev Cancer. 2004;4:861–875. doi: 10.1038/nrc1479. [DOI] [PubMed] [Google Scholar]
- Plastaras JP, Vapiwala N, Ahmed MS, Gudonis D, Cerniglia GJ, Feldman MD, et al. Validation and toxicity of PI3K/Akt pathway inhibition by HIV protease inhibitors in humans. Cancer Biol Ther. 2008;7:628–635. doi: 10.4161/cbt.7.5.5728. [DOI] [PubMed] [Google Scholar]
- Bernstein WB, Dennis PA. Repositioning HIV protease inhibitors as cancer therapeutics. Curr Opin HIV AIDS. 2008;3:666–675. doi: 10.1097/COH.0b013e328313915d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M, Malenke E, Gogel J, Muller H, Ruckrich T, Overkleeft H, et al. Ritonavir induces endoplasmic reticulum stress and sensitizes sarcoma cells toward bortezomib-induced apoptosis. Mol Cancer Ther. 2008;7:1940–1948. doi: 10.1158/1535-7163.MCT-07-2375. [DOI] [PubMed] [Google Scholar]
- Anderson KC. The 39th David A. Karnofsky lecture: bench-to-bedside translation of targeted therapies in multiple myeloma. J Clin Oncol. 2012;30:445–452. doi: 10.1200/JCO.2011.37.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SC, Lau EK, Al-Shabeeb A, Nikolic A, Catalano A, Iland H, et al. Response of myeloma to the proteasome inhibitor bortezomib is correlated with the unfolded protein response regulator XBP-1. Haematologica. 2012;97:64–72. doi: 10.3324/haematol.2011.043331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- Ruckrich T, Kraus M, Gogel J, Beck A, Ovaa H, Verdoes M, et al. Characterization of the ubiquitin-proteasome system in bortezomib-adapted cells. Leukemia. 2009;23:1098–1105. doi: 10.1038/leu.2009.8. [DOI] [PubMed] [Google Scholar]
- Glas R, Bogyo M, McMaster JS, Gaczynska M, Ploegh HL. A proteolytic system that compensates for loss of proteasome function. Nature. 1998;392:618–622. doi: 10.1038/33443. [DOI] [PubMed] [Google Scholar]
- Tebas P, Powderly WG. Nelfinavir mesylate. Expert Opin Pharmacother. 2000;1:1429–1440. doi: 10.1517/14656566.1.7.1429. [DOI] [PubMed] [Google Scholar]
- Berkers CR, Verdoes M, Lichtman E, Fiebiger E, Kessler BM, Anderson KC, et al. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat Methods. 2005;2:357–362. doi: 10.1038/nmeth759. [DOI] [PubMed] [Google Scholar]
- Greiner A, Lautwein A, Overkleeft HS, Weber E, Driessen C. Activity and subcellular distribution of cathepsins in primary human monocytes. J Leukoc Biol. 2003;73:235–242. doi: 10.1189/jlb.0802398. [DOI] [PubMed] [Google Scholar]
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- Mirabella AC, Pletnev AA, Downey SL, Florea BI, Shabaneh TB, Britton M, et al. Specific cell-permeable inhibitor of proteasome trypsin-like sites selectively sensitizes myeloma cells to bortezomib and carfilzomib. Chem Biol. 2011;18:608–618. doi: 10.1016/j.chembiol.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Wilke WW, Taylor EN, Bodeker KL, Hoffman HT, Milhem MM, et al. Signaling pathways in adenoid cystic cancers: implications for treatment. Cancer Biol Ther. 2009;8:1947–1951. doi: 10.4161/cbt.8.20.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Wang S, Zangari M, Xu H, Cao TM, Xu C, et al. Over-expression of CKS1B activates both MEK/ERK and JAK/STAT3 signaling pathways and promotes myeloma cell drug-resistance. Oncotarget. 2010;1:22–33. doi: 10.18632/oncotarget.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezoe T, Saito T, Bandobashi K, Yang Y, Koeffler HP, Taguchi H. HIV-1 protease inhibitor induces growth arrest and apoptosis of human multiple myeloma cells via inactivation of signal transducer and activator of transcription 3 and extracellular signal-regulated kinase 1/2. Mol Cancer Ther. 2004;3:473–479. [PubMed] [Google Scholar]
- Chow J.Clin Oncol 28, 2010, suppl; abstr e13538.
- Kawabata S, Gills JJ, Mercado-Matos JR, Lopiccolo J, Wilson W, Hollander MC, et al. Synergistic effects of nelfinavir and bortezomib on proteotoxic death of NSCLC and multiple myeloma cells. Cell Death Dis. 2012;3:e353. doi: 10.1038/cddis.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke G, Holzhutter HG, Bogyo M, Kairies N, Groll M, de GR, et al. How an inhibitor of the HIV-I protease modulates proteasome activity. J Biol Chem. 1999;274:35734–35740. doi: 10.1074/jbc.274.50.35734. [DOI] [PubMed] [Google Scholar]
- Kraus M, Ruckrich T, Reich M, Gogel J, Beck A, Kammer W, et al. Activity patterns of proteasome subunits reflect bortezomib sensitivity of hematologic malignancies and are variable in primary human leukemia cells. Leukemia. 2007;21:84–92. doi: 10.1038/sj.leu.2404414. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Li B, Cerniglia GJ, Ahmed MS, Hahn SM, Maity A. The HIV protease inhibitor nelfinavir downregulates Akt phosphorylation by inhibiting proteasomal activity and inducing the unfolded protein response. Neoplasia. 2007;9:271–278. doi: 10.1593/neo.07124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan M, Fousek K, Jiang C, Guo S, Synold T, Xi B, et al. Nelfinavir induces liposarcoma apoptosis through inhibition of regulated intramembrane proteolysis of SREBP-1 and ATF6. Clin Cancer Res. 2011;17:1796–1806. doi: 10.1158/1078-0432.CCR-10-3216. [DOI] [PubMed] [Google Scholar]
- Gatti G, Di BA, Casazza R, De PC, Bassetti M, Cruciani M, et al. The relationship between ritonavir plasma levels and side-effects: implications for therapeutic drug monitoring. AIDS. 1999;13:2083–2089. doi: 10.1097/00002030-199910220-00011. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Wolf J, Jakubowiak A, Zonder J, Lonial S, Irwin D, et al. Perifosine plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously treated with bortezomib: results of a multicenter phase I/II trial. J Clin Oncol. 2011;29:4243–4249. doi: 10.1200/JCO.2010.33.9788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.