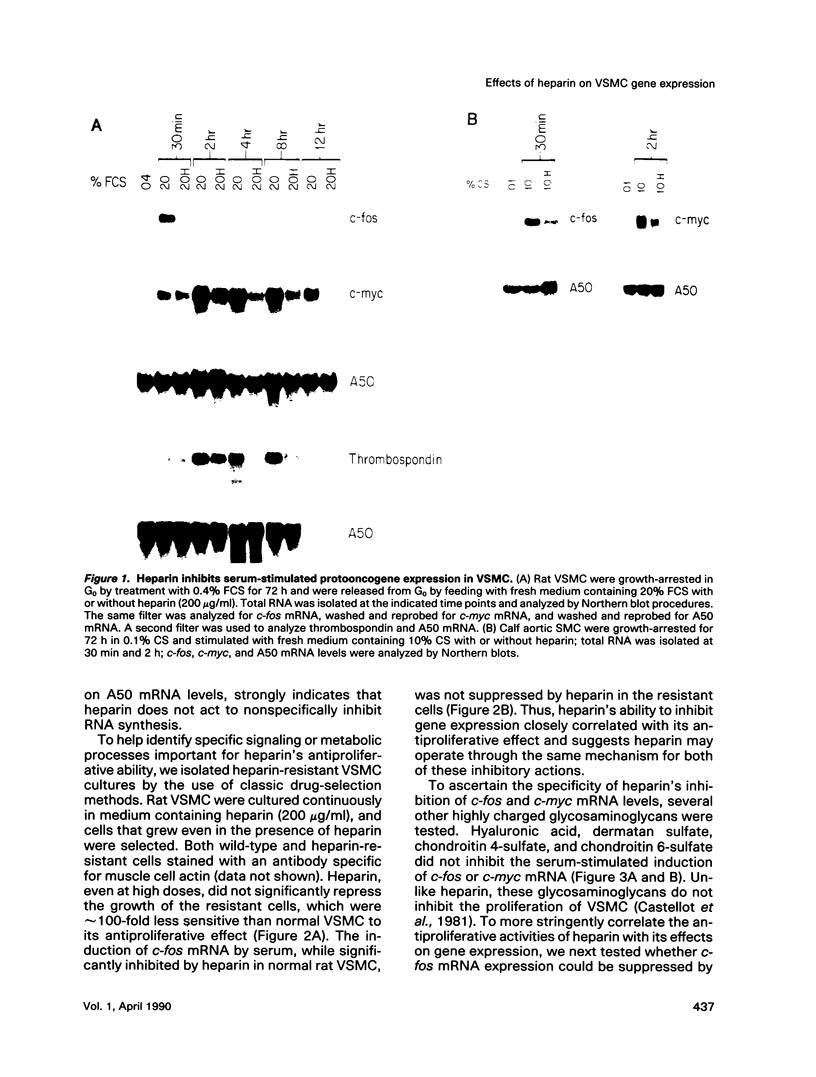
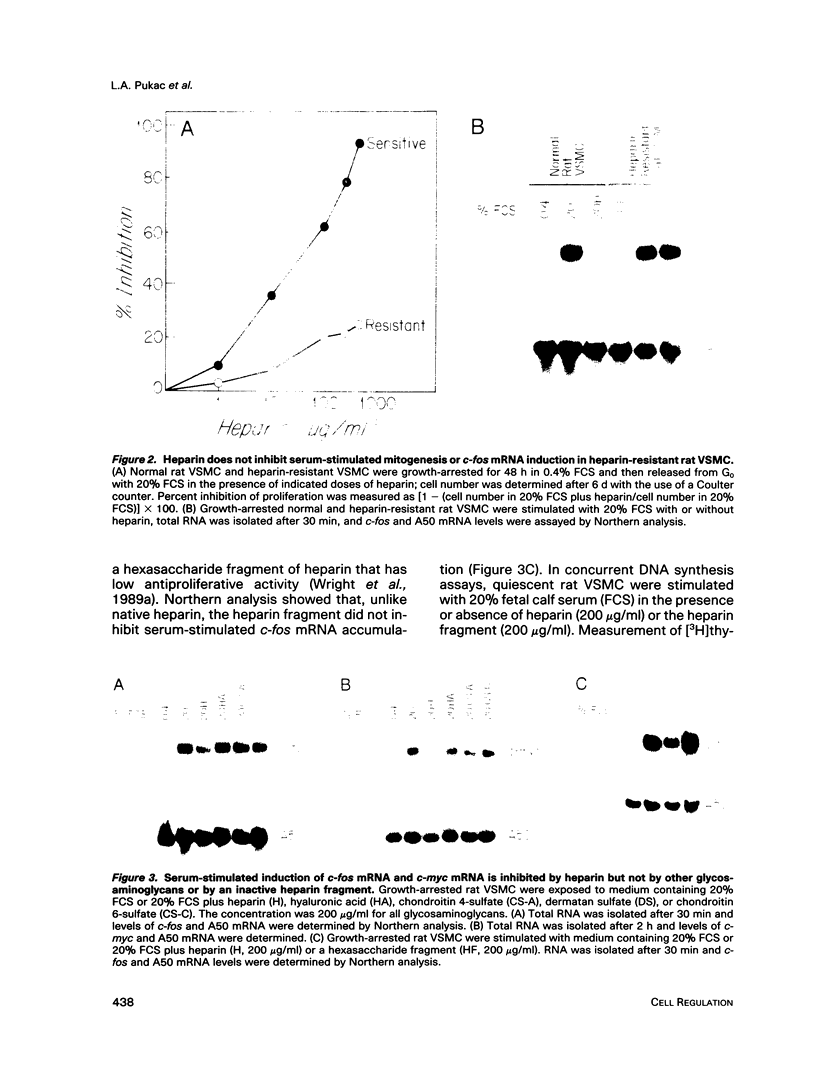
Abstract
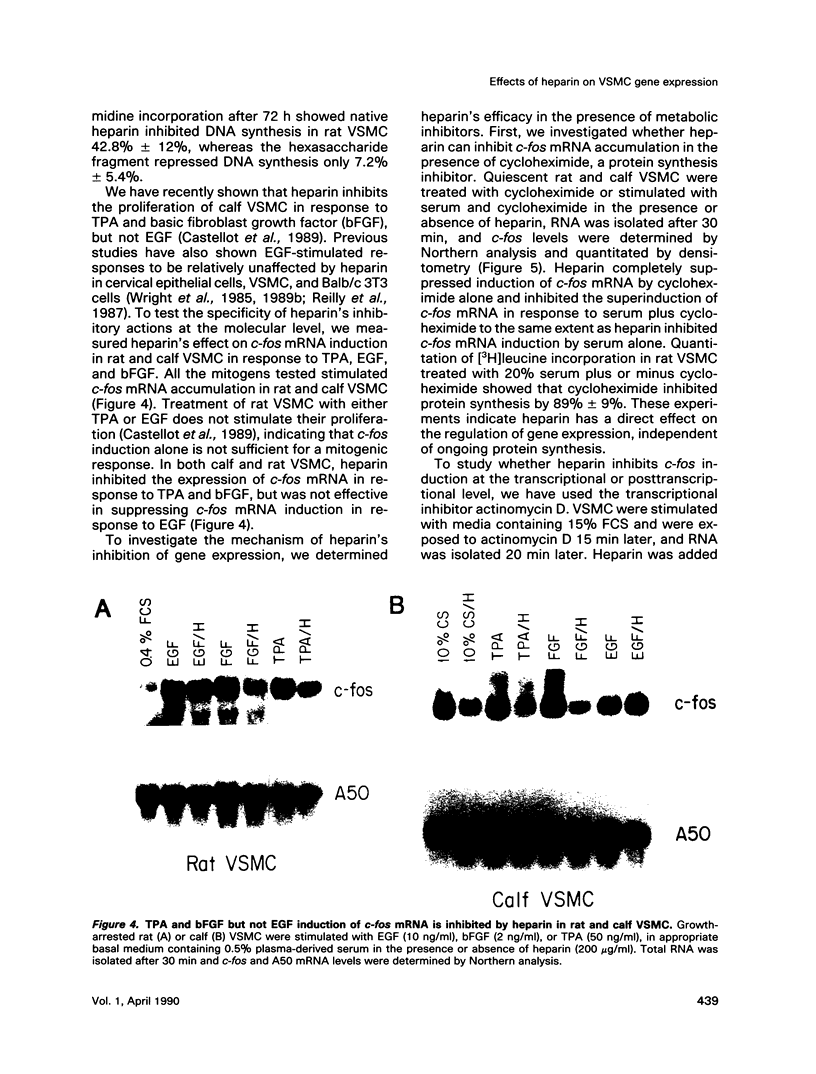
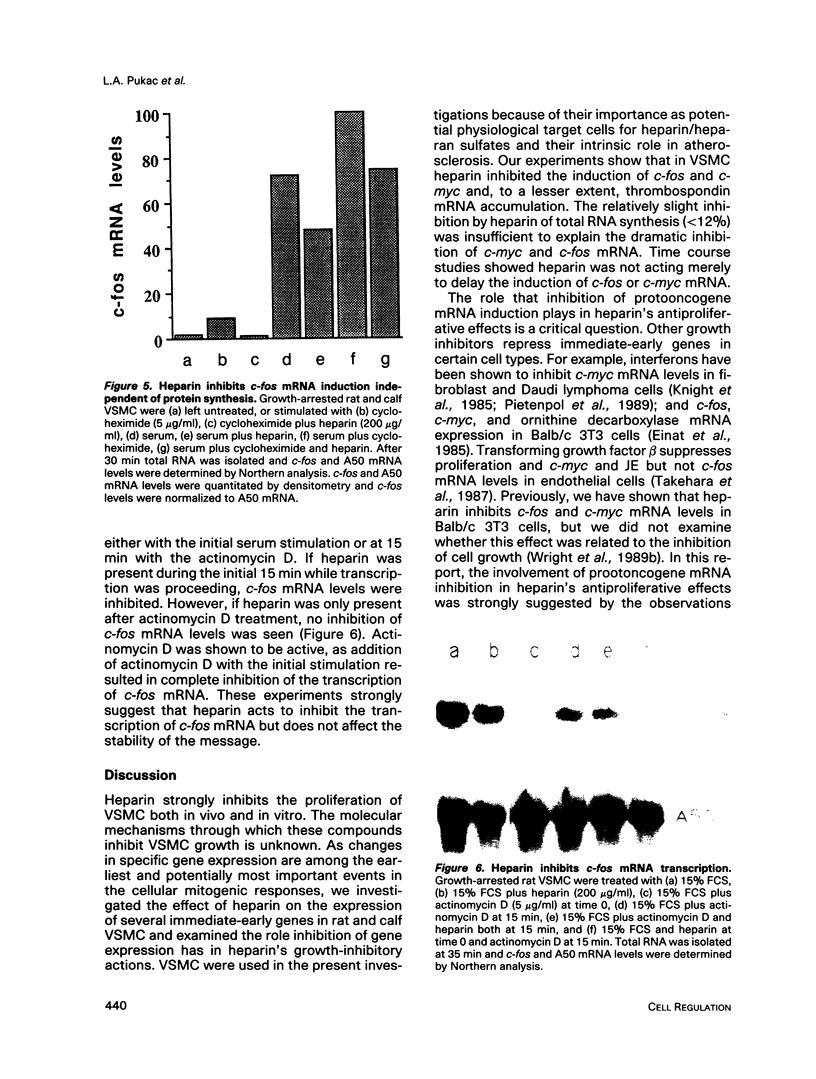
Heparin is a potent inhibitor of vascular smooth muscle cell (VSMC) growth. In this paper we show that heparin suppressed the induction of c-fos and c-myc mRNA in rat and calf VSMC. This effect of heparin is closely associated with its growth-inhibitory activity, as shown by isolating and characterizing a strain of rat VSMC that was resistant to heparin's antiproliferative effect; heparin did not suppress c-fos mRNA induction in these cells. Moreover, neither a nonantiproliferative heparin fragment or other glycosaminoglycans that lack growth-inhibitory activity repressed c-fos or c-myc mRNA levels. The effect of heparin on c-fos mRNA induction was selective for specific mitogens, as heparin inhibited c-fos mRNA induction in phorbol 12-myristate 13-acetate (TPA) stimulated but not epidermal growth factor (EGF) stimulated VSMC. The effect of heparin on gene expression is independent of ongoing protein synthesis, and inhibition of c-fos mRNA is at the transcriptional level. These results suggest that heparin may selectively inhibit a protein kinase C-dependent pathway for protooncogene induction and that this may be one mechanism used by heparin to inhibit cell proliferation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benitz W. E., Lessler D. S., Coulson J. D., Bernfield M. Heparin inhibits proliferation of fetal vascular smooth muscle cells in the absence of platelet-derived growth factor. J Cell Physiol. 1986 Apr;127(1):1–7. doi: 10.1002/jcp.1041270102. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Stumpo D. J., Huang J. K., Nemenoff R. A., Spach D. H. Protein kinase C-dependent and -independent pathways of proto-oncogene induction in human astrocytoma cells. J Biol Chem. 1987 Jun 5;262(16):7774–7781. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Cochran D. L., Karnovsky M. J. Effect of heparin on vascular smooth muscle cells. I. Cell metabolism. J Cell Physiol. 1985 Jul;124(1):21–28. doi: 10.1002/jcp.1041240105. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Favreau L. V., Karnovsky M. J., Rosenberg R. D. Inhibition of vascular smooth muscle cell growth by endothelial cell-derived heparin. Possible role of a platelet endoglycosidase. J Biol Chem. 1982 Oct 10;257(19):11256–11260. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Pukac L. A., Caleb B. L., Wright T. C., Jr, Karnovsky M. J. Heparin selectively inhibits a protein kinase C-dependent mechanism of cell cycle progression in calf aortic smooth muscle cells. J Cell Biol. 1989 Dec;109(6 Pt 1):3147–3155. doi: 10.1083/jcb.109.6.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Cochran D. L., Castellot J. J., Jr, Robinson J. M., Karnovsky M. J. Heparin modulates the secretion of a major excreted protein-like molecule by vascular smooth muscle cells. Biochim Biophys Acta. 1988 Nov 17;967(2):289–295. doi: 10.1016/0304-4165(88)90022-0. [DOI] [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Inhibitory effects of interferon on the expression of genes regulated by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7608–7612. doi: 10.1073/pnas.82.22.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fager G., Hansson G. K., Ottosson P., Dahllöf B., Bondjers G. Human arterial smooth muscle cells in culture. Effects of platelet-derived growth factor and heparin on growth in vitro. Exp Cell Res. 1988 Jun;176(2):319–335. doi: 10.1016/0014-4827(88)90334-5. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Rauscher F. J., 3rd, Josephs S. F., Curran T. The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science. 1988 Mar 4;239(4844):1150–1153. doi: 10.1126/science.2964084. [DOI] [PubMed] [Google Scholar]
- Fritze L. M., Reilly C. F., Rosenberg R. D. An antiproliferative heparan sulfate species produced by postconfluent smooth muscle cells. J Cell Biol. 1985 Apr;100(4):1041–1049. doi: 10.1083/jcb.100.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Heikkila R., Schwab G., Wickstrom E., Loke S. L., Pluznik D. H., Watt R., Neckers L. M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. 1987 Jul 30-Aug 5Nature. 328(6129):445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Heyns A. D., Eldor A., Vlodavsky I., Kaiser N., Fridman R., Panet A. The antiproliferative effect of interferon and the mitogenic activity of growth factors are independent cell cycle events. Studies with vascular smooth muscle cells and endothelial cells. Exp Cell Res. 1985 Dec;161(2):297–306. doi: 10.1016/0014-4827(85)90087-4. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Kindy M. S., Sonenshein G. E. Regulation of oncogene expression in cultured aortic smooth muscle cells. Post-transcriptional control of c-myc mRNA. J Biol Chem. 1986 Sep 25;261(27):12865–12868. [PubMed] [Google Scholar]
- Knight E., Jr, Anton E. D., Fahey D., Friedland B. K., Jonak G. J. Interferon regulates c-myc gene expression in Daudi cells at the post-transcriptional level. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1151–1154. doi: 10.1073/pnas.82.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A. Beta-type transforming growth factor specifies organizational behavior in vascular smooth muscle cell cultures. J Cell Biol. 1987 Jul;105(1):465–471. doi: 10.1083/jcb.105.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Bornstein P. Heparin regulates the collagen phenotype of vascular smooth muscle cells: induced synthesis of an Mr 60,000 collagen. J Cell Biol. 1985 Feb;100(2):613–619. doi: 10.1083/jcb.100.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Castle C. K., Goodman L. V., Weisgraber K. H., Mahley R. W., Shooter E. M., Gebicke-Haerter P. J. Expression of apolipoprotein E by cultured vascular smooth muscle cells is controlled by growth state. J Cell Biol. 1988 Sep;107(3):1207–1213. doi: 10.1083/jcb.107.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombospondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol. 1985 Sep;101(3):1059–1070. doi: 10.1083/jcb.101.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Nguyen H. T., Medford R. M., Nadal-Ginard B. Reversibility of muscle differentiation in the absence of commitment: analysis of a myogenic cell line temperature-sensitive for commitment. Cell. 1983 Aug;34(1):281–293. doi: 10.1016/0092-8674(83)90159-9. [DOI] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Howe P. H., Cunningham M. R., Leof E. B. Interferon alpha/beta modulation of growth-factor-stimulated mitogenicity in AKR-2B fibroblasts. J Cell Physiol. 1989 Dec;141(3):453–460. doi: 10.1002/jcp.1041410302. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta M., Maier J. A., Rusnati M., Ragnotti G. Basic fibroblast growth factor: production, mitogenic response, and post-receptor signal transduction in cultured normal and transformed fetal bovine aortic endothelial cells. J Cell Physiol. 1989 Dec;141(3):517–526. doi: 10.1002/jcp.1041410310. [DOI] [PubMed] [Google Scholar]
- Ran W., Dean M., Levine R. A., Henkle C., Campisi J. Induction of c-fos and c-myc mRNA by epidermal growth factor or calcium ionophore is cAMP dependent. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8216–8220. doi: 10.1073/pnas.83.21.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly C. F., Fritze L. M., Rosenberg R. D. Antiproliferative effects of heparin on vascular smooth muscle cells are reversed by epidermal growth factor. J Cell Physiol. 1987 May;131(2):149–157. doi: 10.1002/jcp.1041310203. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Fritze L. M., Rosenberg R. D. Heparin inhibition of smooth muscle cell proliferation: a cellular site of action. J Cell Physiol. 1986 Oct;129(1):11–19. doi: 10.1002/jcp.1041290103. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Kindy M. S., Brown K. E., Rosenberg R. D., Sonenshein G. E. Heparin prevents vascular smooth muscle cell progression through the G1 phase of the cell cycle. J Biol Chem. 1989 Apr 25;264(12):6990–6995. [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Baur U., Bürgin M., Bühler F. R. Decreased susceptibility of cultured smooth muscle cells from SHR rats to growth inhibition by heparin. J Cell Physiol. 1989 Jan;138(1):137–144. doi: 10.1002/jcp.1041380119. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Vosatka R. J., Ziff E. B., Lamb N. J., Feramisco J. R. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol. 1988 Apr;8(4):1670–1676. doi: 10.1128/mcb.8.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam M., Schmidt L. J., Crutchfield C. E., 3rd, Getz M. J. Negative regulation of serum-responsive enhancer elements. Nature. 1989 Jul 6;340(6228):64–66. doi: 10.1038/340064a0. [DOI] [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Kaibuchi K., Kawahara Y., Fukuzaki H., Takai Y. Induction of protein kinase C activation and Ca2+ mobilization by fibroblast growth factor in Swiss 3T3 cells. FEBS Lett. 1985 Oct 28;191(2):205–210. doi: 10.1016/0014-5793(85)80009-0. [DOI] [PubMed] [Google Scholar]
- Wright T. C., Jr, Johnstone T. V., Castellot J. J., Karnovsky M. J. Inhibition of rat cervical epithelial cell growth by heparin and its reversal by EGF. J Cell Physiol. 1985 Dec;125(3):499–506. doi: 10.1002/jcp.1041250320. [DOI] [PubMed] [Google Scholar]
- Wright T. C., Jr, Pukac L. A., Castellot J. J., Jr, Karnovsky M. J., Levine R. A., Kim-Park H. Y., Campisi J. Heparin suppresses the induction of c-fos and c-myc mRNA in murine fibroblasts by selective inhibition of a protein kinase C-dependent pathway. Proc Natl Acad Sci U S A. 1989 May;86(9):3199–3203. doi: 10.1073/pnas.86.9.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]