Abstract
Brain insults, including traumatic and ischemic injuries, are frequently followed by acute seizures and delayed development of epilepsy. Dysfunction of the blood-brain barrier (BBB) is a hallmark of brain insults and is usually surrounding the core lesion. Recent studies from several laboratories confirmed that vascular pathology is involved in the development of epilepsy and demonstrate a key role for astroglia in this process. In this review, we focus on glia-related mechanisms linking vascular pathology, and specifically BBB dysfunction, to seizures and epilepsy. We summarize molecular and physiological experimental data demonstrating that the function of astrocytes is altered due to direct exposure to serum albumin, mediated by transforming growth factor beta signaling. We discuss the reported changes and their potential role in the observed hyperexcitability as well as potential implications of these findings for the future development of new diagnostic modalities and treatments to allow a full implementation of the gained knowledge for the benefit of patients with epilepsy.
Keywords: buffering, connexins, astroglia, potassium channels, glutamate
BLOOD-BRAIN BARRIER DYSFUNCTION, EPILEPTOGENESIS, AND SEIZURES
Focal epilepsy typically arises either within or adjacent to a cortical lesion (Willoughby, 2000). While the characteristic hypersynchronous activity within the focus has been described extensively, epileptogenesis—the process modifying a functioning neuronal network into an epileptic one is less understood. In light of the high prevalence of acquired epilepsy, the high rate of drug resistance and the consequent neurological impairments, it is crucial to understand the epileptogenesis process, identify patients at risk, and develop antiepileptogenic strategies.
Long-lasting, persistent focal epilepsy can be elicited by inducing developmental cortical malformations (Jacobs et al., 1999; Wong, 2009), repeated electrical stimulation such as in the kindling model (McNamara, 1988) or repeated application of ictogenic agents, such as penicillin (Opdam et al., 2002; Prince and Wilder, 1967) or pentetrazole (Barkai et al., 1990), or by chronic injury (Halpern, 1972; Pitkänen and McIntosh, 2006; Prince and Tseng, 1993). The most common animal models for temporal lobe epilepsy (TLE) involves induction of status-epilepticus (SE) by pilocarpine or kainic acid (Turski et al., 1987) reviewed by (Curia et al., 2008), or by repetitive electrical stimulation (Lothman et al., 1987; for review see Löscher, 2002). In these animal models (similar to patients), a latent period of days to weeks precedes the development of epileptic seizures (Hoffman et al., 1994; Prince and Tseng, 1993). In animal models, the reorganization in nervous tissue during this period ultimately leads to appearance of spontaneous and recurrent seizures. Hence, this period is referred to as epileptogenesis. In clinical practice, this is often not the case and reorganization following a lesion or SE maintains a level of excitability within affected networks which prevent appearance of seizures. This requires studies of mechanisms which shift the balance in reorganizational processes from a condition in which no seizures occur into a condition where every so often seizures emerge. We postulate that dysfunctional blood-brain barrier (BBB) might underlie such a shift in reorganizational balance towards epileptogenesis and/or the occurrence of seizures.
Indeed, clinical data and animal experiments support the hypothesis that primary vascular lesions and, specifically BBB dysfunction, trigger a chain of events leading to epilepsy (For reviews see Friedman et al., 2009; Shlosberg et al., 2010). Significant and long-lasting dysfunction of the BBB are a hallmark of cortical injuries, regardless of etiology (Cervos-Navarro and Lafuente, 1991; Tomkins et al., 2001, 2008). BBB dysfunction is often observed in both human and animal models of traumatic and ischemic brain injuries, within and surrounding brain tumors, infectious and inflammatory brain diseases and neurodegenerative diseases, including Alzheimer’s and vascular dementia (Abbott et al., 2006; Neuwelt, 2004). In epilepsy, magnetic resonance imaging studies in patients with posttraumatic epilepsy demonstrated permeability of BBB to contrast agents, co-localized with the presumed epileptic focus (Tomkins et al., 2008, 2011). Ultrastructural studies on human-resected epileptic tissue show clear BBB abnormalities, including increased micropinocytosis, a thickening of the basal membrane, and the presence of abnormal tight junctions (Cornford, 1999; Cornford and Oldendorf, 1986; Kasantikul et al., 1983). However, no prospective clinical study was performed to test directly to what extent BBB impairment can predict the development of epilepsy in injured patients. In animal studies, a role for BBB opening was suggested in the progression of TLE based on the finding of serum albumin presence in brain parenchyma following SE, and a positive correlation between the extent of BBB opening and the number of seizures (van Vliet et al., 2007). Experimental focal opening of the BBB in the rat neocortex has been shown to result in epileptogenesis, evident by the delayed development of paroxysmal hypersynchronous activity recorded ex vivo (acute slice preparation) and in vivo (Ivens et al., 2007; Seiffert et al., 2004; Tomkins et al., 2007). This epileptogenesis was recapitulated by exposure of brain cortex to serum albumin. Extravasation of serum albumin into the cerebral cortex microenvironment activates a transforming growth factor beta (TGFβ) receptor-mediated signaling cascade in astrocytes (Cacheaux et al., 2009; David et al., 2009; Ivens et al., 2007 and see below).
The possible involvement of serum albumin in astrocytic activation and proliferation is supported by previous studies showing serum albumin inducing proliferation of fibroblasts (Tigyi et al., 1995), calcium signaling as well as DNA synthesis in cultured astrocytes (Nadal et al., 1995). On the basis of their studies, Nadal et al. (1995) concluded that there albumin’s effect is receptor-meditaed. Although albumin is the most abundant protein in the serum, other blood-born proteins may also have a role in the epileptogenic process. For example, it has been recently shown that the serum protein, thrombin, through protease-activated receptor 1 (PAR1), lowers epileptic seizures threshold in the hippocampus CA3 region and produces a long-lasting enhancement of CA1 neurons reactivity to afferent stimulation (Maggio et al., 2008). Thus, it seems plausible that damage to the microvasculature during brain insults and the extravasation of serum proteins, lead to the transformation of the neighboring astrocytes as a primary step in the epileptogenesis. Additionally, increase in vessels permeability to blood proteins leads to antibodies extravasation into brain tissue (van Vliet et al., 2007; Rigau et al., 2007), including autoantibodies to neuronal receptors and channels (Bien and Scheffer, 2011). It is not known to what extent such autoantibodies contribute to the pathogenesis of the disease; however, in some cases a clear respond to immunotherapy has been shown (Vincent et al., 2010).
THE TGFβ PATHWAY AND EPILEPTOGENESIS
TGFβs are pleiotropic cytokines that play a pivotal role in intercellular communication (for review see Massague and Wotton, 2000; Shi and Massague, 2003), and are involved in cell growth, embryogenesis, differentiation, morphogenesis, wound healing, immune response, and apoptosis in a wide variety of cells (Blobe et al., 2000; Gold and Parekh, 1999). TGFβ signaling is mediated mainly by two serine threonine kinase receptors, TGFβRI and TGFβRII, which activate an intracellular signaling system, such as phosphorylation of the Smad protein complex and the p38 mitogen-activated protein kinase (MAPK) pathway. TGFβ is upregulated in many disease conditions (for reviews see Szelenyi, 2001; Vitkovic et al., 2001): TGFβ1 expression is upregulated in the brains of individuals suffering from multiple sclerosis, AIDS, Alzheimer’s disease, stroke, tumors, or trauma. TGFβ has also been shown to be elevated in the cerebro-spinal fluid of some patients following brain injury (Phillips et al., 2006), to be produced in neurons after ischemia (Zhu et al., 2000), to be involved in pericyte-induced alterations in BBB function (Dohgu et al., 2005) and in microglial activation (Schilling and Eder, 2003). Smad3 null mice show reduced glial scarring after cortical stab wound injury (Wang et al., 2007), further supporting the central role of TGFβ in injury. Indeed, while many researchers consider TGFβ1 to be a “protective” cytokine (Brionne et al., 2003; McNeill et al., 1994; Prehn et al., 1993; Zhu et al., 2002), it has also been found to exacerbate excitotoxicity (Mesples et al., 2005; Prehn et al., 1994). These apparent contradicting results in different experimental systems and models can be explained partly by data showing that TGFβ1 actions are dependent on cell type and condition, resulting even in opposing outcomes.
Is TGFβ associated with epileptogenesis? The potential involvement of TGFβ in epileptogenesis is supported by animal experiments showing TGFβ upregulation in neurons from amygdale-kindled rats (Plata-Salaman et al., 2000). Aronica et al. (2000) showed TGFβ expression in astrocytes from the hippocampus of SE-experienced rats. Recent studies in rats in BBB-disrupted animals demonstrated that serum albumin binds to TGFβR and activate TGFβ signaling (Cacheaux et al., 2009; Ivens et al., 2007). Accordingly, transcriptome analysis revealed a strikingly similar transcription modulation patterns in animals exposed to BBB dysfunction, serum-derived albumin or following direct brain exposure to physiological levels of TGFβ1. However, the detailed mechanisms and cellular pathways bridging TGFβ signaling to seizures in different cell types are still a matter of investigation.
THE ROLE OF ASTROCYTES IN EPILEPTOGENESIS FOLLOWING BBB DYSFUNCTION
The interactions observed between serum albumin and TGFβ receptors and the uptake of serum albumin preferentially into glia cells within a few hours following BBB opening and prior to the development of seizures (Ivens et al., 2007) raised the hypothesis that glia functions and dysfunctions play a key role in the generation of the epileptic network. Furthermore, direct neocortical application of TGFβ1 on the brain surface and activation of TGFβ signaling, resulted in a prominent transcriptional-mediated change in expression levels of astrocytic genes (David et al., 2009).
Changes in glia morphology and function are a hallmark in the epileptic tissue in many of the patients (for reviews see Binder and Steinhauser, 2006; Heinemann et al., 2000; Jabs et al., 2008; Wetherington et al., 2008) and are summarized in detail in this special issue. Recent studies demonstrating a broad range of physiological effects of brain glia on neuronal activity, including the direct modulation of synaptic transmission and plasticity, point to a potential role of glia in epileptogenesis. It is thus hypothesized that the “transformation” or “activation” of glia in the presence of dysfunctional BBB leads to the reorganization of the neuronal network, which characterizes the epileptic brain. The term “activated” glia is often used, but this is unfortunately a very imprecise term and it is not clear what “activation” really means, to what extent it is a “single uniform” state, and how persistant is it. Is it just the increased expression of glial fibrillary acidic protein (GFAP), or is it associated with alterations in specific functions such as the expression of pumps and ion channels? How the connectivity between astrocytes is altered and what role activation of astrocytes has in the synthesis of cytokines and their release? Since cells which express GFAP and therefore termed “astrocytes” include radial glia such as Müller and Bergmann cells, but also astrocytes which have been termed glutamate transporter or passive cells as well as astrocytes which express AMPA receptors and voltage gated ion channels (Glu-R cells or complex astrocytes), it is unclear what activation of astrocytes really means and to what extent all types of glia (or even astrocytes) are affected in the same way. Different “types” of glia thus different not only in morphology, but also in functions (e.g. in the extent of electrical coupling between them, spatial buffering of extracellular potassium, transport of glutamate and attachment to vessels). Here, we will summarize physiological changes observed in activated astrocytes following BBB opening, and from these observations we will deduce a working hypothesis on their role in remodeling the neuronal network towards lowering its threshold to large-scale synchronization, development and propagation of seizures.
Several functional changes in astrocytic properties, which may be relevant to the epileptogenesis process, have been found in the BBB-impaired cortex. These include: (1) Reduced expression of potassium inward rectifying channels (Kir4.1) and water channels (aquaporin 4, AQP4); (2) Reduced expression of gap junctions; and (3) impaired glutamate metabolism.
Reduced Kir 4.1 and AQP4
BBB opening, brain exposure to albumin or to TGFβ1 resulted in downregulation of both Kir4.1 and AQP4 (David et al., 2009; Perillan et al., 2002). Both channels are co-localized most abundantly in astrocytic endfeet and considered crucial for the regulation of the brain’s extracellular potassium ([K+]o) and water fluxes. Spatial buffering of [K+]o occurs when its concentrations increases nonhomogenously throughout the different cortical layers. This is usually the case during physiological activation and even more so during repetitive activation (Dietzel et al., 1989; Pumain and Heinemann, 1985). In the hippocampus, [K+]o is usually maximally increased in pyramidal layer of ammon’s horn or in the granular layer of the dentate gyrus (Krnjevic et al., 1982). In the neocortex, [K+]o accumulation is often maximal in the deep layers (Hablitz and Heinemann, 1987). Local increase in [K+]o depolarizes astrocytes which carry large resting conductance to potassium. If these are spatially extended and or electrically coupled (Wallraff et al., 2006) this depolarization will spread through the glial network; Consequently, the local depolarization does not correspond to the local accumulation of [K+]o, leading to a driving force for potassium influx at sites of maximal [K+]o elevation and efflux at remote sites. This mechanism underlies the observed mismatch between transient and focal increase in [K+]o and the associated glial depolarization (Futamachi and Pedley, 1976). This prevents excess increase in [K+]o by dissipating it much faster than expected from pure diffusion (Gardner-Medwin and Nicholson, 1983). The influx of potassium into glia also contributes to the generation of slow negative potentials at the site of maximal K+ uptake which may affect neurons by field effects mediating ephaptic interactions. Finally, due to differences in ion transport numbers, transglial influx of potassium is associated with a shrinkage of the extracellular space at sites of maximal neuronal activity: As K+ ions enter the glial compartment, potential gradient emerge; The corresponding current in the extracellular space is predominantly carried by Na+ and Cl−, the predominant ions. Thus, Na+ is transported to the site of K+ accumulation while Cl− moves away. The Cl− and K+ ions are only partially replaced by Na+, resulting in a decrease of extracellular osmolarity, leading to a water flux into the cells, and a shrinkage of the extracellular space. At remote sites, the opposite effect is expected due to K+ outflow from glia and Cl− transport to these sites (Lux et al., 1986). Which K+ channels account for the potassium movements into astrocytes? It is likely that Kir 4.1 channels are very important in this regard. These ion channels are very sensitive to low concentrations of barium which significantly leads to depolarization of astrocytes. In addition, it is likely that two pore domain K+ channels also contribute to the influx of K+ (Pasler et al., 2007). These channels are also sensitive to barium but require higher concentrations. These differences in pharmacological sensitivity to barium permit testing changes in functional expression of Kir 4.1 channels during epileptogenesis in the dysfunctional BBB model. Indeed, while in control cortex low concentrations of barium increased [K+]o levels and slowed the decay of [K+]o in response to iontophoretic application, this effect was almost absent in the epileptogenic tissue (i.e. 24 h after BBB opening) and resembled the situation found in human epileptic cortex (Jauch et al., 2002). These results, together with mRNA and protein levels analysis confirmed that downregulation of Kir 4.1 channels characterizes transformed astrocytes in the epileptogenic tissue (David et al., 2009; Ivens et al., 2007). Loss of Ikir was also demonstrated in cortical astrocytes following exposure to fluid percussion model of traumatic brain injury with serum extravasation (Stewart et al., 2010). Several lines of evidence support a role for Kir 4.1 down regulation in epileptogenesis: (1) mice with glial specific Kir 4.1 deletions suffer from epilepsy and die relatively soon after birth (Djukic et al., 2007); (2) homozygous missense mutations in the KCNJ10 gene (encoding for the Kir 4.1 channel) has been recently described in five children with EAST (epilepsia, ataxia, moderate sensorineural deafness, and a renal salt losing tubulopathy) syndrome (Bockenhauer et al., 2009); (3) Impaired [K+]o buffering due to downregulation of Kir4.1 channels has been reported in the hippocampus of pilocarpine-treated epileptic rats (Gabriel et al., 1998) and in the sclerotic hippocampus of TLE patients (Jauch et al., 2002; Kivi et al., 2000; Schroder et al., 2000).
Reduced expression of aquaporin channels may also contribute to the epileptogenic role of transformed astrocytes. AQP4 is expressed in astrocytes throughout the central nervous system, particularly at the BBB. AQP4-null mice indeed show reduced [K+]o buffering and prolonged duration of induced-seizures (Binder et al., 2006; Strohschein et al., 2011).
Role of Gap Junctions
Gap junctions are functional channels between cells that allow for controlled transcellular movement of molecules between cells, and are comprised of connexin proteins. As described above, astrocytes are coupled via gap junctions to form large cellular networks which facilitate spatial buffering of small molecules (e.g., K+). Interestingly, the expression of the astrocytic gap junction proteins connexin 30 and 43 is reduced in the BBB-induced epileptogenic cortex. This reduction may contribute to the activity-dependant accumulation of [K+]o (David et al., 2009). Notably, while block of Kir 4.1 and 2 Pore domain channels have dramatic effects on K+ homeostasis, the effects of loss of connexins are less pronounced. Mice lacking connexin 30 and 43 in astrocytes show only mild disturbances in [K+]o homeostasis. Nevertheless, afferent stimulation results in a larger rise in [K+]o in these mice compared with controls, lower threshold for seizures, accelerated propagation of spreading depolarization, and enhanced locomotor activity (Theis et al., 2003; Wallraff et al., 2006).
Impaired Glutamate Metabolism
Within the neurovascular unit, glial cells have a key role in the uptake and metabolism of glutamate. Following BBB dysfunction activated astrocytes show reduced levels of mRNA encoding for the astrocytic glutamate transporters of the solute carrier family 1, subfamily A members SLC1A2 and SLC1A3 (Chaudhry et al., 1995; Su et al., 2003). The effect was specific to astrocytes since SLC1A1 (preferentially expressed in neurons; see (Rothstein et al., 1994) did not show significant changes in expression levels. In addition, the astrocytic glutaminase and glutamine synthetase (Derouiche and Frotscher, 1991) were also downregulated. Astrocytic buffering of glutamate (by glutamine synthetase) plays a lead role in neuronal hyperexcitability regulation (Eid et al., 2008). Data showing reduced glutamate buffering capacity during epileptogenesis further stress the potential role of altered glutamate metabolism in astrocytes in the development of epilepsy (David et al., 2009). In addition, astrocytic glutamate release has been described in several preparations and implicated to contribute to a slow, TTX resistant, NMDA sensitive neuronal inward current (Tian et al., 2005). It is not clear, however, to what extent such release contributes to epileptogenesis under BBB dysfunction. There is no direct evidence for increased glutamate release from transformed astrocytes, although one potential mechanism is the upregulation of TNFα which is a prominent modulator of glutamate release (Bezzi et al., 2004). Finally, reduced glutamate uptake in transformed astrocytes may also interfere with the production of glutathione: astrocytes use glutamate to uptake cystine, which is used to synthesize glutamyl–cysteine, which is released from astrocytes for synthesis of glutathione in neurons. Downregulation of neuronal and glial glutathione would weaken defense mechanisms against free radicals and would be expected to result in increased damage (Schuchmann and Heinemann, 2000). Interference with glutamate transport into astrocytes will also affect the detoxification of glutamate to glutamine and thus might interfere with detoxification of ammonium. Ammonium disturbs Cl− transporters and thus might contribute to a reduced efficacy of GABA-mediated synaptic inhibition.
SUMMARY
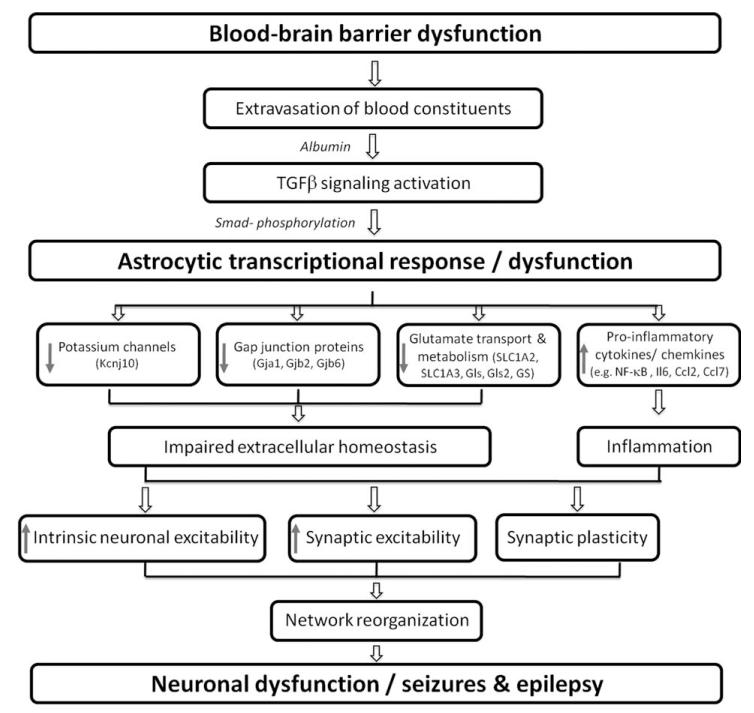
Figure 1 summarizes the role played by vascular injury and transformed (activated) astrocytes in reducing seizure threshold. However, it is important to distinguish between seizure generation and epileptogenicity—which carries a more complex and long-lasting network modifications. The response of astrocytes to vascular injury is rapid, and seems to be directly related to the diffusion of the most common serum protein, albumin, via the dysfunctional barrier. Thus, it is proposed that transformation of astrocytes starts during the latent period of epileptogenesis and is regulated by TGFβ signaling. Under these conditions, astrocytes show a less “rigid” control on potassium and glutamate levels, most significantly during repetitive activation. We postulate that transformed astrocytes may allow enhanced axonal sprouting and synaptogenesis as part of the brain response to injury. One view may thus be that epilepsy develops when the normal network response to injury has not been properly controlled due to lasting dysfunction of the local microvasculature. This hypothesis, while still requiring careful experimental control and appropriate experimental design, may change our view on the mechanisms underlying epileptogenesis and potential targets for its prevention. If there is a critical time window in which BBB dysfunction predicts epileptogenesis following insult, it may become an early biomarker for the development of epilepsy and would facilitate studies on its prevention (Pitkänen, 2010). To address BBB dysfunction as a biomarker for the prediction of epilepsy, large prospective human studies will be required. Quantitative methods for evaluating focal BBB damage noninvasively are mandatory for such a study. While few imaging approaches have been proposed (Tomkins et al., 2001, 2008, 2011) there is still an urgent need for the development of such methods. In parallel, animal studies are required for better understanding the mechanisms underlying BBB damage and repair following insult, to allow a selective and efficient targeting and modification of these processes.
Fig. 1.
Summary diagram on the role of BBB dysfunction and astroglia functions in epileptogenesis. The damage to endothelial cells leads to dysfunction of the BBB and the extravasation of serum proteins into the neuropil. Albumin activates TGFβ signaling in astrocytes leading to transcriptional response associated with their transformation into “active” astrocytes. Transcriptional response includes the down regulation of Kcnj10 (inward rectifier 4.1 potassium channel) together with Gja1, Gjb2, and Gjb6 (gap junction proteins, connexins 43, 26, and 30, respectively). In addition, genes associated with glutamate metabolism are downregulated, including the mRNA coding for the astrocytic glutamate transporters of the solute carrier family 1, subfamily A members SLC1A2 and SLC1A3. Glutaminase (Gls, Gls2) and glutamine synthetase (GS) are also downregulated. Together, homeostasis of the extracellular brain environment is impaired, leading to enhanced neuronal excitability. In addition, upregulation of proinflammatory cytokines and chemokines a, as part as the local inflammatory response, may also contribute to the increase in neuronal excitability, associated with reorganization of the local neuronal network, typical hallmark of the epileptic brain.
REFERENCES
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- Barkai E, Golan H, Grossman Y, Gutnick MJ. Pentylenetetrazole-induced kindling is prevented by prior treatment with cysteamine. Eur J Pharmacol. 1990;182:167–169. doi: 10.1016/0014-2999(90)90507-3. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bien CG, Scheffer IE. Autoantibodies and epilepsy. Epilepsia. 2011;52(Suppl 3):18–22. doi: 10.1111/j.1528-1167.2011.03031.x. [DOI] [PubMed] [Google Scholar]
- Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al MO, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40:1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, Heinemann U, Friedman A, Kaufer D. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci. 2009;29:8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervos-Navarro J, Lafuente JV. Traumatic brain injuries: Structural changes. J Neurol Sci. 1991;103:3–14. doi: 10.1016/0022-510x(91)90002-o. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, Lookeren Campagne Mv, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: Highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Cornford EM. Epilepsy and the blood brain barrier: Endothelial cell responses to seizures. Adv Neurol. 1999;79:845–862. [PubMed] [Google Scholar]
- Cornford EM, Oldendorf WH. Epilepsy and the blood-brain barrier. Adv Neurol. 1986;44:787–812. [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D, Friedman A. Astrocytic dysfunction in epileptogenesis: Consequence of altered potassium and glutamate homeostasis? J Neurosci. 2009;29:10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouiche A, Frotscher M. Astroglial processes around identified glutamatergic synapses contain glutamine synthetase: Evidence for transmitter degradation. Brain Res. 1991;552:346–350. doi: 10.1016/0006-8993(91)90103-3. [DOI] [PubMed] [Google Scholar]
- Dietzel I, Heinemann U, Lux HD. Relations between slow extracellular potential changes, glial potassium buffering, and electrolyte and cellular volume changes during neuronal hyperactivity in cat brain. Glia. 1989;2:25–44. doi: 10.1002/glia.440020104. [DOI] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Eid T, Ghosh A, Wang Y, Beckstrom H, Zaveri HP, Lee TS, Lai JC, Malthankar-Phatak GH, de Lanerolle NC. Recurrent seizures and brain pathology after inhibition of glutamine synthetase in the hippocampus in rats. Brain. 2008;131:2061–2070. doi: 10.1093/brain/awn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: Novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85:142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futamachi KJ, Pedley TA. Glial cells and extracellular potassium: Their relationship in mammalian cortex. Brain Res. 1976;109:311–322. doi: 10.1016/0006-8993(76)90532-1. [DOI] [PubMed] [Google Scholar]
- Gabriel S, Kivi A, Kovacs R, Lehmann TN, Lanksch WR, Meencke HJ, Heinemann U. Effects of barium on stimulus-induced changes in [K+]o and field potentials in dentate gyrus and area CA1 of human epileptic hippocampus. Neurosci Lett. 1998;249:91–94. doi: 10.1016/s0304-3940(98)00420-0. [DOI] [PubMed] [Google Scholar]
- Gardner-Medwin AR, Nicholson C. Changes of extracellular potassium activity induced by electric current through brain tissue in the rat. J Physiol. 1983;335:375–392. doi: 10.1113/jphysiol.1983.sp014540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LI, Parekh TV. Loss of growth regulation by transforming growth factor-beta (TGF-beta) in human cancers: Studies on endometrial carcinoma. Semin Reprod Endocrinol. 1999;17:73–92. doi: 10.1055/s-2007-1016214. [DOI] [PubMed] [Google Scholar]
- Hablitz JJ, Heinemann U. Extracellular K+ and Ca2+ changes during epileptiform discharges in the immature rat neocortex. Brain Res. 1987;433:299–303. doi: 10.1016/0165-3806(87)90036-8. [DOI] [PubMed] [Google Scholar]
- Halpern L, Purpura D. Chronically isolated aggregates of mammalian cerebral cortical neurons studied in-situ. In: Penry J, Tower D, Woodbury D, Walter R, editors. Experimental Models of Epilepsy. Raven; New York: 1972. pp. 197–221. [Google Scholar]
- Heinemann U, Gabriel S, Jauch R, Schulze K, Kivi A, Eilers A, Kovacs R, Lehmann TN. Alterations of glial cell function in temporal lobe epilepsy. Epilepsia. 2000;41(Suppl 6):S185–S189. doi: 10.1111/j.1528-1157.2000.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Hoffman SN, Salin PA, Prince DA. Chronic neocortical epileptogenesis in vitro. J Neurophysiol. 1994;71:1762–1773. doi: 10.1152/jn.1994.71.5.1762. [DOI] [PubMed] [Google Scholar]
- Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- Jabs R, Seifert G, Steinhauser C. Astrocytic function and its alteration in the epileptic brain. Epilepsia. 2008;(49Suppl 2):3–12. doi: 10.1111/j.1528-1167.2008.01488.x. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Hwang BJ, Prince DA. Focal epileptogenesis in a rat model of polymicrogyria. J Neurophysiol. 1999;81:159–173. doi: 10.1152/jn.1999.81.1.159. [DOI] [PubMed] [Google Scholar]
- Jauch R, Windmuller O, Lehmann TN, Heinemann U, Gabriel S. Effects of barium, furosemide, ouabaine and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) on ionophoretically-induced changes in extracellular potassium concentration in hippocampal slices from rats and from patients with epilepsy. Brain Res. 2002;925:18–27. doi: 10.1016/s0006-8993(01)03254-1. [DOI] [PubMed] [Google Scholar]
- Kasantikul V, Brown WJ, Oldendorf WH, Crandall PC. Ultrastructural parameters of limbic microvasculature in human psychomotor epilepsy. Clin Neuropathol. 1983;2:171–178. [PubMed] [Google Scholar]
- Kivi A, Lehmann TN, Kovacs R, Eilers A, Jauch R, Meencke HJ, von Deimling A, Heinemann U, Gabriel S. Effects of barium on stimulus-induced rises of [K+]o in human epileptic non-sclerotic and sclerotic hippocampal area CA1. Eur J Neurosci. 2000;12:2039–2048. doi: 10.1046/j.1460-9568.2000.00103.x. [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Morris ME, Reiffenstein RJ, Ropert N. Depth distribution and mechanism of changes in extracellular K+ and Ca2+ concentrations in the hippocampus. Can J Physiol Pharmacol. 1982;60:1658–1671. doi: 10.1139/y82-244. [DOI] [PubMed] [Google Scholar]
- Löscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50:105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bennett JP, Perlin JB. Alterations in neurotransmitter amino acids in hippocampal kindled seizures. Epilepsy Res. 1987;1:313–320. doi: 10.1016/0920-1211(87)90055-6. [DOI] [PubMed] [Google Scholar]
- Lux HD, Heinemann U, Dietzel I. Ionic changes and alterations in the size of the extracellular space during epileptic activity. Adv Neurol. 1986;44:619–639. [PubMed] [Google Scholar]
- Maggio N, Shavit E, Chapman J, Segal M. Thrombin induces long-term potentiation of reactivity to afferent stimulation and facilitates epileptic seizures in rat hippocampal slices: Toward understanding the functional consequences of cerebrovascular insults. J Neurosci. 2008;28:732–736. doi: 10.1523/JNEUROSCI.3665-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO. Pursuit of the mechanisms of kindling. Trends Neurosci. 1988;11:33–36. doi: 10.1016/0166-2236(88)90047-1. [DOI] [PubMed] [Google Scholar]
- McNeill H, Williams C, Guan J, Dragunow M, Lawlor P, Sirimanne E, Nikolics K, Gluckman P. Neuronal rescue with transforming growth factor-beta 1 after hypoxic-ischaemic brain injury. Neuroreport. 1994;5:901–904. doi: 10.1097/00001756-199404000-00012. [DOI] [PubMed] [Google Scholar]
- Mesples B, Fontaine RH, Lelievre V, Launay JM, Gressens P. Neuronal TGF-beta1 mediates IL-9/mast cell interaction and exacerbates excitotoxicity in newborn mice. Neurobiol Dis. 2005;18:193–205. doi: 10.1016/j.nbd.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Nadal A, Fuentes E, Pastor J, McNaughton PA. Plasma albumin is a potent trigger of calcium signals and DNA synthesis in astrocytes. Proc Natl Acad Sci USA. 1995;92:1426–1430. doi: 10.1073/pnas.92.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt EA. Mechanisms of disease: The blood-brain barrier. Neurosurgery. 2004;54:131–140. doi: 10.1227/01.neu.0000097715.11966.8e. [DOI] [PubMed] [Google Scholar]
- Opdam HI, Federico P, Jackson GD, Buchanan J, Abbott DF, Fabinyi GC, Syngeniotis A, Vosmansky M, Archer JS, Wellard RM, Bellomo R. A sheep model for the study of focal epilepsy with concurrent intracranial EEG and functional MRI. Epilepsia. 2002;43:779–787. doi: 10.1046/j.1528-1157.2002.04202.x. [DOI] [PubMed] [Google Scholar]
- Pasler D, Gabriel S, Heinemann U. Two-pore-domain potassium channels contribute to neuronal potassium release and glial potassium buffering in the rat hippocampus. Brain Res. 2007;1173:14–26. doi: 10.1016/j.brainres.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Perillan PR, Chen M, Potts EA, Simard JM. Transforming growth factor-beta 1 regulates Kir2.3 inward rectifier K+ channels via phospholipase C and protein kinase C-delta in reactive astrocytes from adult rat brain. J Biol Chem. 2002;277:1974–1980. doi: 10.1074/jbc.M107984200. [DOI] [PubMed] [Google Scholar]
- Phillips DJ, Nguyen P, Adamides AA, Bye N, Rosenfeld JV, Kossmann T, Vallance S, Murray L, Morganti-Kossmann MC. Activin a release into cerebrospinal fluid in a subset of patients with severe traumatic brain injury. J Neurotrauma. 2006;23:1283–1294. doi: 10.1089/neu.2006.23.1283. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Therapeutic approaches to epileptogenesis—Hope on the horizon. Epilepsia. 2010;51(Suppl 3):2–17. doi: 10.1111/j.1528-1167.2010.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, McIntosh TK. Animal models of post-traumatic epilepsy. J Neurotrauma. 2006;23:241–261. doi: 10.1089/neu.2006.23.241. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Romanovitch AE, Kelly ME, Bureau Y, Anisman H, McIntyre DC. Kindling modulates the IL-1beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Mol Brain Res. 2000;75:248–258. doi: 10.1016/s0169-328x(99)00306-x. [DOI] [PubMed] [Google Scholar]
- Prehn JH, Bindokas VP, Marcuccilli CJ, Krajewski S, Reed JC, Miller RJ. Regulation of neuronal Bcl2 protein expression and calcium homeostasis by transforming growth factor type beta confers wide-ranging protection on rat hippocampal neurons. Proc Natl Acad Sci USA. 1994;91:12599–12603. doi: 10.1073/pnas.91.26.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn JH, Peruche B, Unsicker K, Krieglstein J. Isoform-specific effects of transforming growth factors-beta on degeneration of primary neuronal cultures induced by cytotoxic hypoxia or glutamate. J Neurochem. 1993;60:1665–1672. doi: 10.1111/j.1471-4159.1993.tb13389.x. [DOI] [PubMed] [Google Scholar]
- Prince DA, Tseng GF. Epileptogenesis in chronically injured cortex: In vitro studies. J Neurophysiol. 1993;69:1276–1291. doi: 10.1152/jn.1993.69.4.1276. [DOI] [PubMed] [Google Scholar]
- Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Arch Neurol. 1967;16:194–202. doi: 10.1001/archneur.1967.00470200082007. [DOI] [PubMed] [Google Scholar]
- Pumain R, Heinemann U. Stimulus- and amino acid-induced calcium and potassium changes in rat neocortex. J Neurophysiol. 1985;53:1–16. doi: 10.1152/jn.1985.53.1.1. [DOI] [PubMed] [Google Scholar]
- Rigau V, Morin M, Rousset MC, de BF, Lebrun A, Coubes P, Picot MC, Baldy-Moulinier M, Bockaert J, Crespel A, Lerner-Natoli M. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Schilling T, Eder C. Effects of kinase inhibitors on TGF-beta induced upregulation of Kv1.3 K+ channels in brain macrophages. Pflugers Arch. 2003;447:312–315. doi: 10.1007/s00424-003-1155-3. [DOI] [PubMed] [Google Scholar]
- Schroder W, Hinterkeuser S, Seifert G, Schramm J, Jabs R, Wilkin GP, Steinhauser C. Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia. 2000;41(Suppl 6):S181–S184. doi: 10.1111/j.1528-1157.2000.tb01578.x. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Heinemann U. Diminished glutathione levels cause spontaneous and mitochondria-mediated cell death in neurons from trisomy 16 mice: A model of Down’s syndrome. J Neurochem. 2000;74:1205–1214. doi: 10.1046/j.1471-4159.2000.741205.x. [DOI] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TH, Eastman CL, Groblewski PA, Fender JS, Verley DR, Cook DG, D’Ambrosio R. Chronic dysfunction of astrocytic inwardly rectifying K+ channels specific to the neocortical epileptic focus after fluid percussion injury in the rat. J Neurophysiol. 2010;104:3345–3360. doi: 10.1152/jn.00398.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohschein S, Huttmann K, Gabriel S, Binder DK, Heinemann U, Steinhauser C. Impact of aquaporin-4 channels on K+ buffering and gap junction coupling in the hippocampus. Glia. 2011;59:973–980. doi: 10.1002/glia.21169. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proc Natl Acad Sci U S A. 2003;100:1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelenyi J. Cytokines and the central nervous system. Brain Res Bull. 2001;54:329–338. doi: 10.1016/s0361-9230(01)00428-2. [DOI] [PubMed] [Google Scholar]
- Theis M, Jauch R, Zhuo L, Speidel D, Wallraff A, Doring B, Frisch C, Sohl G, Teubner B, Euwens C, Huston J, Steinhauser C, Messing A, Heinemann U, Willecke K. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin 43. J Neurosci. 2003;23:766–776. doi: 10.1523/JNEUROSCI.23-03-00766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu QW, Peng WG, Lin J, Oberheim N, Lou NH, Wang XH, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigyi G, Hong L, Yakubu M, Parfenova H, Shibata M, Leffler CW. Lysophosphatidic acid alters cerebrovascular reactivity in piglets. Am J Physiol. 1995;268:H2048–H2055. doi: 10.1152/ajpheart.1995.268.5.H2048. [DOI] [PubMed] [Google Scholar]
- Tomkins O, Feintuch A, Benifla M, Cohen A, Friedman A, Shelef I. Blood-brain barrier breakdown following traumatic brain injury: A possible role in posttraumatic epilepsy. Cardiovasc Psychiatry Neurol. 2011;765923:1–11. doi: 10.1155/2011/765923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins O, Friedman O, Ivens S, Reiffurth C, Major S, Dreier JP, Heinemann U, Friedman A. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25:367–377. doi: 10.1016/j.nbd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Tomkins O, Kaufer D, Korn A, Shelef I, Golan H, Reichenthal E, Soreq H, Friedman A. Frequent blood-brain barrier disruption in the human cerebral cortex. Cell Mol Neurobiol. 2001;21:675–691. doi: 10.1023/a:1015147920283. [DOI] [PubMed] [Google Scholar]
- Tomkins O, Shelef I, Kaizerman I, Eliushin A, Afawi Z, Misk A, Gidon M, Cohen A, Zumsteg D, Friedman A. Blood-brain barrier disruption in post-traumatic epilepsy. J Neurol Neurosurg Psychiatry. 2008;79:774–777. doi: 10.1136/jnnp.2007.126425. [DOI] [PubMed] [Google Scholar]
- Turski L, Cavalheiro EA, Czuczwar SJ, Turski WA, Kleinrok Z. The seizures induced by pilocarpine: Behavioral, electroencephalographic and neuropathological studies in rodents. Pol J Pharmacol Pharm. 1987;39:545–555. [PubMed] [Google Scholar]
- van Vliet EA, da Costa AS, Redeker S, van SR, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- Vincent A, Irani SR, Lang B. The growing recognition of immunotherapy-responsive seizure disorders with autoantibodies to specific neuronal proteins. Curr Opin Neurol. 2010;23:144–150. doi: 10.1097/WCO.0b013e32833735fe. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, Maeda S, Sternberg E. Anti-inflammatory cytokines: Expression and action in the brain. Neuroimmunomodulation. 2001;9:295–312. doi: 10.1159/000059387. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26:5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Moges H, Bharucha Y, Symes A. Smad3 null mice display more rapid wound closure and reduced scar formation after a stab wound to the cerebral cortex. Exp Neurol. 2007;203:168–184. doi: 10.1016/j.expneurol.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby JO. Mechanisms underlying partial (focal, or lesional) epilepsy. J Clin Neurosci. 2000;7:291–294. doi: 10.1054/jocn.1999.0221. [DOI] [PubMed] [Google Scholar]
- Wong M. Animal models of focal cortical dysplasia and tuberous sclerosis complex: Recent progress toward clinical applications. Epilepsia. 2009;50(Suppl 9):34–44. doi: 10.1111/j.1528-1167.2009.02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Roth-Eichhorn S, Braun N, Culmsee C, Rami A, Krieglstein J. The expression of transforming growth factor-beta1 (TGF-beta1) in hippocampal neurons: A temporary upregulated protein level after transient forebrain ischemia in the rat. Brain Res. 2000;866:286–298. doi: 10.1016/s0006-8993(00)02240-x. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yang GY, Ahlemeyer B, Pang L, Che XM, Culmsee C, Klumpp S, Krieglstein J. Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage. J Neurosci. 2002;22:3898–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]