Abstract
Finding an ideal biomaterial with the proper mechanical properties and biocompatibility has been of intense focus in the field of soft tissue engineering. This paper reports on the synthesis and characterization of a novel crosslinked urethane-doped polyester elastomer (CUPOMC), which was synthesized by reacting a previously developed photocrosslinkable poly (octamethylene maleate citrate) (POMC) prepolymers (pre-POMC) with 1,6-hexamethylene diisocyanate (HDI) followed by thermo- or photo-crosslinking polymerization. The mechanical properties of the CUPOMCs can be tuned by controlling the molar ratios of pre-POMC monomers, and the ratio between the prepolymer and HDI. CUPOMCs can be crosslinked into a 3D network through polycondensation or free radical polymerization reactions. The tensile strength and elongation at break of CUPOMC synthesized under the known conditions range from 0.73±0.12MPa to 10.91±0.64MPa and from 72.91±9.09% to 300.41±21.99% respectively. Preliminary biocompatibility tests demonstrated that CUPOMCs support cell adhesion and proliferation. Unlike the pre-polymers of other crosslinked elastomers, CUPOMC pre-polymers possess great processability demonstrated by scaffold fabrication via a thermally induced phase separation method. The dual crosslinking methods for CUPOMC pre-polymers should enhance the versatile processability of the CUPOMC used in various conditions. Development of CUPOMC should expand the choices of available biodegradable elastomers for various biomedical applications such as soft tissue engineering.
Keywords: Biodegradable Elastomer, Polyester, Soft Tissue Engineering, Thermo-Crosslinking, UV-Crosslinking
1. INTRODUCTION
Finding an ideal biomaterial is one of the major goals in the field of tissue engineering. Many of the native tissues in the human body have elastomeric properties. Thus, the biomaterials selected to repair these tissues should have similar elastic properties in order to sustain and recover from multiple deformations without causing irritation to the surrounding tissues (Wang, 2002). It has been proven that mechanical stimuli can enhance cellular growth, alignment, and extracellular matrix production. Previous research has also shown that the appropriate mechanical constraints can help yield both fibril alignment and the geometry of a native heart valve (Neidert & Tranquillo, 2006; Robinson, 2008). Moreover, the addition of mechanical stimuli has also been shown to have an influence on the stem cell differentiation (Engelmayr, 2006; Zhao, 2009). Mechanical mismatch between host blood vessels and vascular grafts may contribute to the development of myointimal hyperplasia, a major reason for graft failure (Yang, 2005). Therefore, a suitable biomaterial for soft tissue engineering should have the appropriate mechanical properties similar to the target tissue, and be capable of transmitting mechanical stimulus to the seeded cells.
Recently, many researchers have focused their work on the development of biodegradable polyester and polyurethane elastomers for soft tissue engineering. Biodegradable polyurethanes (BPUs) are a family of elastomers, which have been used in a wide variety of biomedical applications due to their good mechanical properties (up to 29 MPa tensile strength) and elasticity (up to 895% elongation) (Guan, 2002, 2004; Guan & Wagner, 2005; Guelcher, 2008; Zhang, 2008). However, due to their aliphatic nature BPUs are susceptible to permanent creep under cyclic mechanical loading. Therefore, the potential long-term success of polyurethanes as scaffold materials for dynamic tissues like blood vessels and ligament is still questionable (Dey, 2008).
Elastomeric polyesters are another family of polymers that have attracted interest due to the appropriate biodegradability, biocompatibility, and elasticity for various biomedical applications (Webb, 2004; Yang, 2009). Thereof, crosslinked polyester elastomers have attracted much attention recently due to their excellent elasticity without permanent deformation under cyclic deformation such as poly (glycerol sebacate) (PGS) and poly (octamethylene citrate) (POC) (Wang, 2002; Yang, 2004, 2006; Chen, 2008). PGS and POC have shown excellent biocompatibility in vitro and in vivo. The crosslinking nature of PGS and POC also confer excellent elasticity to these polymers. However, the mechanical strength of PGS and POC are still relatively weak which range from 0.5±0.2 MPa to 2.9±0.1 MPa, especially when they are made into porous scaffolds. The reported tensile mechanical strength of POC porous scaffolds are only 0.3±0.1 MPa (Yang, 2005). The processability of PGS and POC is also limited with only a salt-leaching method reported for this type of polymer. A commonly used thermally induced phase separation (TIPS) or freeze-drying method for scaffold fabrication cannot be applied to these polymers due to the sticky nature of their low molecular weight pre-polymers, which are the only processable forms for these polymers.
To improve the mechanical properties of POC but retain the excellent elasticity, we have recently reported a crosslinked urethane-doped polyester (CUPE) elastomer. 1,6-hexamethylene diisocyanate (HDI) is used to extend the POC pre-polymer chains to obtain a pre-CUPE polymer. Pre-CUPE can be further thermally crosslinked into a urethane-doped polyesters (CUPE) elastomeric network. The tensile strength of CUPE was as high as 41.07±6.85 MPa with corresponding elongation at break of 222.66±27.84% (Dey, 2008).
Given that there has been great interest in using photopolymerization techniques for various biomedical applications such as 3-dimensional (3-D) tissue construction and cell entrapment, (Khademhosseini et al., 2006; Du, 2008; Yu & Ding, 2008) an in situ crosslinkable biodegradable polyester network which was referred to as poly(octamethylene maleate citrate) (POMC) has been recently synthesized based on POC (Gyawali, 2010). Unsaturated maleic acid is reacted with citric acid and 1,8-octanediol to form a photocrosslinkable POMC pre-polymer (pre-POMC). However, similar to POC in addition to the photocross-linkability, POMC is still relatively weak and the processability of pre-POMC is still limited due to its low molecular weight.
Aiming at synthesizing a biodegradable elastomer with photocrosslinkability and excellent processability, we have synthesized and characterized a new family of polymers, cross-linked urethane-doped poly (octamethylene maleate citrate) (CUPOMC), in the present study. The rationale behind this polymer is: (1) the major chemical structure of CUPOMC is composed of urethane and ester bonds that have been used in many biodegradable polymer designs (Webb, 2004; Guelcher, 2008). (2) The presence of vinyl groups makes it possible for free radical polymerization such as photocrosslinking. (3) The unused pendant functional groups (-COOH and -OH) after free radical polymerization can be used for post polymerization through polycondensation or for bioconjugation (Yang, 2004). (4) The urethane-doped structure should result in a material with strong mechanical properties similar to CUPE (Dey, 2008). In addition, controlling the feeding ratio of the different monomers should create a material with tunable the mechanical properties and degradation rates. In this work, we describe the synthesis, characterization and cytocompatibility of the CUPOMCs. Porous scaffolds are also fabricated using a thermally induced phase separation (TIPS) method to demonstrate the processability and potential of CUPOMC in soft tissue engineering applications.
2. MATERIALS AND METHODS
2.1. Materials
All the chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless noted otherwise and used as received. Cell culture media was obtained from HyClone (Logan, UT).
2.2. Polymer Synthesis
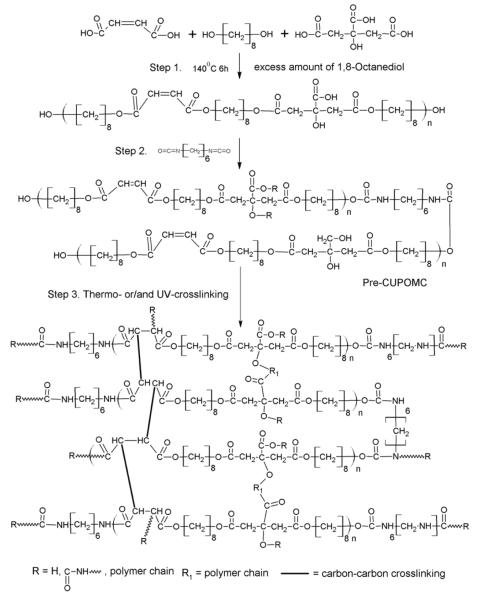
The synthesis of the CUPOMCs was carried out in following steps. The synthesis schematic is shown in Figure 1. Briefly, 0.11 mole of 1,8-octanediol, 0.8 mole of maleic acid, and 0.2 mole of citric acid were melted in a round bottom reaction flask under 160°C. Once all the monomers had been melted, the temperature was reduced to 140°C, and the mixture was bulk polymerized through polycondensation for 4h to obtain the POMC prepolymer. The prepolymer was purified by drop wise precipitation in deionized water. After purification, the prepolymer was then lyophilized. The molecular weight of the POMC prepolymer was characterized by Matrix assisted laser desorption/ionization mass spectroscopy (MALDI-MS) as 680 Da. 0.02 mole of POMC prepolymer was dissolved in 1,4-dioxane to obtain a 3% W/V solution. Next, 0.02 mole of 1,6-hexamethylene diisocyanate (HDI) was added into reaction system to synthesize pre-CUPOMC. The reaction was carried out under constant stirring with stannous octoate as a catalyst (0.1% wt). The reaction temperature was maintained at 55°C. A small amount of reaction solution was taken out and checked by Fourier transform infrared (FT-IR) after 48h. The reaction was terminated when the isocyanate peak at 2267cm−1 disappeared.
Figure 1.
Synthesis schematic of CUPOMC polymers. The monomers citric acid, 1,8-octanediol, and maleic acid underwent polycondensation to produce hydroxyl group capped Pre-POMC in step 1. In step 2, 1,6-hexamethyl diisocyanate was used to extend the Pre-POMC chain. In step 3, the Pre-CUPOMC was thermo- and/or UV-crosslinked to obtain CUPOMC network.
To obtain a thermo-crosslinked polymer, the pre-CUPOMC polymer solution was cast into a Teflon mold and dried in a chemical hood equipped with laminar airflow to evaporate all the solvent. The resulting thermo-CUPOMC film was placed into an oven under 80°C for predetermined time periods.
The free radical reaction was carried out by UV-crosslinking. Briefly, the pre-CUPOMC solution was mixed with 0.3% w/v Irgacure 784, which was used as the photoinitiator. Then the polymer solution was casted into a Teflon mold and placed under UV light for predetermined time period to synthesize photo-CUPOMC.
Polymers with different feeding ratios of 1,8-octanediol, citric acid, and HDI were synthesized using the same steps. Different CUPOMCs were abbreviated as follows: CUPOMC-ratio of citric acid-maleic acid-1,8-octanediol-HDI (ex. CUPOMC-0.2-0.8-1.1-1.0). The prepolymer of CUPOMCs was abbreviated as Pre-CUPOMC. In our following study, CUPOMC represents thermo-CUPOMC unless otherwise specified.
2.3. Polymer Characterization
2.3.1 Mechanical Tests
The mechanical tests of different Pre-CUPOMC and CUPOMC films were conducted on an MTS Insight 2 machine equipped with a 500N load cell. The testing samples were cut into a dog bone shape samples, as per ASTM D412a (25×6×0.5mm, length x width x thickness). The testing sample was pulled at a rate of 500mm/ min and elongated to failure. The initial modulus was calculated from the initial slope. 8-10 samples were measured and averaged.
2.3.2. In Vitro Degradation Study
For the in vitro degradation study, the polymer films were cut into disk-shaped specimens 7mm in diameter and 0.5mm in thickness. The degradation studies were conducted in both phosphate buffer saline (PBS, pH 7.4) and 0.05M NaOH solutions. To rapidly obtain relative degradation rates, each specimen was placed in a clean glass tube containing 10ml NaOH solution, and then incubated under 37°C for predetermined time points. At each time point, the samples were washed three times with deionized water and lyophilized. The mass loss was calculated by comparing the initial mass (W0) with the mass measured after lyophilized (Mt), as shown in equation:
| (1) |
The final result was obtained from the average value of six individual samples.
2.3.3. In Vitro Cytotoxicity Evaluation of Degradation Products
NIH 3T3 fibroblasts (3T3) were cultured in a 50ml culture flask with Dulbecco’s modified eagle’s medium (DMEM) supplemented with 5% fetal bovine serum. Cell culture was maintained in a water-jacket incubator equilibrated with 5% CO2 and 95% humidity at 37°C. After confluence of cell proliferation, the cells were trypsinized, centrifuged, and suspended in culture media before seeding. According a method described previously (Timmer, 2003) the cytotoxicity of the CUPOMC degradation products was carried out by completely degrading the materials and exposing the degraded product solution to the cultured cells. Polymers were hydrolytically degraded in accelerated conditions. The difference between CUPOMC (urethane-doped POMC) and CUPE (urethane-doped polyester)(Dey, 2008) with 2d oven crosslinking was compared. Poly(D,L-lactide-co-glycolide) (PLGA 75/25) was used as control. All polymers were placed in 50ml 1N NaOH solution and incubated under 37°C. The polymers took 24h to be completely degraded. The solution was then filtered through a cellulose acetate membrane filter (0.2 μm pore diameter). The pH was adjusted to 7.4 with 1N HCl. The solution was filtered again for sterilization and then diluted by 2, 10, 50 and 100 times with culture media. Then the solutions with culture media were added to the cultured cells in 96 well plates (100μl/well) and incubated at 37°C and 5% CO2 for 24h. After incubation, cell viability was testified using methylthiazole tetrazolium (MTT) assay. Six samples were tested for each polymer.
2.4. In Vitro Cell Proliferation
Both CUPOMC and CUPE films were oven-crosslinked for 2d and cut into discs with a diameter of 6mm. PLGA films were selected as a control. All the samples were sterilized by incubation in 70% v/v ethanol for 15min followed by UV light exposure for 1h. 3T3’s were seeded on the films at a density of 2.0×105 cells/ ml in a 96-well plate. After 1h pre-incubation, 200μl of culture media was added into each well. After incubation for predetermined time points, the cell viability on each sample was testified using MTT assay. The result was obtained from average value of six samples. The morphology of 3T3 on the CUPOMC films was observed directly under microscopy.
2.5. Scaffold Fabrication
The CUPOMCs were fabricated into porous scaffolds by a thermally induced phase separation (TIPS) method as described previously (Guan, 2007). Briefly, a 3% w/v pre-CUPOMC solution in 1,4-dioxane was frozen under -80°C for 2h and then freeze-dried to obtain a porous scaffold. The morphology of the TIPS scaffold was observed by scanning electron microscope (SEM).
2.6. Statistical Methods
Data were expressed as the mean ± standard deviation. The statistical significance between two sets of data was calculated using a One-way ANOVA. Data were considered to have significant difference, when a p-value of 0.05 or less was obtained.
3. RESULTS AND DISCUSSIONS
3.1. Synthesis and Characterization of CUPOMC
The FT-IR spectra of pre-CUPOMC-0.8-1.1-1.0, pre-POMC, and pre-CUPE were compared in Figure 2. All the three polymers had a sharp peak at 1730cm−1, which was assigned to the carbonyl group (C=O). Amide I at 1670cm−1 and amide II at 1560cm−1 vibrations were found on the spectra of pre-CUPOMC and pre-CUPE, which indicates the successful doping of urethane (Dey, 2008). The characteristic peak of the double bond at 1647cm−1 was overlapped by the amide I peak. However, the peak of trans-double bond at 980cm−1 could be observed from the spectra of the pre-POMC and pre-CUPOMC. These results indicated that the double bond was maintained after the chain extension reaction. It also demonstrated that during the synthesis of pre-POMC, the cis- structure of double bond from maleic acid had turned into trans- structure.
Figure 2.
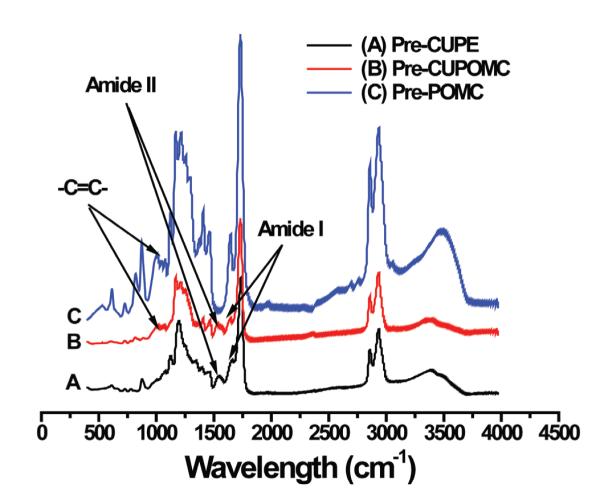
FT-IR spectra of representative prepolymers. (A) Pre-CUPE; (B) Pre-CUPOMC; (C) Pre-POMC.
3.2. Mechanical Test
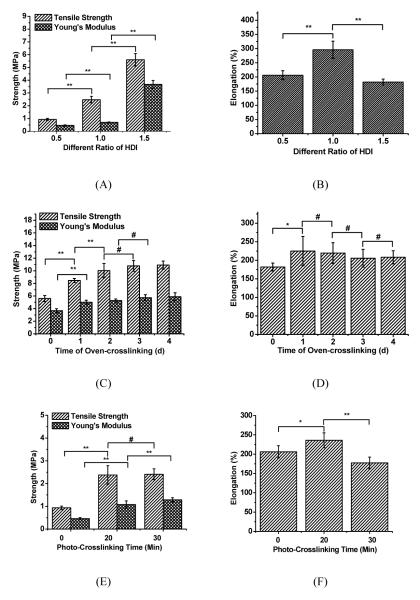
The mechanical properties of different polymers were compared through tensile tests. To evaluate the impact of the HDI ratio, mechanical tests were performed on prepolymers of CUPOMC-0.2-0.8-1.1-0.5, CUPOMC-0.2-0.8-1.1-1.0 and CU - POMC-0.2-0.8-1.1-1.5. The results are shown in Figures 3A and 3B. With the increase of the HDI, the tensile strength and initial modulus ranged from 0.94±0.08 and 5.60±0.48 respectively. Compared with POMCs (photocrosslinked), the tensile strength of which was lower than 1MPa (Gyawali, 2010) the presence of the urethane bond dramatically increased the tensile strength of the materials, even at the pre-polymer stage. The elongation of these prepolymers ranged from 181.92±10.64% and 295.85±30.06, which covers the elongation of native arteries and veins (~260%).
Figure 3.
Tensile mechanical tests on pre-CUPOMC and CUPOMC. (A and B) Tensile strength, Young’s Modulus and elongation of pre-CUPOMC-0.2-0.8-1.1-0.5, pre-CUPOMC-0.2-0.8-1.1-1.0 and pre-CUPOMC-0.2-0.8-1.1-1.5 (various HDI molar ratio: 0.5, 1.0, and 1.5); (C and D) Tensile strength, Young’s Modulus and elongation of CUPOMC-0.2-0.8-1.1-1.5 with different thermo-crosslinking time ranging from 1d to 4d. (E and F) Tensile strength, Young’s Modulus, Elongation of CUPOMC-0.2-0.8-1.1-0.5 with different UV-crosslinking time. **p<0.01, *p<0.05, #p>0.05; N=8.
CUPOMC-0.2-0.8-1.1-1.5 was selected to analyze the impact of different thermo-cross-linking times on the material mechanical properties. From Figure 3C, it showed an increasing trend with the increasing thermo-crosslinking times from 0 day to two days. Since there was only 0.2 molar ratio of citric acid in the polymer, there was no significant difference for the tensile strength (up to 10.91±0.63MPa) and initial modulus after two-day thermo-crosslinking suggesting the crosslinking reactions were completed within two days. The elongation of the polymers remained similar after one-day crosslinking (Figure 3D).
CUPOMC-0.2-0.8-1.1-0.5 was UV-crosslinked with Irgacure 784 for 20 and 30min. As shown in Figures 3E and 3F, the polymer crosslinked for 20min under UV irradiation was almost three times as strong as the original pre-polymer. However, the additional UV-crosslinking times seemed to not change the mechanical properties significantly. Since UV-crosslinking only consumed vinyl groups from the polymer chain, the functional groups on the pendent chain can be saved for thermo-polymerization or further chemical modification. The UV-crosslinking is a much milder polymer processing method than the thermo-crosslinking providing advantages when thermo-sensitive biomolecules have to be incorporated or conjugated to the polymers.
The mechanical properties of the selected biodegradable elastomers and soft tissues were listed in Table 1. The tensile strength of pre-CUPOMC and CUPOMCs under the known synthesis conditions ranged from 0.73±0.12 and 10.91±0.63MPa (Figures 3A and 3C), which are similar to POC and much stronger than PGS and POMC. Importantly, the elongation range of pre-CUPOMC and CUPOMCs covered all the selected polymers and the soft tissues in Table 1. These results indicated that doping urethane bonds in POMCs created strong CUPOMCs without losing the elasticity. It should be noted that even the tensile strength of pre-CUPOMCs synthesized under the known conditions could range from 0.73±0.12 and 5.60±0.48. This is very different from the other published pre-POC, pre-PGS and pre-POMC polymers which could not even be tested for their tensile mechanical properties due to their sticky nature. In other words, pre-CUPOMCs can be used as implant materials for various applications without even a further post-polymerization (thermo- or photo-). The tunable mechanical properties of CUPOMCs suggested that CUPOMCs may be viable candidate materials for soft tissue engineering applications.
Table 1.
Mechanical properties of selected biodegradable elastomers and soft tissues
| Tissue/Polymer | Tensile Strength (MPa) |
Young’s Modulus (MPa) |
Elongation (%) |
Reference |
|---|---|---|---|---|
| Human Bladder | 0.27±0.14 | 0.25±0.18 | 0.69±0.17 | (Dahms, 1998) |
| Smooth muscle relaxed | N/A | 0.006 | 300 | (Chandran, 1992) |
| Smooth muscle contracted | N/A | 0.01 | 300 | (Chandran, 1992) |
| Aortic valve leaflet (circumfer ential) |
N/A | 15±6 | 21±12 | (Balguid, 2007) |
| Ulnar cadaveric peripheral nerve | 9.8 – 21.6 | N/A | 8 – 21 | (Millesi, 1995) |
| Medial cadaveric peripheral nerve | 9.8 – 30.4 | N/A | 6 – 22 | (Millesi, 1995) |
| Cerebral artery | N/A | 15.7 | 50 | (Monson, 2003) |
| Cerebral vein | N/A | 6.85 | 83 | (Monson, 2003) |
| Poly (octanediol-citrate) | ~ 5.80±0.76 | 6.44±0.28 | 367±15 | (Yang, 2006) |
| Poly (glycerol sebacate) | >0.5 | 0.282±0.025 | >267 | (Wang, 2002) |
| PLGA | 41.4 – 55.2 | 1.4 – 2.8 | 3 - 10 | (Yang, 2001) |
| Caprolactone soft segment poly urethane |
1.5 | 3.7 | 60 | (Yeganeh, 2005) |
3.3. In Vitro Degradation of Pre-CUPOMCs
The degradation rates of pre-CUPOMCs could be varied by changing the feeding ratio of monomers. As shown in Figure 4A, pre-CUPOMC-0.2-0.8-1.1-1.0 polymers were fully degraded after 14 weeks of incubation in PBS. Increasing the HDI ratio and decreasing the diol ratio resulted in slower degradation rates. This was explained that increasing the HDI ratio and decreasing the diol ratio could both increase more hydrolysis-resistant urethane and amides bonds than ester bonds, which were in agreement with previous findings on POC and CUPE (Yang, 2004, 2006; Dey, 2008). Compared to the long degradation times for some polyesters (18-60 months for PLLA and 24 months for PCL), the 14-week in vitro degradation time period for pre-CUPOMC can potentially be more suitable for some tissue engineering applications. Normally, polyurethanes undergo even longer degradation times (Amsden, 2007). The degradation study in base solution also provided strong evidence that the degradation rates of CUPOMCs are tunable.
Figure 4.
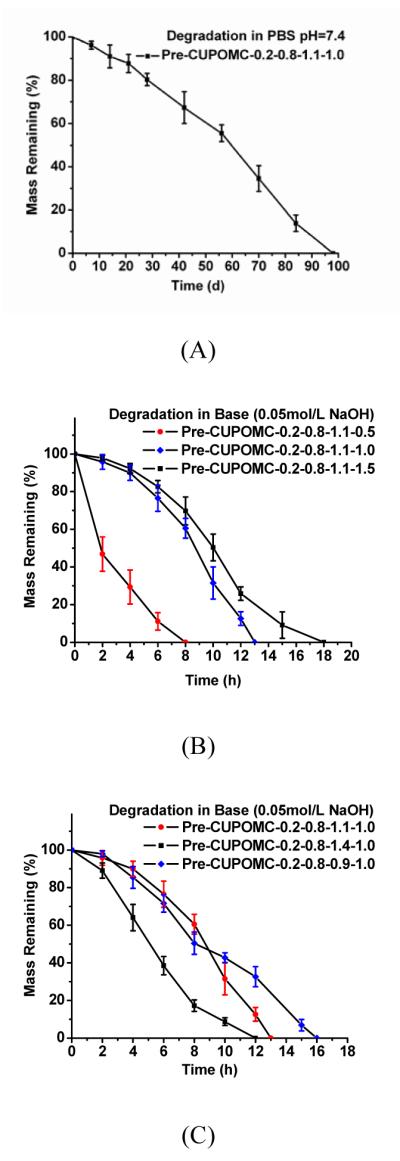
(A) In vitro degradation of Pre-CUPOMC-0.2-0.8-1.1-1.0 in PBS (pH=7.4) at 37°C (n=6). (B) In vitro degradation of Pre-CUPOMC with different ratio of HDI in NaOH (0.05mol/L) at 37°C (n=6). (C) In vitro degradation of Pre-CUPOMC with different ratio of 1,8-octanediol in NaOH (0.05mol/L) at 37°C (n=6).
3.4. Cell Culture
As pre-CUPOMCs themselves may be used as materials. Therefore, a preliminary cell attachment study was conducted on a selected pre-CUPOMC film. The cell morphology of 3T3 fibroblasts on pre-CUPOMC-0.2-0.8-1.1-1.0 films are shown in Figures 5A and 5B with different magnifications. It was observed that 3T3 fibroblasts had a stretched morphology on Pre-CUPOMC-0.2-0.8-1.1-1.0 films, which indicates that this polymer supported 3T3 fibroblast adhesion.
Figure 5.
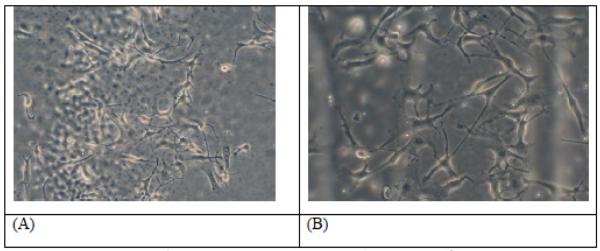
Images of 3T3 mouse fibroblasts on the Pre-CUPOMC-0.2-0.8-1.1-1.0 film observed under microscopy with 20× (A) and 32× (B) magnification.
To study the cytotoxicity of the CUPOMC degradation products, the maximum release of degradation products was achieved by an accelerated degradation in strong base solution (Timmer, 2003). The degradation solution of the polymers was then incubated with 3T3 fibroblasts for MTT assay. All the values of absorbance were normalized to the PLGA at 100x dilution. The results are shown in Figure 6A. The viability of 3T3s cultured in the presence of the CUPOMC degradation products was 1.80±0.07%, 5.66±0.66%, 40.53±4.63%, and 81.96±11.23% for 2×, 10×, 50×, and 100× dilutions, respectively. The results indicated that CUPOMC had a dose-dependent cytotoxic effect. CUPOMC degradation products produced similar or slightly higher cytotoxicity compared to the PLGA degradation products at 100× dilution suggesting an acceptable cytotoxicity of CUPOMC degradation products.
Figure 6.
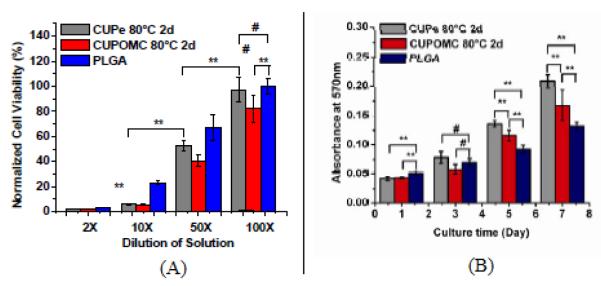
(A) Cytotoxicity evaluation of degradation products of CUPOMC-0.2-0.8-1.1-1.0 with 2d thermo-crosslinking under 80°C at 2×, 10×, 50×, and 100× dilutions. CUPe with same thermo-crosslinking condition and PLGA 75/25 were used as control. (B) Cell viability and proliferation assay (MTT assay) for 3T3 fibroblasts cultured on CUPOMC-0.2-0.8-1.1-1.0 film with 2d thermo-crosslinking under 80°C. CUPe with same thermo-crosslinking condition and PLGA 75/25 were used as control.
The cytocompatibility of CUPOMC was evaluated by cell adhesion and proliferation on CUPOMC-0.2-0.8-1.1-1.0 films. From the MTT assay results (Figure 6B), PLGA had a higher cell adhesion than both CUPe and CUPOMC during the initial phase of cell adhesion and proliferation. After 3 days cell culture, there was no significant difference in the cell number between CUPOMC and PLGA. CUPOMC displayed higher cell viability than PLGA after 5 and 7 days cell culture, but CUPe was even higher. The results suggested that CUPOMCs supported higher cell proliferation rates than PLGA. The cytotoxicity studies demonstrated that CUPOMCs have the potential to be used as cell delivery carrier such as in tissue engineering applications.
3.5. Scaffold Fabrication or Pre-CUPOMC
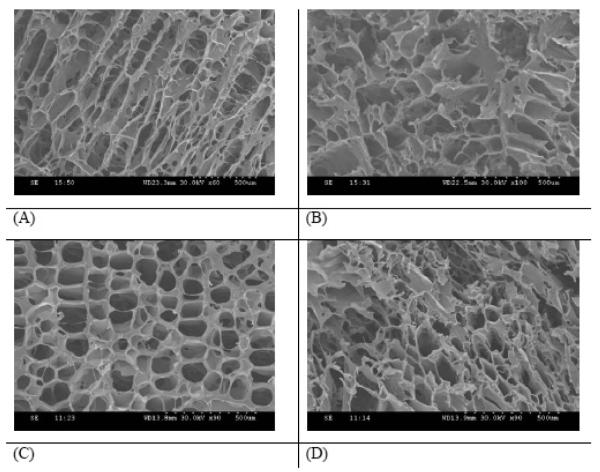
As we have studied, pre-CUPOMC possessed excellent mechanical properties even without further crosslinking. We have also studied the processability of pre-CUPOMC by scaffold fabrication. The morphology of the TIPS scaffold fabricated from pre-CUPOMC-0.2-0.8-1.1-1.0 was observed on a Hitachi 3000 SEM. CU-POMC-0.2-0.8-1.1-1.0 TIPS scaffolds are shown in Figure 7. Pictures of both the surface and cross section were taken. Figures 7A and 7B show that the TIPS fabrication technique could be applied to pre-CUPOMC to prepare a scaffold with a highly interconnected porous structure. The morphology of the scaffold was also observed after 1d thermo-crosslinking. The porous structure was maintained in both the surface and cross section of the scaffold as shown in Figures 7C and 7D. Tensile mechanical tests on the above pre-CUPOMC-0.2-0.8-1.1-1.0 TIPS scaffold confirmed the soft and elastic nature of the scaffolds (Young’s modulus: 0.09±0.01MPa, elongation: 192.44±24.76%).
Figure 7.
(A and B) SEM images of the surface (A) and cross section (B) of a pre-CU-POMC-0.2-0.8-1.1-1.0 scaffolds fabricated by a thermally induced phase separation (TIPS) method. (C and D) SEM images of the surface (C) and cross section (D) of a CUPOMC-0.2 -0.8-1.1-1.0 scaffold thermally crosslinked under 80 þC, 1 day.
4. CONCLUSION
We have developed a new family of polyester elastomers, poly (octamethylene maleate citrate) urethane (CUPOMCs). CUPOMCs possess tunable mechanical properties and degradation rate. CUPOMCs could be either thermo-crosslinkable and/or UV-crosslinkable providing flexibility to use these polymers in various applications. CUPOMC prepolymers exhibited excellent processability offering advantages over the previous developed biodegradable elastomers. Preliminary biocompatibility evaluation in vitro supported that CUPOMCs may be good candidate materials for cell delivery carriers. The development of CUPOMCs should expand the choices of available biodegradable elastomers for broad biomedical applications such as soft tissue engineering.
ACKNOWLEDGMENTS
This work was supported in part by a R01 award (1R01EB012575-01A1) from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institute of Health (NIH), and a National Science Foundation (NSF) CAREER award 0954109.
Contributor Information
Yi Zhang, University of Texas at Arlington, USA.
Richard T. Tran, University of Texas at Arlington, USA
Dipendra Gyawali, University of Texas at Arlington, USA.
Jian Yang, University of Texas at Arlington, USA.
REFERENCES
- Amsden B. Curable, biodegradable elastomers: emerging biomaterials for drug delivery and tissue engineering. Soft Matter. 2007;3(11):1335–1348. doi: 10.1039/b707472g. doi:10.1039/b707472g. [DOI] [PubMed] [Google Scholar]
- Balguid A, Rubbens MP, Mol A, Bank RA, Bogers AJJC, Van Kats JP. The role of collagen cross-links in biomechanical behavior of human aortic heart valve leaflets - Relevance for tissue engineering. Tissue Engineering. 2007;13(7):1501–1511. doi: 10.1089/ten.2006.0279. doi:10.1089/ten.2006.0279. [DOI] [PubMed] [Google Scholar]
- Chandran KB. Cardiovascular biomechanics. New York University Press; New York, NY: 1992. [Google Scholar]
- Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29(1):47–57. doi: 10.1016/j.biomaterials.2007.09.010. doi:10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Dahms SE, Piechota HJ, Dahiya R, Lue TF, Tanagho EA. Composition and biomechanical properties of the bladder acellular matrix graft: comparative analysis in rat, pig and human. British Journal of Urology. 1998;82(3):411–419. doi: 10.1046/j.1464-410x.1998.00748.x. doi:10.1046/j.1464-410X.1998.00748.x. [DOI] [PubMed] [Google Scholar]
- Dey J, Xu H, Shen J, Thevenot P, Gondi SR, Nguyen KT. Development of biodegradable crosslinked urethane-doped polyester elastomers. Biomaterials. 2008;29(35):4637–4649. doi: 10.1016/j.biomaterials.2008.08.020. doi:10.1016/j.biomaterials.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(28):9522–9527. doi: 10.1073/pnas.0801866105. doi:10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmayr GC, Jr, Sales VL, Mayer JE, Jr, Sacks MS. Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: Implications for engineered heart valve tissues. Biomaterials. 2006;27(36):6083–6095. doi: 10.1016/j.biomaterials.2006.07.045. doi:10.1016/j.biomaterials.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Guan J, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. Journal of Biomedical Materials Research. 2002;61(3):493–503. doi: 10.1002/jbm.10204. doi:10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- Guan J, Sacks MS, Beckman EJ, Wagner WR. Biodegradable poly(ether ester urethane) urea elastomers based on poly(ether ester) triblock copolymers and putrescine: synthesis, characterization and cytocompatibility. Biomaterials. 2004;25(1):85–96. doi: 10.1016/s0142-9612(03)00476-9. doi:10.1016/S0142-9612(03)00476-9. [DOI] [PubMed] [Google Scholar]
- Guan J, Stankus JJ, Wagner WR. Biodegradable elastomeric scaffolds with basic fibroblast growth factor release. Journal of Controlled Release. 2007;120(1-2):70–78. doi: 10.1016/j.jconrel.2007.04.002. doi:10.1016/j.jconrel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J, Wagner WR. Synthesis, characterization and cytocompatibility of polyurethaneurea elastomers with designed elastase sensitivity. Biomacromolecules. 2005;6(5):2833–2842. doi: 10.1021/bm0503322. doi:10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelcher SA. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Engineering. Part B, Reviews. 2008;14(1):3–17. doi: 10.1089/teb.2007.0133. doi:10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- Gyawali D, Tran RT, Guleserian KJ, Tang L, Yang J. Citric acid-derived photocrosslinked biodegradable elastomers. Journal of Biomaterials Science. Polymer Edition. 2010;21(13):1761–1782. doi: 10.1163/092050609X12567178204169. doi:10.1163/092050609X12567178204169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademhosseini A, Eng G, Yeh J, Fukuda J, Blumling J, Langer R, Burdick JA. Micromolding of photocroslinkable hyaluronic acid for cell encapsulation and entrapment. Journal of Biomedical Materials Research. 2006;79A:522–532. doi: 10.1002/jbm.a.30821. doi:10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- Millesi H, Zoch G, Reihsner R. Mechanical-properties of peripheral-nerves. Clinical Orthopaedics and Related Research. 1995;(314):76–83. [PubMed] [Google Scholar]
- Monson KL, Goldsmith W, Barbaro NM, Manley GT. Axial mechanical properties of fresh human cerebral blood vessels. Journal of Biomechanical Engineering-Transactions of the Asme. 2003;125(2):288–294. doi: 10.1115/1.1554412. doi:10.1115/1.1554412. [DOI] [PubMed] [Google Scholar]
- Neidert MR, Tranquillo RT. Tissue-engineered valves with commissural alignment. Tissue Engineering. 2006;12(4):891–903. doi: 10.1089/ten.2006.12.891. doi:10.1089/ten.2006.12.891. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Johnson SL, Evans MC, Barocas VH, Tranquillo RT. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Engineering. Part A. 2008;14(1):83–95. doi: 10.1089/ten.a.2007.0148. doi:10.1089/ten.a.2007.0148. [DOI] [PubMed] [Google Scholar]
- Timmer MD, Shin H, Horch RA, Ambrose CG, Mikos AG. In vitro cytotoxicity of injectable and biodegradable poly(propylene fumarate)-based networks: unreacted macromers, cross-linked networks, and degradation products. Biomacromolecules. 2003;4(4):1026–1033. doi: 10.1021/bm0300150. doi:10.1021/bm0300150. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nature Biotechnology. 2002;20(6):602–606. doi: 10.1038/nbt0602-602. doi:10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- Webb AR, Yang J, Ameer GA. Biodegradable polyester elastomers in tissue engineering. Expert Opinion on Biological Therapy. 2004;4(6):801–812. doi: 10.1517/14712598.4.6.801. doi:10.1517/14712598.4.6.801. [DOI] [PubMed] [Google Scholar]
- Yang J, Motlagh D, Webb AR, Ameer GA. Novel biphasic elastomeric scaffold for small-diameter blood vessel tissue engineering. Tissue Engineering. 2005;11(11-12):1876–1886. doi: 10.1089/ten.2005.11.1876. doi:10.1089/ten.2005.11.1876. [DOI] [PubMed] [Google Scholar]
- Yang J, Webb AR, Ameer GA. Novel citric acid-based biodegradable elastomers for tissue engineering. Advanced Materials (Deerfield Beach, Fla.) 2004;16(6):511–516. doi:10.1002/adma.200306264. [Google Scholar]
- Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials. 2006;27(9):1889–1898. doi: 10.1016/j.biomaterials.2005.05.106. doi:10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang Y, Guatam S, Liu L, Dey J, Chen W. Development of aliphatic biodegradable photoluminescent polymers. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(25):10086–10091. doi: 10.1073/pnas.0900004106. doi:10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Leong KF, Du ZH, Chua CK. The design of scaffolds for use in tissue engineering. Part 1. Traditional factors. Tissue Engineering. 2001;7(6):679–689. doi: 10.1089/107632701753337645. doi:10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- Yeganeh H, Lakouraj MM, Jamshidi S. Synthesis and characterization of novel biodegradable epoxy-modified polyurethane elastomers. Journal of Polymer Science. Part A, Polymer Chemistry. 2005;43(14):2985–2996. doi:10.1002/pola.20789. [Google Scholar]
- Yu L, Ding JD. Injectable hydrogels as unique biomedical materials. Chemical Society Reviews. 2008;37(8):1473–1481. doi: 10.1039/b713009k. doi:10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wen X, Vyavahare NR, Boland T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials. 2008;29(28):3781–3791. doi: 10.1016/j.biomaterials.2008.06.009. doi:10.1016/j.biomaterials.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Lin H, Zhang J, Chen B, Sun W, Wang X. Crosslinked three-dimensional demineralized bone matrix for the adipose-derived stromal cell proliferation and differentiation. Tissue Engineering. Part A. 2009;15(1):13–21. doi: 10.1089/ten.tea.2008.0039. doi:10.1089/ten.tea.2008.0039. [DOI] [PubMed] [Google Scholar]