Abstract
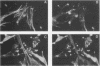
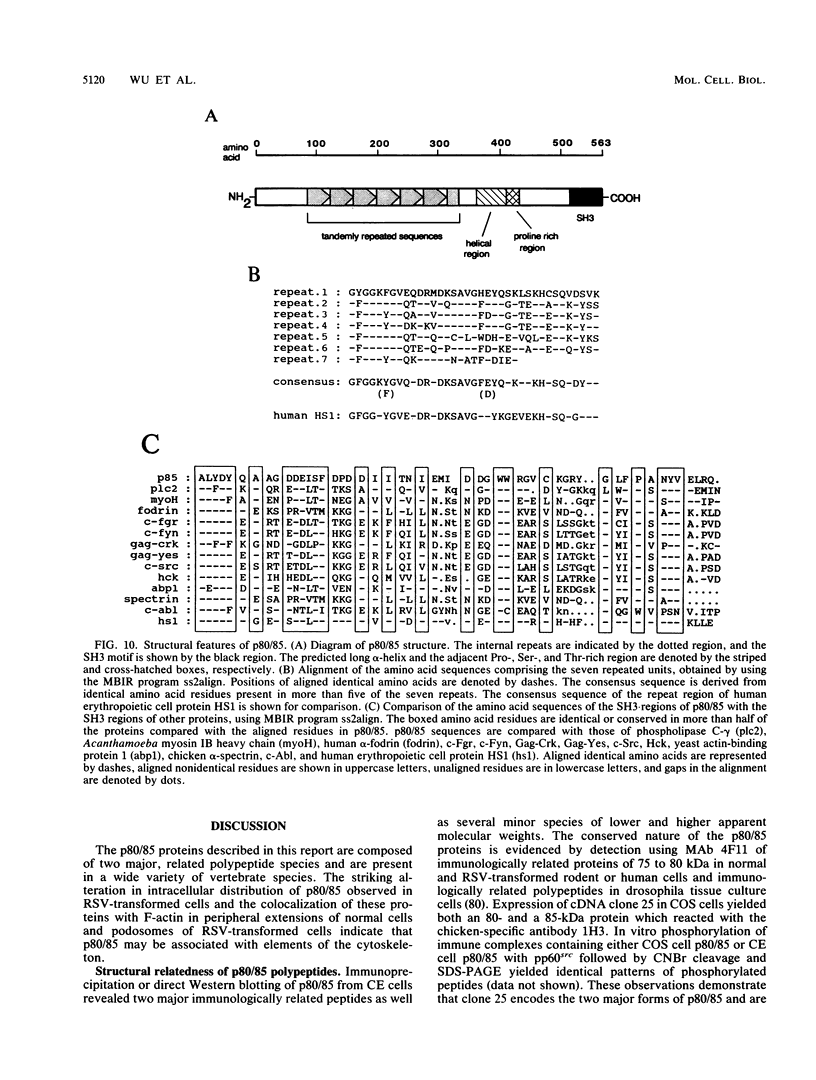
Transformation of cells by the src oncogene results in elevated tyrosine phosphorylation of two related proteins, p80 and p85 (p80/85). Immunostaining with specific monoclonal antibodies revealed a striking change of subcellular localization of p80/85 in src-transformed cells. p80/85 colocalizes with F-actin in peripheral extensions of normal cells and rosettes (podosomes) of src-transformed cells. Sequence analysis of cDNA clones encoding p80/85 revealed an amino-terminal domain composed of six copies of a direct tandem repeat, each repeat containing 37 amino acids, a carboxyl-terminal SH3 domain, and an interdomain region composed of a highly charged acidic region and a region rich in proline, serine, and threonine. The multidomain structure of p80/85 and its colocalization with F-actin in normal and src-transformed cells suggest that these proteins may associate with components of the cytoskeleton and contribute to organization of cell structure.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M., Norman D., Willis A., Campbell I. D. Structure of the fibronectin type 1 module. Nature. 1990 Jun 14;345(6276):642–646. doi: 10.1038/345642a0. [DOI] [PubMed] [Google Scholar]
- Beckerle M. C. Identification of a new protein localized at sites of cell-substrate adhesion. J Cell Biol. 1986 Nov;103(5):1679–1687. doi: 10.1083/jcb.103.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton A. H., Kanner S. B., Vines R. R., Wang H. C., Gibbs J. B., Parsons J. T. Transformation by pp60src or stimulation of cells with epidermal growth factor induces the stable association of tyrosine-phosphorylated cellular proteins with GTPase-activating protein. Mol Cell Biol. 1991 Feb;11(2):945–953. doi: 10.1128/mcb.11.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brzeska H., Lynch T. J., Martin B., Korn E. D. The localization and sequence of the phosphorylation sites of Acanthamoeba myosins I. An improved method for locating the phosphorylated amino acid. J Biol Chem. 1989 Nov 15;264(32):19340–19348. [PubMed] [Google Scholar]
- Burr J. G., Dreyfuss G., Penman S., Buchanan J. M. Association of the src gene product of Rous sarcoma virus with cytoskeletal structures of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3484–3488. doi: 10.1073/pnas.77.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Gould K., Sefton B. M. The absence of myristic acid decreases membrane binding of p60src but does not affect tyrosine protein kinase activity. J Virol. 1986 May;58(2):468–474. doi: 10.1128/jvi.58.2.468-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987 Apr 10;49(1):83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Chen J. M., Parsons S. J., Parsons J. T. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature. 1985 Jul 11;316(6024):156–158. doi: 10.1038/316156a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Pfeuty T., Singer S. J. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Martin G. S. Linker insertion-deletion mutagenesis of the v-src gene: isolation of host- and temperature-dependent mutants. J Virol. 1989 Feb;63(2):542–554. doi: 10.1128/jvi.63.2.542-554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorit R. L., Schoenbach L., Gilbert W. How big is the universe of exons? Science. 1990 Dec 7;250(4986):1377–1382. doi: 10.1126/science.2255907. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Mulholland J., Zhu Z. M., Botstein D. Homology of a yeast actin-binding protein to signal transduction proteins and myosin-I. Nature. 1990 Jan 18;343(6255):288–290. doi: 10.1038/343288a0. [DOI] [PubMed] [Google Scholar]
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990 Jan 25;343(6256):377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Navankasattusas S., Kavanaugh W. M., Milfay D., Fried V. A., Williams L. T. cDNA cloning of a novel 85 kd protein that has SH2 domains and regulates binding of PI3-kinase to the PDGF beta-receptor. Cell. 1991 Apr 5;65(1):75–82. doi: 10.1016/0092-8674(91)90409-r. [DOI] [PubMed] [Google Scholar]
- Fukui Y., O'Brien M. C., Hanafusa H. Deletions in the SH2 domain of p60v-src prevent association with the detergent-insoluble cellular matrix. Mol Cell Biol. 1991 Mar;11(3):1207–1213. doi: 10.1128/mcb.11.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Bretscher A., Esch F. S., Hunter T. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 1989 Dec 20;8(13):4133–4142. doi: 10.1002/j.1460-2075.1989.tb08598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Grandori C., Hanafusa H. Phosphorylation of cellular proteins in Rous sarcoma virus-infected cells: analysis by use of anti-phosphotyrosine antibodies. Mol Cell Biol. 1988 Aug;8(8):3035–3042. doi: 10.1128/mcb.8.8.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Varmus H. E. Site-directed mutagenesis of the SH2- and SH3-coding domains of c-src produces varied phenotypes, including oncogenic activation of p60c-src. Mol Cell Biol. 1990 Apr;10(4):1307–1318. doi: 10.1128/mcb.10.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Kinoshita N., Morikawa K., Yanagida M. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell. 1990 Jan 26;60(2):319–328. doi: 10.1016/0092-8674(90)90746-2. [DOI] [PubMed] [Google Scholar]
- Iba H., Takeya T., Cross F. R., Hanafusa T., Hanafusa H. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4424–4428. doi: 10.1073/pnas.81.14.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kanner S. B., Parsons S. J., Parsons J. T., Gilmer T. M. Activation of pp60c-src tyrosine kinase specific activity in tumor-derived Syrian hamster embryo cells. Oncogene. 1988 Apr;2(4):327–335. [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Parsons J. T. Tyrosine phosphorylation of a 120-kilodalton pp60src substrate upon epidermal growth factor and platelet-derived growth factor receptor stimulation and in polyomavirus middle-T-antigen-transformed cells. Mol Cell Biol. 1991 Feb;11(2):713–720. doi: 10.1128/mcb.11.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990 May;87(9):3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Wang H. C., Vines R. R., Parsons J. T. The SH2 and SH3 domains of pp60src direct stable association with tyrosine phosphorylated proteins p130 and p110. EMBO J. 1991 Jul;10(7):1689–1698. doi: 10.1002/j.1460-2075.1991.tb07693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D., Kaneko H., Miyagoe Y., Ariyasu T., Watanabe T. Isolation and characterization of a novel human gene expressed specifically in the cells of hematopoietic lineage. Nucleic Acids Res. 1989 Nov 25;17(22):9367–9379. [PMC free article] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma L. M., Weber M. J. Constitutive phosphorylation of the receptor for insulinlike growth factor I in cells transformed by the src oncogene. Mol Cell Biol. 1990 Jul;10(7):3626–3634. doi: 10.1128/mcb.10.7.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto T. L., Lomax K. J., Volpp B. D., Nunoi H., Sechler J. M., Nauseef W. M., Clark R. A., Gallin J. I., Malech H. L. Cloning of a 67-kD neutrophil oxidase factor with similarity to a noncatalytic region of p60c-src. Science. 1990 May 11;248(4956):727–730. doi: 10.1126/science.1692159. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Linder M. E., Burr J. G. Nonmyristoylated p60v-src fails to phosphorylate proteins of 115-120 kDa in chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2608–2612. doi: 10.1073/pnas.85.8.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990 Mar 1;344(6261):36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Teti A., Zambonin-Zallone A., Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987 Mar;169(1):202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988 Mar 17;332(6161):272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Giebelhaus D. H., Champion J. E., Bailes J. A., Lacey S., Carritt B., Henchman S. K., Moon R. T. cDNA cloning, sequencing and chromosome mapping of a non-erythroid spectrin, human alpha-fodrin. Differentiation. 1987;34(1):68–78. doi: 10.1111/j.1432-0436.1987.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Nemeth S. P., Fox L. G., DeMarco M., Brugge J. S. Deletions within the amino-terminal half of the c-src gene product that alter the functional activity of the protein. Mol Cell Biol. 1989 Mar;9(3):1109–1119. doi: 10.1128/mcb.9.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Expression of v-src and chicken c-src in rat cells demonstrates qualitative differences between pp60v-src and pp60c-src. Cell. 1984 May;37(1):131–139. doi: 10.1016/0092-8674(84)90308-8. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Weber M. J. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr Top Microbiol Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- Pawson T. Non-catalytic domains of cytoplasmic protein-tyrosine kinases: regulatory elements in signal transduction. Oncogene. 1988 Nov;3(5):491–495. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Potts W. M., Reynolds A. B., Lansing T. J., Parsons J. T. Activation of pp60c-src transforming potential by mutations altering the structure of an amino terminal domain containing residues 90-95. Oncogene Res. 1988;3(4):343–355. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. B., Kanner S. B., Wang H. C., Parsons J. T. Stable association of activated pp60src with two tyrosine-phosphorylated cellular proteins. Mol Cell Biol. 1989 Sep;9(9):3951–3958. doi: 10.1128/mcb.9.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. B., Roesel D. J., Kanner S. B., Parsons J. T. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989 Feb;9(2):629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. B., Vila J., Lansing T. J., Potts W. M., Weber M. J., Parsons J. T. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO J. 1987 Aug;6(8):2359–2364. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M. The viral tyrosine protein kinases. Curr Top Microbiol Immunol. 1986;123:39–72. doi: 10.1007/978-3-642-70810-7_3. [DOI] [PubMed] [Google Scholar]
- Shalloway D., Coussens P. M., Yaciuk P. Overexpression of the c-src protein does not induce transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7071–7075. doi: 10.1073/pnas.81.22.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Boguski M. S., Goebl M., Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990 Jan 26;60(2):307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Margolis B., Mohammadi M., Lowenstein E., Fischer R., Drepps A., Ullrich A., Schlessinger J. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell. 1991 Apr 5;65(1):83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Tarone G., Cirillo D., Giancotti F. G., Comoglio P. M., Marchisio P. C. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985 Jul;159(1):141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Trahey M., Wong G., Halenbeck R., Rubinfeld B., Martin G. A., Ladner M., Long C. M., Crosier W. J., Watt K., Koths K. Molecular cloning of two types of GAP complementary DNA from human placenta. Science. 1988 Dec 23;242(4886):1697–1700. doi: 10.1126/science.3201259. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Hieda Y., Tsukita S. A new 82-kD barbed end-capping protein (radixin) localized in the cell-to-cell adherens junction: purification and characterization. J Cell Biol. 1989 Jun;108(6):2369–2382. doi: 10.1083/jcb.108.6.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C. E., Glenney J. R., Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990 Sep;111(3):1059–1068. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel U. S., Dixon R. A., Schaber M. D., Diehl R. E., Marshall M. S., Scolnick E. M., Sigal I. S., Gibbs J. B. Cloning of bovine GAP and its interaction with oncogenic ras p21. Nature. 1988 Sep 1;335(6185):90–93. doi: 10.1038/335090a0. [DOI] [PubMed] [Google Scholar]
- Wang H. C., Parsons J. T. Deletions and insertions within an amino-terminal domain of pp60v-src inactivate transformation and modulate membrane stability. J Virol. 1989 Jan;63(1):291–302. doi: 10.1128/jvi.63.1.291-302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilenko W. J., Payne D. M., Fitzgerald D. L., Weber M. J. Phosphorylation and activation of epidermal growth factor receptors in cells transformed by the src oncogene. Mol Cell Biol. 1991 Jan;11(1):309–321. doi: 10.1128/mcb.11.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler P. A., Boschelli F. Src homology 2 domain deletion mutants of p60v-src do not phosphorylate cellular proteins of 120-150 kDa. Oncogene. 1989 Feb;4(2):231–236. [PubMed] [Google Scholar]