Abstract
Forty-nine patients from the Burns Unit at the QECH had swabs taken from various sites in order to determine the bacterial profile and antibiotic susceptibilities in burn wounds colonized by bacteria. The mean age was 16 years (range 1–70 years); 27 (55 %) of the study population were female and 22 (45%) were male. Twenty-four (49%) patients were epileptic. Open fire (41%) was the most common cause of burn injuries among epileptics while hot water burns (29%) were commonest among non-epileptics. Burn injury and percentage total burn surface area (% TBSA) injuries decreased with age, and the upper and lower limbs, trunk, head and neck were the most commonly affected sites. Staphylococcus aureus was the commonest isolate (23%), followed by Proteus mirabilis (22.7%), Streptococci spp (15.9%), Pseudomonas aeruginosa (4.5%) and 3.4% for Escherichia coli, Salmonella and Klebsiella spp. There was a significant trend of bacterial growth with increasing % TBSA (p<0.001). Bacterial growth was significantly more common in more recent burns of less than 20 days compared to burns of longer duration (OR 4.1 [95% CI 1.58–10.99]). Broad-spectrum antibiotics are required as first-line therapy for burns-related sepsis but there is need for surveillance of antibiotic susceptibility to help determine appropriate therapy.
Introduction
The skin forms a protective barrier against invasion by bacteria, fungi and viruses and any breach in this barrier provides easy access for microbial invasion. The burn wound is initially sterile; however, Gram-positive bacteria from hair follicles and sweat glands, which may survive thermal injury, colonize the wound within 48 hours of injury.1 Following the initial period of shock, sepsis is the major complication in burns2–4 and it has been estimated that about 75% of the mortality associated with burn injuries is related to sepsis especially in developing countries.1–5 In addition, overcrowding in Burns Units is an important cause of crossinfection6 which necessitates a regular monitoring of bacterial species and their antibiotic susceptibilities because significant shifts in these data may be correlated with changes in clinical management with respect to drug choice for therapy.7
A retrospective study at the QECH for the period 19941999 showed that S.aureus, P.aeruginosa and beta-haemolytic streptococci were the commonest isolates.8 There was a high level of non-susceptibility to commonly available antibiotics. Susceptibility to penicillin was 33% for S.aureus and 64% for beta-haemolytic streptococci while 53% of P.aeruginosa were susceptible to gentamicin. Since bacterial species may change over time9,10 due to cross-infections, overcrowding and changes in use and availability of antibiotic therapy, we conducted a prospective study to determine the current bacterial flora of burn wounds in the Burns Unit at the QECH.
Patients And Methods
This was a prospective study based in the Burns Unit of QECH and the Department of Microbiology, College of Medicine. The study was conducted over a 2-month period (June–August, 2003). Patients in the unit who gave consent at the time of study were enrolled. A brief history of age, sex, cause, duration at time of study, site, extent of the burn, depth of the burn and the use of antibiotics was taken. Some information was also extracted from patient files. Swab specimens were taken, Gram stained and cultured as previously described.8 Data were analyzed using the Excel Computer Package. Permission to carry out this study was granted by the College of Medicine Research and Ethics Committee (COMREC).
Results
Demographics
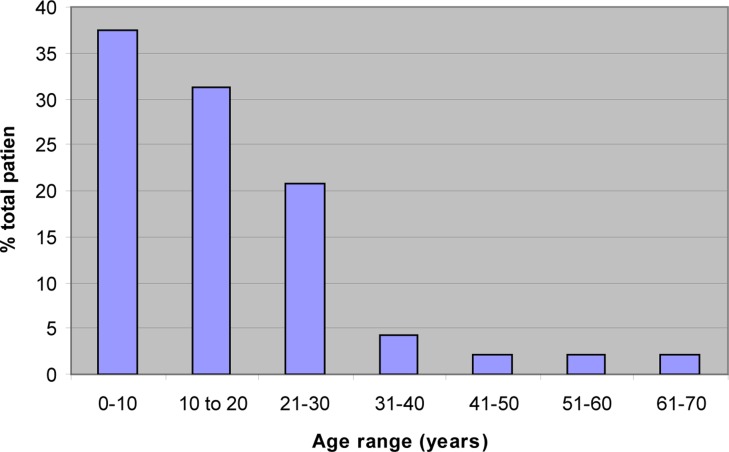
Within the study period, 58 patients were admitted to the Burns Unit. Of these 51 patients consented to participate but 2 were discharged before swabs could be taken. From the remaining 49 patients, 117 swabs were taken from various sites. Results for 109 swabs were available and 28 of these showed no growth. Figure 1 shows the age distribution of patients: mean age 16 years (range 1–70 years). Twenty-seven (55%) of the patients were female and 22 (45%) were male. Twenty-four patients (49%) were epileptic.
Figure 1.
Distribution of burn injuries by age
Characteristics of burns
Table 1 shows the distribution of burn injuries by total body surface area (TBSA) and relationship of TBSA with bacterial colonization. The table also shows a significant increase in the ratio of bacteria grown per swab in relation to the increasing TBSA % burned (Chi-squared for trend 12.9: p<0.001). There were no patients with superficial burn injuries. Seventeen (38%) had partial thickness burns, 20 (44%) had full thickness burns while 8 (18%) had burns of mixed depth. The common sites were the upper limb (32%), lower limb (26%), anterior trunk (19%) and head and neck (12%). The back (8%), buttocks (2%) and perineum (1%) were less commonly affected. Table 2 shows the duration of burn wound at the time of specimen collection. Proportion with bacterial colonization was significantly greater in those with burns of less than 20 days (OR 4.1 [1.58–10.99]; p<0.01)
Table 1.
Distribution of burn injuries by total body surface area and bacterial colonization
| TBSA % | Patients (n) |
Swabs taken |
Bacteria growth |
Bacterial growth per swab (%) |
| 1–5 | 18 | 33 | 17 | 52% |
| 6–10 % | 9 | 30 | 23 | 77% |
| 11–15 % | 10 | 31 | 27 | 87% |
| 15–20 % | 6 | 13 | 12 | 92% |
| 1–35 % | 1 | 2 | 2 | 100% |
| Total | 44 | 109 | 81 | 74 |
Table 2.
Duration of burn injury at time of specimen collection and bacterial colonization
| Duration of burn (days) |
Patients (n) |
Swabs taken |
Bacterial growths |
Bacterial growth/swab taken |
| 0–10 | 19 | 46 | 37 | 80 |
| 11–20 | 6 | 14 | 14 | 100 |
| 21.30 | 2 | 3 | 1 | 33 |
| 31–40 | 20 | 30 | 21 | 70 |
| 41–50 | 5 | 9 | 1 | 11 |
| >50 | 7 | 15 | 10 | 67 |
Bacterial isolates
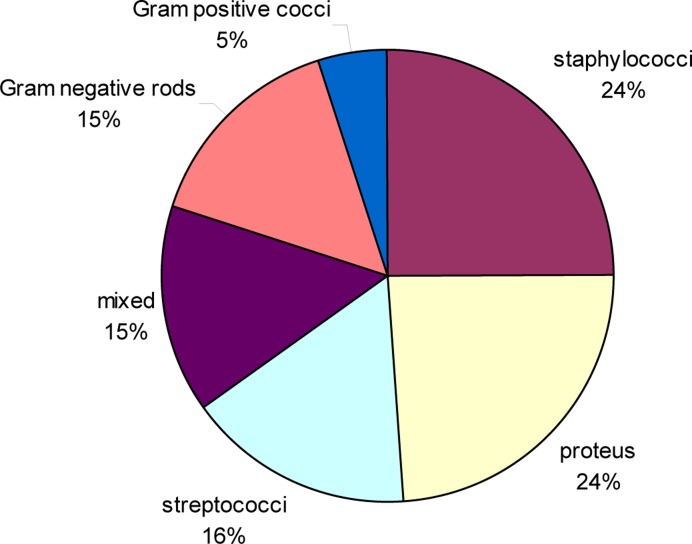
Figure 2 shows the range of bacteria isolated from burn wounds. S. aureus, P. mirabilis and streptococci were the commonest isolates. The other Gram negatives were P.aeruginosa (4.5%). Salmonella, E. coli and Klebsiella spp.
Figure 2.
Bacterial species in burn injuries
Discussion
The epidemiology of burns reported from this study is similar to that reported in an earlier and larger study of burns at QECH.11 Children and epileptics are at greatest risk of getting burn injuries in Malawi. The limbs (upper and lower) trunk, head and neck are more prone to burn injuries than other parts of the body although buttocks and perineum are more likely to grow bacteria due to their proximity to the anus. This predisposes to infection by Gram negative bacteria from the gastrointestinal and genitourinary tracts.
Staphylococcus aureus remains the commonest organism associated with burn injuries at the QECH consistent with the previous study at QECH.8 The second most common organism was Proteus in contrast to findings from the previous study and from studies elsewhere.6,8–13 Other studies have found that Pseudomonas and Streptococci are more common. To be able to determine those bacteria is extremely important before grafting is undertaken. We are not certain why Proteus was so common and Pseudomonas much less common than expected.
The study also showed an increasing rate of bacterial isolation with increasing total burn surface area. This has also been shown in other studies14,15 This is because an increasing TBSA increases the area left exposed, unprotected by epithelial covering. It also shows an increasing risk of colonization by microbes in partial thickness burns compared to full thickness burns. This may be due to the presence of epithelium in partial thickness burn wounds.
The changes in bacterial spectrum are not unexpected because of cross infections, resistance to drugs and introduction of new bacteria from other places. In the 1920's and 30's, streptococcal infections were the commonest but the incidence fell with the introduction of penicillin. Staphylococcus aureus was the next commonest organism but with introduction of topical antibiotics, fungi and viruses became more important.16–20 With increasing range of antibiotics available, resistant Gram negatives become more prevalent.
The resistance of organisms to antibiotics poses a challenge to burn care because it reduces effectiveness of treatment and may increase morbidity and mortality. We know from prior studies of bacterial sepsis at QECH that antibiotic resistance is common for pathogens that were common in this study.21 Penicillin resistance was 96% in 84 isolates of S.aureus and gentamicin resistance was 14% for S.aureus, 92% for 52 isolates of Group A streptococci and 32% for 341 isolates of enteric Gram negatives.21 It becomes even more challenging in a resource limited setting like Malawi where cultures and antibiotic sensitivities are not routinely done and the choice of antibiotics is limited.
Conclusions
Bacterial colonization is very common in burns at QECH making infection control measures extremely important. Regular surveillance of bacterial profile and their antibiotic susceptibilites should be encouraged to help guide first-line therapy for burns-related sepsis.
Acknowledgements
We gratefully acknowledge the technical assistance of Messrs N Chilewani and Dan Banda of the Department of Microbiology and Mr Chilingulo of the Burns Unit, QECH. The support of Prof E Borgstein and Dr E J van Hasselt of the department of Surgery is also appreciated. We thank College of Medicine for funding the project.
References
- 1.Vindenes H, Bjerknes R. Microbial colonization of large wounds. Burns. 1995;21:575–579. doi: 10.1016/0305-4179(95)00047-f. [DOI] [PubMed] [Google Scholar]
- 2.Yemul VL, Sengupta SR. Bacteriology of burns. Burns. 1980;7:190–193. [Google Scholar]
- 3.Signori M, Grappolini S, Magliano E, Donati I. Updated evaluation of the activity of antibiotics in a burn center. Burns. 1992;18:500–503. doi: 10.1016/0305-4179(92)90185-w. [DOI] [PubMed] [Google Scholar]
- 4.Manson WL, Peron PCJ, Fidler V, Sauer EW, Klase HJ. Colonization of burns and duration of hospital say of severely burnt patients. J Hosp Infect. 1992;22:55–63. doi: 10.1016/0195-6701(92)90130-e. [DOI] [PubMed] [Google Scholar]
- 5.Donati L, Scammazo F, Gervasoni M, Magliano A, Stankow B, Fraschini Infection and antibiotic therapy in 4000 burned patients in Milani Italy between 1976 and 1988. Burns. 1993;4:345–348. doi: 10.1016/0305-4179(93)90125-r. [DOI] [PubMed] [Google Scholar]
- 6.Gupta M, Gupta OK, Yadu Vanshi RK, Upadhyaya J. Burn epidemiology: the Pink City scene. Burns. 1993;19:47–51. doi: 10.1016/0305-4179(93)90100-m. [DOI] [PubMed] [Google Scholar]
- 7.Revathi G, Puri J, Jain BK. Bacteriology of burns. Burns. 1998;24:347–349. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 8.Komolafe OO, James J, Kalongolera L, Makoka M. Bacteriology of burns at the Queen Elizabeth Central Hospital, Blantyre, Malawi. Burns. 2003;1568:1–4. doi: 10.1016/s0305-4179(02)00273-5. [DOI] [PubMed] [Google Scholar]
- 9.Bowser-Wallace BH, Graves DB, Caldwell FT. An epidemiological profile and trend analysis of wound flora in burned children: 7 years' experience. Burns Incl Therm Inj. 1984;11:16–25. doi: 10.1016/0305-4179(84)90156-6. [DOI] [PubMed] [Google Scholar]
- 10.Panit DV, Gore MA, Saileshwar N, Deodhar LP. Laboratory data from the surveillance of a burns ward for the detection of hospital infection. Burns. 1993;19:52–55. doi: 10.1016/0305-4179(93)90101-d. [DOI] [PubMed] [Google Scholar]
- 11.Komolafe OO, James J, Kalongolera L, Makoka M. Epidemiology and mortality of burns at the Queen Elizabeth Central Hospital, Blantyre, Malawi. Cent Afr Med J. 2002;49:130–134. [PubMed] [Google Scholar]
- 12.Atoyebi OA, Sowemimo GOA, Odugbemi T. Bacterial flora of burn wounds in Lagos, Nigeria: a prospective study. Burns. 1992;18:448–451. doi: 10.1016/0305-4179(92)90175-t. [DOI] [PubMed] [Google Scholar]
- 13.Taylor GD, Kibsey P, Kirkland T, Burroghs E, Tredget E. Predominance of Staphylococcal organisms in infections occurring in a burns intensive care unit. Burns. 1992;18:332–335. doi: 10.1016/0305-4179(92)90158-q. [DOI] [PubMed] [Google Scholar]
- 14.Reig A, Tegerina C, Codina J, Miradet V. Infections in burn patients. Ann MBC. 5(2) [Google Scholar]
- 15.McManus AT, Mason AD, McManus WF. A decade of reduced gram-negative infections and mortality associated with improved isolation of burned patients. Arch Surg. 1994;129:1306–1309. doi: 10.1001/archsurg.1994.01420360096013. [DOI] [PubMed] [Google Scholar]
- 16.Aldrich RH. The role of infections in burns: the theory and treatment with special reference to gentian violet. New Engl J Med. 1933;208:299. [Google Scholar]
- 17.Cruickshank R. The bacterial infections of burns. J Path Bacteriol. 1935;31:376. [Google Scholar]
- 18.Moncrief JA, Teplitz C. Changing concepts in burn sepsis. J Trauma. 1964;4:233. doi: 10.1097/00005373-196403000-00011. [DOI] [PubMed] [Google Scholar]
- 19.McManus AT, Mason AD, McManus WF, Pruitt BA. 25-year review of Pseudomonas bacteraemia in a burn center. Eur J Clin Micro. 1985;4:219–223. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- 20.McMillan BG. The problem of infections in burns. In: Mummel RP, editor. Clinical burn therapy. Bristol: Wright; 1982. p. 335. [Google Scholar]
- 21.Phiri A, Milledge J, Calis JCJ, et al. Aetiology of neonatal sepsis at QECH, Blantyre: 1996–2001. Malawi Med J. 2005;17:92–96. [Google Scholar]