Abstract
Although synaptic plasticity is believed to comprise the cellular substrate for learning and memory, limited direct evidence exists that hippocampus-dependent learning actually triggers synaptic plasticity. It is likely, however, that long-term potentiation (LTP) works in concert with its counterpart, long-term depression (LTD) in the creation of spatial memory. It has been reported in rats that weak synaptic plasticity is facilitated into persistent plasticity if afferent stimulation is coupled with a novel spatial learning event. It is not known if this phenomenon also occurs in other species. We recorded from the hippocampal CA1 of freely behaving mice and observed that novel spatial learning triggers endogenous LTD. Specifically, we observed that LTD is enabled when test-pulse afferent stimulation is given during the learning of object constellations or during a spatial object recognition task. Intriguingly, LTP is significantly impaired by the same tasks, suggesting that LTD is the main cellular substrate for this type of learning. These data indicate that learning-facilitated plasticity is not exclusive to rats and that spatial learning leads to endogenous LTD in the hippocampus, suggesting an important role for this type of synaptic plasticity in the creation of hippocampus-dependent memory.
Keywords: behavior, hippocampus, in vivo, learning, LTD, LTP, synaptic plasticity
Introduction
Bidirectional synaptic plasticity, in the form of synaptic potentiation and depression, has been proposed as the mechanism that underlies learning and memory formation (Bear 1996; Martin et al. 2000; Malenka and Bear 2004). Whilst a number of reports have shown changes in synaptic plasticity arising from motor learning (Rioult-Pedotti et al. 1998, 2000) and fear memory acquisition (Whitlock et al. 2006), slow progress has been made in establishing a direct link between learning and synaptic plasticity in the hippocampus. One approach to address this is through real-time electrophysiological recordings from synapses in task-performing animals. Of particular interest is the hippocampus, which is known to be critically involved in the formation of declarative memories in humans (Eichenbaum 2001). In adult rats, the coupling of afferent stimulation either as test-pulses or weak synaptic plasticity protocols, with specific spatial learning events facilitates the expression of persistent long-term potentiation (LTP) or long-term depression (LTD) (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004, 2007, 2008a). Learning-facilitated plasticity of this nature is not noticeably influenced through generalized behavioral activity as passive visual spatial novelty without active exploration elicits similar synaptic changes (Kemp and Manahan-Vaughan 2011). Furthermore, fear-context learning elicits changes in synaptic strength that share cellular mechanisms with electrically induced LTP (Whitlock et al. 2006). Taken together, these observations support a tight association between synaptic plasticity and hippocampus-dependent learning.
Hippocampus-dependent organization of stored information in the form of memory contains both spatial (Morris et al. 1982; Eichenbaum 1999a) as well as nonspatial components (Maren et al. 1997; McEchron et al. 1998). Declarative memory refers to assimilated information concerning facts and events in relation to previously encountered elements. Both spatial and recognition memory are forms of declarative memory in humans. Whilst behavioral paradigms designed to test for spatial memory question a subject’s ability to remember a location, the recognition test probes the capacity to identify a previously encountered item. Object recognition (OR) is therefore a crucial part of declarative memory and has arguably both spatial and nonspatial elements. This concept is in line with the behavioral observations by others who suggest that the hippocampus not only serves as a cognitive map for spatial (O'Keefe and Nadel 1978) but also for object memory through the incorporation of converging spatial and nonspatial inputs (Manns and Eichenbaum 2006, 2009). The OR task was originally construed to test preverbal infants (Fagan 1990). In humans, the importance of an intact OR ability can be seen from the debilitating effects of the failure to distinguish between familiar and new objects in neurological disorders such as Parkinson’s and Alzheimer’s disease and visual agnosia in brain trauma patients (Farah 1992; Grady et al. 2001; Laatu et al. 2004). In rodents, OR memory can be tested and quantified by capitalizing on their innate preference to explore newer objects in comparison with previously encountered items (Clark and Martin 2005). One could refer to the task in this context as episodic-like, bearing in mind that declarative memory is believed to encompass conscious awareness (Eichenbaum 1999b) and acknowledging the difficulty of precisely ascertaining whether rodents engage in episodic memory formation (Suzuki 2003; Zentall 2006). To date, studies have yielded conflicting results in terms of whether OR requires the hippocampus. Whereas some lesion studies in humans and animals have suggested that the hippocampus is irrelevant for OR (Murray and Mishkin 1998; Baxter and Murray 2001; Mumby 2001), others have provided evidence for a significant role of the hippocampus through lesion and pharmacological studies (Reed and Squire 1997; Beason-Held et al. 1999; Clark et al. 2000; Zola et al. 2000; Broadbent et al. 2004). Recently, however, it has become apparent that both spatial and “temporal order” aspects of OR are likely to be strongly hippocampus dependent (Fortin et al. 2002; Manns and Eichenbaum 2006, 2009; Manns et al. 2007; Farovik et al. 2010).
Our study had 2 main goals. On the one hand, we addressed if learning-facilitated plasticity occurs in the hippocampus of the freely behaving mouse. We feel this question is of fundamental importance in clarifying whether this phenomenon is a common property of synaptic information encoding shared by different species or is a unique rat-specific phenomenon (Kemp and Manahan-Vaughan 2007). On the other hand, we explored the relationship between spatial OR memory and the expression of persistent synaptic plasticity in the hippocampus. Here, our goal was to investigate whether synaptic plasticity serves as a cellular substrate for the encoding of spatial OR and if so, which form of synaptic plasticity may be responsible.
Materials and Methods
The present study was carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) for care of laboratory animals and after approval of the local government ethics committee. All efforts were made to minimize the number of animals used.
Surgery
Male C57BL/6 mice (7–8 weeks, Charles River, Germany) were anesthetized (sodium pentobarbital 60 mg/kg, intraperitoneal) and underwent stereotaxic chronic implantation of bipolar stimulating electrodes in the right Schaffer collateral pathway of the dorsal hippocampus (anterioposterior (AP): −2.0 mm; mediolateral (ML): 2.0 mm from bregma; dorsoventral (DV): ∼1.4 mm from brain surface) and monopolar recording electrodes in the right ipsilateral CA1 stratum radiatum (AP: −1.9; ML: 1.4; DV: ∼1.2) to monitor the evoked potentials at the Schaffer Collateral—CA1 synapses. The stimulating and recording electrodes were made of polyurethane-coated stainless steel wire (100 μm diameter; Gündel, BioMedical Intruments, Germany) and were lowered into the brain through a hole drilled on the skull. On the contralateral side, 2 holes were drilled on the skull into which anchor screws were inserted. The anchor screws were attached to stainless steel wires (A-M Systems, Washington, USA) that served as reference and ground electrodes. The 5 wires were secured on a 6-pin socket (Conrad Electronic SE, Germany), and the whole assembly was stabilized on the skull using dental cement. Test-pulse recordings during surgery aided depth adjustment of the electrodes, which was later verified by postmortem histology. After surgery, mice were housed individually and given at least 7 days recovery time before experiments began. Electrophysiological recordings and behavioral paradigms were performed in 20 (L) × 20 (W) × 30 (H) cm topless recording chambers wherein the mice could move freely and had access to food and water ad libitum. Animals were transferred in their cages into the experiment room 1 day before the start of experiments to ensure adequate acclimatization to the gross environment.
Measurement of Evoked Potentials
Each mouse had its socket connected via swivel connector to the recording/stimulation system wires suspended above the recording chamber to enable monitoring of evoked potentials while the animal freely behaved and performed tasks. The field excitatory postsynaptic potential (fEPSP) was employed as a measure of excitatory synaptic transmission in the CA1 region. To obtain these measurements, an evoked response was generated in the stratum radiatum by stimulating the Schaffer collateral at low frequency (0.025 Hz) with single biphasic square waves of 0.2 ms duration per half-wave, generated by a constant current isolation unit (World Precision Instruments, Florida, USA). The fEPSP signal was amplified using a differential AC amplifier (A-M Systems) and digitalized through a data acquisition unit (Cambridge Electronic Design, UK). For each time point measured during the experiments, 5 consecutively evoked fEPSP responses at 40 s intervals were averaged. The first 6 time-points, which were recorded at 5-min interval, were averaged, and all time points were expressed as a mean percentage (±standard error of the mean [SEM]) of this value. Plasticity-inducing electrical stimulation, together with the relevant behavioral task (when appropriate) was applied immediately after the sixth time point, and synaptic transmission was recorded for another 4 h (240 min). A further 1 h recording was performed the next day, roughly 24 h after the experiment began to determine the degree of persistency of any changes in synaptic transmission. fEPSP was quantified by measuring the slope obtained on the first negative deflection of the evoked potential. By means of an input–output curve determination conducted before every experiment, the largest obtainable fEPSP was found for each individual animal (maximum intensity used 125 μA). The intensity that elicited 40% of the maximum fEPSP was used for recordings. Electroencephalography (EEG) activity was monitored throughout the course of the experiment for the occurrence of seizure activity. No behavioral signs or EEG activity indicating seizures were observed.
Electrical Induction of Synaptic Plasticity
All animals were first tested in a “baseline” experiment without any external behavioral stimuli to ensure that the recordings were stable. LTP was electrically induced by using 100 Hz high-frequency stimulation (HFS; 50 stimuli). Test-pulse stimulation (0.025 Hz, 2 trains of 5 stimuli, 5-min intertrain interval) refers to 2 sets of test-pulse recordings. The terms “electrically induced” plasticity and “learning-facilitated” plasticity are used to distinguish between synaptic plasticity that is induced solely through electrical stimulation and plasticity that is strengthened by the concurrency of the electrical stimulation and the behavioral task.
OR Protocol
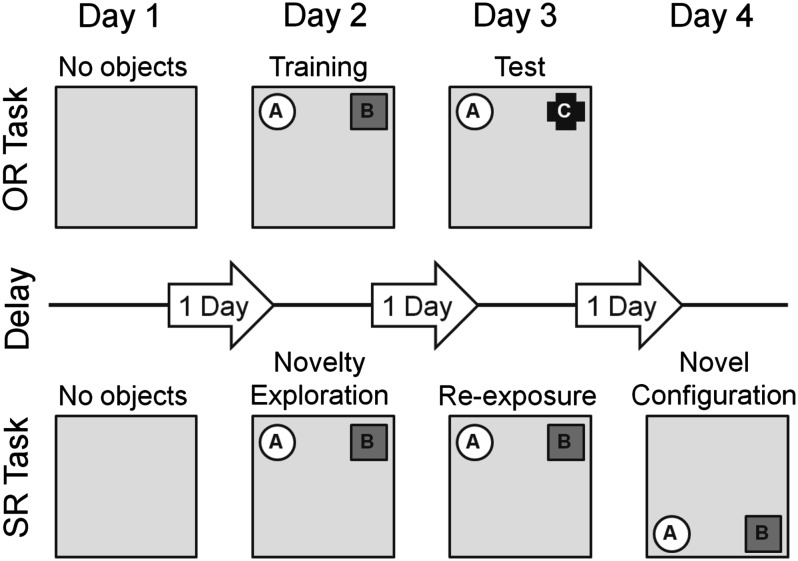
The OR experiments were typically run for 3 consecutive days with the same animals (Fig. 1). An electrical stimulation-only experiment was performed as a control of the synaptic plasticity profile without OR on day 1. This also served as a habituation phase to the recording chamber directly preceding the OR task. Two novel objects (i.e., A and B) were then presented on day 2 (training phase) and one familiar and one novel object (i.e., A and C) was presented 24 h later on day 3 (test phase) to test for OR memory. The presentation of objects lasted for 10 min, where the animals were left to explore freely, and were removed from the recording chamber after the presentation. The presentation of objects always coincided with the start of the electrical stimulation (i.e., HFS, test-pulse stimulation). Recordings were made during all phases of the behavioral training and testing. The role of the familiar and novel objects, as well as their relative positions, were randomly assigned for each animal. The objects and the recording chambers were cleaned thoroughly between task trials to ensure the absence of olfactory cues. The objects were distinctly different from one another and heavy so that they could not be moved by the mice. Several copies of each object were present.
Figure 1.
Schematic summary of the experimental designs for the OR task and SR task. On day 1 in both tasks, animals were given either test-pulse stimulation or HFS in the absence of any objects in the recording chamber to obtain the synaptic transmission profile of electrically induced plasticity without the tasks. After day 1, the tasks were presented simultaneously with test-pulse stimulation or HFS. In the OR task, the mice were exposed to 2 novel objects on day 2 (training phase) and to 1 familiar and 1 novel object on day 3 (test phase) at the same locations in the chamber to test for OR. Mice in the spatial object recognition (SR) task were exposed to 2 novel objects on day 2 (novelty exploration), then reexposed to the same objects at the same positions on day 3 (reexposure), and on day 4 (novel configuration), the same objects were repositioned to the other half of the contextually polarized chamber to test for SR of the objects. The tasks after day 1 were given simultaneously together with either test-pulse stimulation or HFS. The labels above the chambers indicate the different phases of the OR and SR tasks. Gray arrows represent the delay period between the individual phases of the tasks.
Spatial Recognition Protocol
In the spatial recognition (SR) experiments, the novelty of the object position in space, rather than the object itself, was examined (Fig. 1). The recording chambers were completely (3 walls and the base) gray in color except for the front wall, which was white. This served to polarize the environment and provide a gross contextual feature to the recording chambers. The SR experiments were performed 4 days in a row with the same animals. A baseline experiment without the SR task was run on the day 1 which also served to habituate the mice. On day 2 (novelty exploration), 2 objects (i.e., A and B) were presented against the gray back wall similar to the OR training. The mice were then reexposed to the objects in the same locations on the day 3 (reexposure), and on day 4 (novel configuration), the objects were shifted in a parallel fashion to the white front wall to test for object–space recognition memory. The objects were always presented for 10 min simultaneously with the test-pulse stimulation and removed from the recording chamber after the presentation.
Data Analysis
A separate group of different animals was used in each set of experiments to obtain the electrophysiological and corresponding behavioral data (i.e., the animals used to obtain data portrayed in Figs. 2–4 come from separate sets of animals, respectively). To analyze the electrophysiological data between groups, a 2-way analysis of variance (ANOVA) with repeated measures was applied. The fEPSPs from the period after electrical stimulation with or without the behavioral paradigm to the end of the experiment was compared between groups in order to assess the statistical difference in any change of synaptic strength as a consequential effect. The significance level was set at P < 0.05.
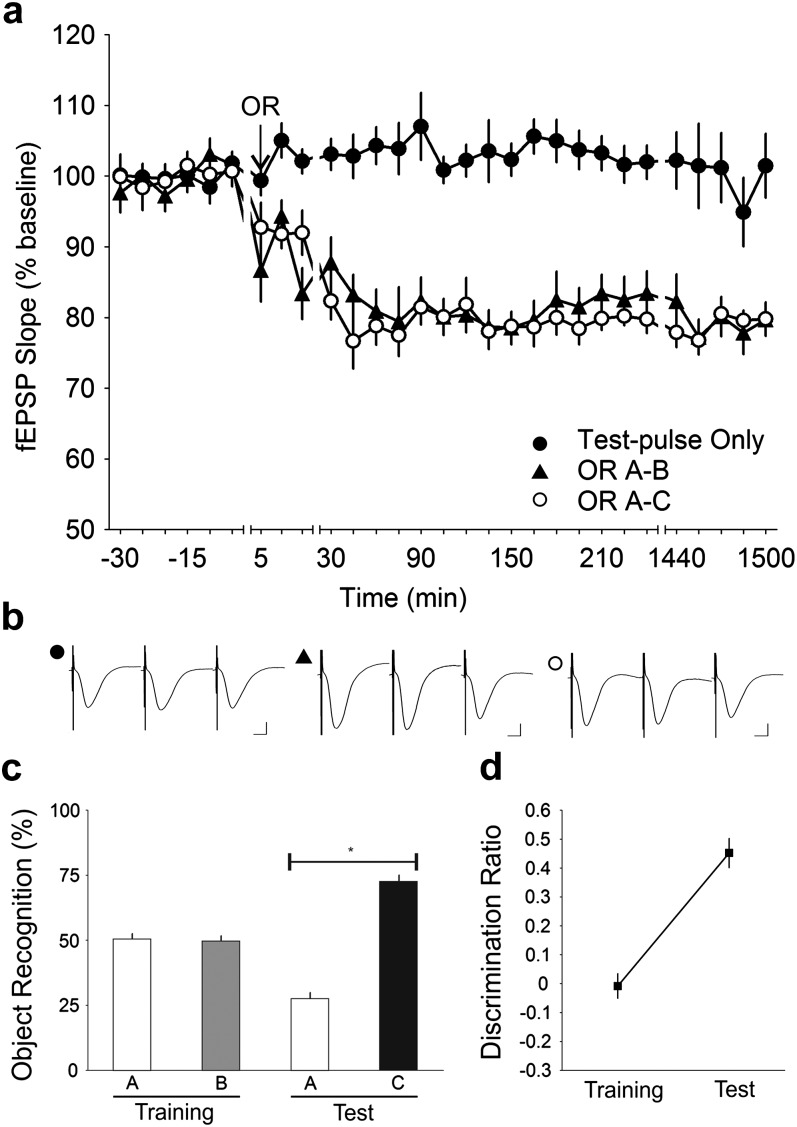
Figure 2.
OR training and test cause an intrinsic decrease in synaptic strength. (a) Stable baseline recording was obtained when only test-pulses were applied to the Schaffer collateral—CA1 stratum radiatum synapses in the freely moving mice (n = 10). When test-pulse stimulation was coupled with OR training (OR A–B), a progressive decrease in synaptic strength that stabilized at ∼45 min and lasted for at least 24 h was observed. A similar trend was also observed during the test phase (OR A–C). The decrease in the training and the test phases were both significant compared with test-pulse only (ANOVA, P < 0.0001). (b) Analog traces illustrate the Schaffer collateral—CA1 field potentials at pretask, 5 min and 24 h (1440 min). Vertical scale bar corresponds to 2 mV and horizontal scale bar corresponds to 5 ms. (c) Behavioral analysis showed that the mice performed the OR task successfully with a delay period of 24 h between phases. The mice explored both objects A and B equally during the training phase but explored the novel object C to a significantly greater extent during the test phase. *t-test, P < 0.0001. (d) Analysis of the discrimination ratio revealed that the mice showed no preference for either object during the training phase but a strong preference for the novel object during the test phase (ANOVA, P < 0.0001).
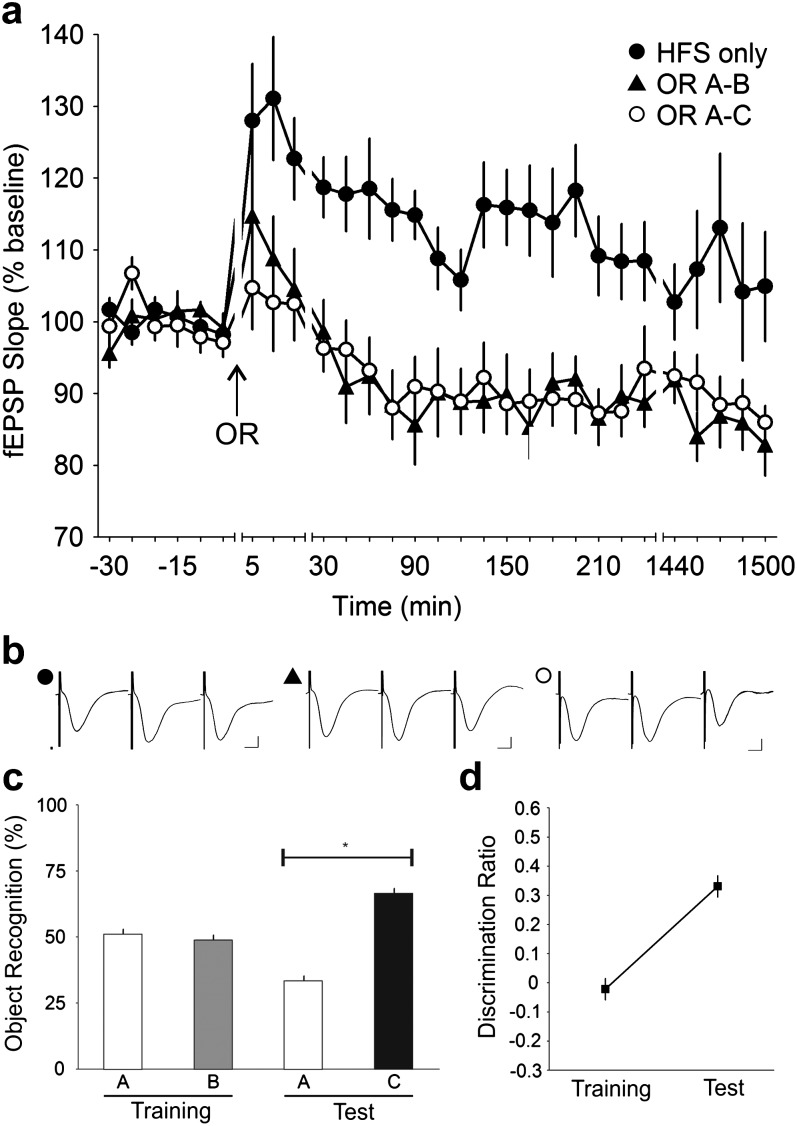
Figure 3.
OR training and test impairs LTP in the CA1. (a) Application of 100 Hz HFS to the Schaffer collateral—CA1 stratum radiatum synapses in freely moving mice led to LTP (n = 10). When HFS was given simultaneously with OR training (OR A–B) and test (OR A–C), the potentiation was completely abolished (ANOVA, P < 0.0001) in both phases. The inhibition of LTP fell below basal levels and persisted for more than 24 h (1440 min). (b) Analog traces illustrate the Schaffer collateral—CA1 field potentials at pretask, 5 min and 24 h (1440 min). Vertical scale bar corresponds to 2 mV and horizontal scale bar corresponds to 5 ms. (c) Behavioral analysis showed that the mice performed the OR task successfully when given concurrently with HFS. The mice explored both objects A and B equally during the training phase but explored the novel object C to a significantly greater extent during the test phase. *t-test, P < 0.0001. (d) Analysis of the discrimination ratio revealed that the mice showed no preference for either object during the training phase but a strong preference for the novel object during the test phase (ANOVA, P < 0.0001).
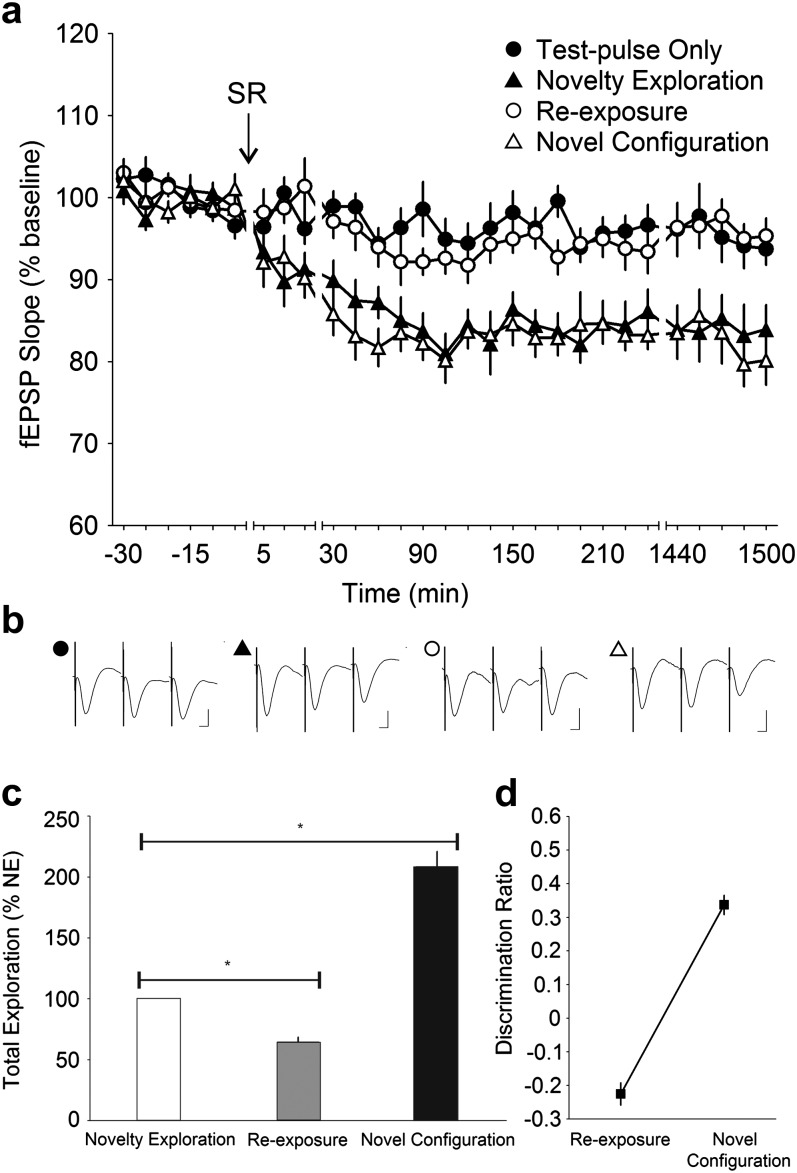
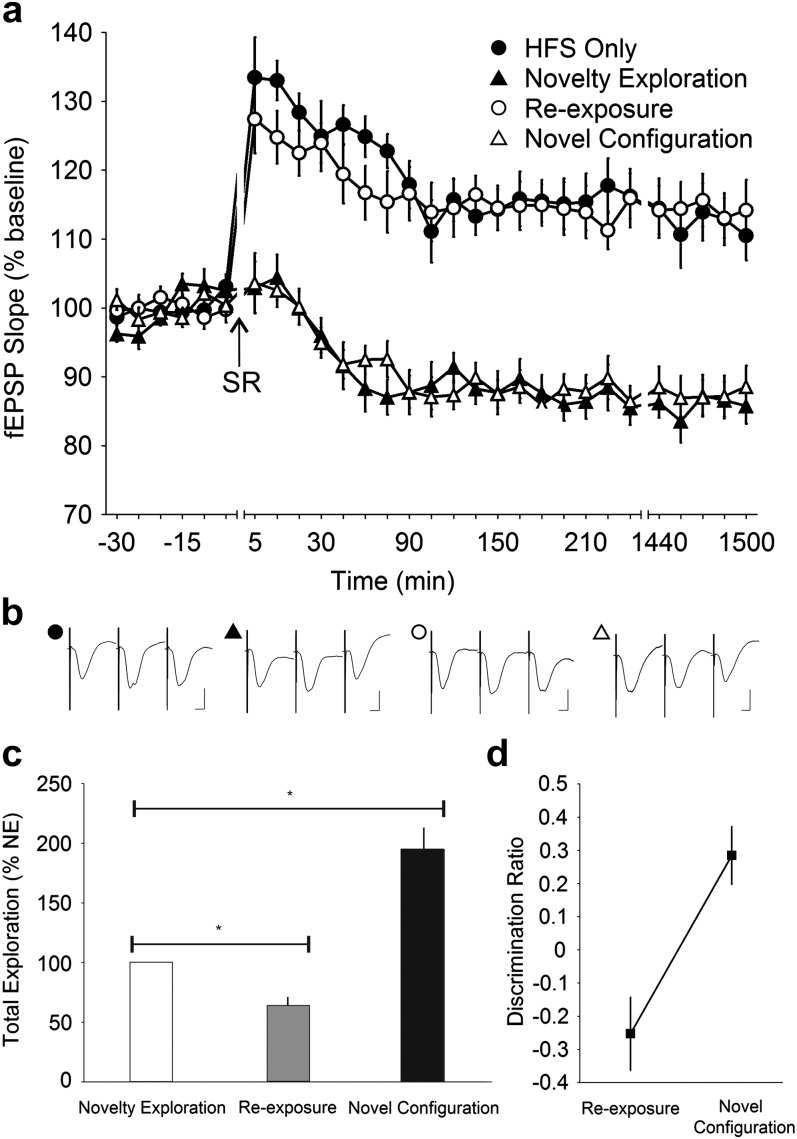
Figure 4.
Object–space novelty during SR and not OR per se determines SC—CA1 synaptic depression. (a) Stable baseline recording was obtained when only test-pulses were applied to the Schaffer collateral—CA1 stratum radiatum synapses in the freely moving mice (n = 14). When test-pulse stimulation was paired with spatial object recognition (SR), a progressive decrease in synaptic strength that plateaued and lasted for at least 24 h (1440 min) was observed (ANOVA, P < 0.0001). No decrease in synaptic strength was observed when the mice were reexposed to the same objects at the same locations the next day. When a novel spatial configuration of the same objects was presented, however, the synaptic depression reemerged (ANOVA, P < 0.0001). (b) Analog traces illustrate the Schaffer collateral—CA1 field potentials at pretask, 5 min and 24 h (1440 min). Vertical scale bar corresponds to 2 mV and horizontal scale bar corresponds to 5 ms. (c) Behavioral analysis showed that the mice explored the objects significantly less during reexposure at the old positions but explored to a much greater extent when the objects were arranged in a novel configuration. Reexposure and novel configuration data are expressed as a percentage of novelty exploration. *t-test, P < 0.0001. (d) Analysis of the discrimination ratio revealed that the mice showed a decreased preference when the objects where presented at the familiar locations during reexposure and a strong increment in preference when the objects were relocated during novel configuration (ANOVA, P < 0.0001).
Behavioral data for the OR and SR experiments were recorded from cameras positioned above the chambers and digitally stored. Exploration of the objects was then analyzed post-hoc using the within-object area scoring system which was defined as sniffing of the object (with nose contact or head directed to the object) within ∼2 cm radius of the object. (Bevins and Besheer 2006) Standing, sitting, or leaning on the object was not scored as object exploration. OR data were expressed as a percentage of the total exploration time for each object per experiment, and the SR data were expressed as a percentage of the total exploration time on SR novelty exploration (Clarke et al. 2010). The results across animals were expressed in terms of mean ± SEM. The data were then statistically assessed using the Student’s t-test by comparing group means with the fixed value of 50%, which represents no differentiation between objects. The significance level was set at P < 0.05 (Clarke et al. 2010).
In addition, task performance was measured by calculating the discrimination ratio. In the OR task, this referred to the proportion of total exploration time spent exploring the novel object (i.e., the difference between the time spent exploring the novel and the familiar object divided by the total time spent exploring both objects). In the SR task, this referred to the proportion of exploration time spent exploring objects in the familiar location during reexposure or objects in the new location during novel configuration (i.e., the difference between the time spent exploring the familiar or new location and novelty exploration divided by the total time spent exploring the familiar or new location and novelty exploration). This measure takes into account individual differences in the total amount of exploration time. A discrimination ratio of zero represents no preference for either object in OR or location in SR, a positive ration indicates preference for the novel object or novel location and a negative ratio reflects a preference for the familiar object or location. The data were assessed using ANOVA with the significance level set at P < 0.05.
Results
OR Results in Persistent Synaptic Depression
In the past, we have reported that the coupling of electrical stimulus that usually leads to weak or no plasticity, with a spatial learning event leads to robust long-lasting plasticity in the adult rat (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004, 2007, 2008a). We therefore explored whether OR facilitates synaptic plasticity in the adult mouse.
In this study, the training phase consisted of the exposure to 2 novel objects. During the test phase 24 h later, one familiar and one novel object were presented (Fig. 1). We found that during the training phase, the mice explored both objects equally (A: 50.41 ± 2.15%, B: 49.59 ± 2.15%; t-test, P = 0.7916; n = 10), whereas during the test-phase, the mice explored the novel object to a significantly larger extent in comparison with the familiar object (A: 27.42 ± 2.52%, C: 72.58 ± 2.52%) in the test phase (t-test, P < 0.0001; n = 10) (Fig. 2c). Given that 24 h intervened between the training and test phases, this was a clear behavioral indication that the mice successfully formed a memory of the objects during the training phase. Moreover, the discrimination ratio revealed that the mice had a significantly greater preference (ANOVA, F1,9 = 146.74, P < 0.0001) for the novel object during the test phase (Fig. 2d).
Test-pulse stimulation given to the Schaffer collaterals resulted in fEPSPs that were highly stable during the 25 h recording period. When the animals engaged in OR during test-pulse stimulation, immediately after cessation of the task, fEPSPs became persistently depressed (n = 10). Notably, significant synaptic depression was observed after both the training phase (ANOVA, F1,414 = 450.02, P < 0.0001) and test phase (ANOVA, F1,414 = 604.66, P < 0.0001) (Fig. 2a). The reduction in synaptic transmission was stable and persisted for at least 25 h in the training (79.68 ± 1.93% at t = 1500 min) and test (79.78 ± 2.36% at t = 1500 min) phases.
OR Impairs LTP
If the performance of the OR task relied on an intrinsic decrease in Schaffer collateral – CA1 synaptic efficacy (Fig. 2a), it could be hypothesized that the threshold needed to induce LTP at these synapses would be negatively affected by OR. We investigated this possibility in a study (Fig. 3a) where HFS was applied whilst the mice performed the OR task. HFS alone induced a strong initial potentiation that slowly returned to near baseline values by the next day. When the OR task was paired with HFS, a significant impairment of the potentiation was seen: the initial potentiation (t = 5 min) was reduced in both training and test phases compared with test-pulse only controls (ANOVA, F1,411 = 176.55, P < 0.0001 and F1,408 = 179.99, P < 0.0001, respectively; n = 10). The impairment was not significantly different between the 2 phases (ANOVA, F1,411 = 0.10122, P = 0.7505; n = 10). Successful acquisition of the OR task was evident from the significantly greater exploration of the novel object (A: 33.47 ± 1.86%, C: 66.53 ± 1.86%) in the test phase (t-test, P < 0.0001; n = 10) compared with the equal exploration of both objects (A: 51.11 ± 1.86%, B: 48.89 ± 1.86%) during training (t-test, P = 0.4107; n = 10) (Fig. 3c). This observation was furthered by the strong increment (ANOVA, F1,9 = 24.956, P < 0.001) in the discrimination ratio during the test phase which indicated a strong preference for the novel object (Fig. 3d).
Novel Object–Space Learning Underlies Facilitation of Synaptic Depression by OR
We were puzzled by the observation that synaptic depression was facilitated both during the training and test phases of OR. We felt at first that this result was rather counterintuitive, since one would expect the OR process in the test phase to elicit a synaptic change different from when recognition was not involved (i.e., training phase).
The hippocampus has been proposed to construct a cognitive map that mediates the representation of physical space (O'Keefe and Nadel 1978; Moser et al. 2008), and its circuitry is believed to be specifically tuned for novelty detection (Lisman and Otmakhova 2001; Vinogradova 2001). Given this, we hypothesized that synaptic changes observed in OR were elicited by the configurative novelty of object–space rather than object novelty per se. This implies that object and space are recognized as a unit rather than separately and that changes arising in any component of this representation would sufficiently constitute spatial novelty. The decrease in synaptic strength during OR training and testing would then have derived from the detection of the new spatial configuration of novel objects (i.e., novel object–space pairing) in both phases, even though initially behaviorally quantified as the mere recognition of the novel object.
To test whether the spatial novelty of the object alone (i.e., no novel object, just novel position) would evoke the same synaptic changes as described in Figure 2, we simply shifted the positions of the objects within the recording chamber in the SR task (see protocol described in Fig. 1). Here, we found a distinct decrease in CA1 synaptic strength similar to that observed in OR, but always only when the objects were spatially relocated (ANOVA, F1,586 = 205.45, P < 0.0001; n = 14) (Fig. 4a). Reexposure of the same objects in the now familiar location resulted in no change in synaptic strength (ANOVA, F1,592 = 3.3969, P = 0.06582; n = 14) compared with test-pulse only values. When the same objects were shifted anew to a novel configuration, a significant persistent reduction in synaptic efficacy was once more observed (ANOVA, F1,593 = 223.48, P < 0.0001; n = 14), which was not significantly different from the change observed during novelty exploration (ANOVA, F1,587 = 2.5229, P = 0.11274; n = 14). This indicated that spatial novelty alone in object–space pairings is necessary and sufficient to evoke CA1 synaptic depression.
Our behavioral data were congruent with the electrophysiological results. When compared with novelty exploration, the total amount of exploration toward both objects was greatly reduced during reexposure (64.56 ± 4.02%; t-test, P < 0.0001; n = 14) but was significantly increased when the objects were spatially rearranged (208.54 ± 12.35%; t-test, P < 0.0001; n = 14) (Fig. 4c). Furthermore, the discrimination ratio showed that the mice had a reduced preference for objects at the familiar locations and a significant increment in preference (ANOVA, F1,13 = 154.03, P < 0.0001) when the objects were shifted to a novel location (Fig. 4d). Evidently, the mice were not only capable of recognizing novel objects but also learned the positions of the objects, whereby the novel locations demanded greater exploratory attention compared with the familiar locations. Whereas recognition of a novel spatial configuration resulted in a corresponding decrease in synaptic efficacy at the Schaffer collateral—CA1 synapse, the realization that objects were not spatially relocated required no such synaptic change.
Novel Object–Space Learning Impairs LTP
To further examine if equal mechanisms underlie the synaptic depression elicited after exploring a new object in OR and a novel location in SR, the SR task was executed during HFS to examine if the inhibition of HFS-induced LTP which occurred during OR is also observed in SR. When HFS was paired with SR novelty exploration, a significant impairment of the LTP was observed (ANOVA, F1,684 = 846.32, P < 0.0001; n = 16) (Fig. 5a). Reexposing the mice to the objects together with HFS in their familiar location did not evoke any impairment but resulted in a synaptic potentiation not dissimilar to that elicited with HFS alone (ANOVA, F1,683 = 2.6946, P = 0.10115). Relocating the objects to a novel configuration again resulted in a significant abolishment (ANOVA, F1,681 = 856.84, P < 0.0001) of the HFS-induced LTP, which had a similar profile to that which was elicited during novelty exploration (ANOVA, F1,687 = 0.73195, P = 0.39255). The electrophysiological data thus indicates that similar cellular processes underlie the synaptic changes that occur during both the OR and the SR tasks.
Figure 5.
Object–space novelty during SR impairs LTP in the CA1 (a) Application of 100 Hz HFS to the Schaffer collateral—CA1 stratum radiatum synapses in freely moving mice led to LTP (n = 16). When HFS stimulation was paired with spatial object recognition (SR), the potentiation was completely abolished and fell below basal levels and then persisted for more than 24 h (1440 min; ANOVA, P < 0.0001). No inhibition in synaptic potentiation was observed when the mice were reexposed to the same objects at the same locations the next day together with HFS. When a novel spatial configuration of the same objects was presented with HFS, however, the abolishment of synaptic potentiation reemerged (ANOVA, P < 0.0001). (b) Analog traces illustrate the Schaffer collateral—CA1 field potentials at pretask, 5 min and 24 h (1440 min). Vertical scale bar corresponds to 2 mV and horizontal scale bar corresponds to 5 ms. (c) Behavioral analysis showed that the mice explored the objects significantly less during reexposure at the old positions but explored to a much greater extent when the objects were arranged in a novel configuration. Reexposure and novel configuration data are expressed as a percentage of novelty exploration. *t-test, P < 0.0001. (d) Analysis of the discrimination ratio revealed that the mice showed a decreased preference when the objects where presented at the familiar locations during reexposure and a strong increment in preference when the objects were relocated in a novel location (ANOVA, P < 0.0001).
Behaviorally, the data showed that the mice explored the objects in the familiar location to a significantly reduced extent during reexposure (63.81 ± 6.97%; t-test, P < 0.0001; n = 16) when compared with novelty exploration (Fig. 5c). During novel configuration, the mice explored to newly located objects to a significantly greater degree (194.69 ± 18.10%; t-test, P < 0.0001). In addition, analysis of the discrimination ratio revealed that the mice exhibited a stronger preference for the objects in the novel configuration (ANOVA, F1,16 = 77.010, P < 0.0001) compared with when they were familiar (Fig. 5d).
Discussion
In this study, we show for the first time that intrinsic and endogenous synaptic depression occurs during OR in the mouse hippocampus. We report that OR involves a spatial component that elicits changes in synaptic strength in the hippocampus and results in LTD. Hippocampal LTP is prevented by OR. Furthermore, our data support that learning-facilitated LTD may be a common property shared across multiple species that is used for synaptic information storage in association with hippocampus-dependent learning events.
OR is believed to be processed by the perirhinal cortex (Mumby and Pinel 1994; Murray and Mishkin 1998; Baxter and Murray 2001; Mumby 2001; Winters et al. 2004). This has been demonstrated, for example, through studies where the presentation of objects without a distinct context facilitates the expression of the immediate early gene cFos in the perirhinal cortex but not in the hippocampus (Zhu et al. 1995, 1996). However, in the environment where objects are presented, a spatial component is always present in the OR task, as each object has a metric coordinate. Our data support that the hippocampus encodes this spatial component of OR: each time an object pair is presented synaptic depression occurs, the depression only fails if familiar objects are presented in familiar locations. This finding may help clarify the controversy in the scientific literature. Studies in humans, nonhuman primates, and other mammals have reported that the hippocampus is irrelevant for OR (Murray and Mishkin 1998; Baxter and Murray 2001; Mumby 2001), whereas others have found significant effects of hippocampal lesions on recognition memory (Reed and Squire 1997; Beason-Held et al. 1999; Clark et al. 2000; Zola et al. 2000; Broadbent et al. 2004). Whilst these prior studies contribute invaluable resource to our understanding, our current data support the idea that it is the spatial aspect of OR that appears to be processed by the hippocampus. This finding is in line with reports that the perirhinal cortex is responsible for nonspatial aspects of OR (Brown and Aggleton 2001; Aggleton and Brown 2005).
The hippocampus is known to play an essential role in novelty detection, despite controversy over the precise subregional localization of mismatch detection (Lisman and Otmakhova 2001; Vinogradova 2001; Lee et al. 2005). The major hippocampal subregions, the CA1, CA3, and the dentate gyrus, respond to novelty-elicited synaptic changes (Vianna et al. 2000; Straube, Korz, Balschun, et al. 2003; Lee et al. 2005). Whilst LTP, particularly in the dentate gyrus, is elicited through changes in novel empty space (Straube, Korz, Balschun, et al. 2003; Straube, Korz, Frey 2003; Kemp and Manahan-Vaughan 2007, 2008a), LTD appears to engage in a different form of novel information processing. In the CA1 in rats, novel constellation of small feature-conferring objects within an environment facilitates LTD (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004), whereas in the dentate gyrus such facilitation of LTD surfaces predominantly through the novelty of disproportionately large landmark cues which polarize the environment (Kemp and Manahan-Vaughan 2008a). In line with prior observations, our current results show that the novelty of either component of the object–space pairing (novelty of a new object in old space, or of an old object in new space), which effectively translates into spatial novelty, elicits endogenous LTD in the CA1 region in mice. In the CA1 region, behavioral activity alone is insufficient to trigger the synaptic changes that arise from the acquisition of novel spatial features, as active exploration of a featureless empty environment does not elicit persistent plasticity (Kemp and Manahan-Vaughan 2004). Moreover, the LTD facilitation through active exploration in a spatial task can be mimicked through a similar spatial task in which the subjects remain stationary (Kemp and Manahan-Vaughan 2011).
LTP was prevented by either the OR or the spatial OR task. The profile of synaptic depression that occurred after induction of LTP remained very similar to that seen in naive synapses. In both cases, synapses depressed by roughly 20%, which amounted to the same relative change as that obtained in naive synapses. Learning performance was also unaffected by the induction of LTP. This suggests that LTP may not play a significant part in the processing of this kind of information.
The finding that learning-facilitated plasticity is conserved across species is an important observation. Here, we describe for the first time that learning-facilitated plasticity occurs in the mouse. In rats, novel spatial context facilitates hippocampal LTD (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004, 2007, 2008a) and inhibits LTP (Kemp and Manahan-Vaughan 2004). Here, we show that OR facilitates hippocampal LTD and impairs LTP. This is an important finding, as it highlights that LTP cannot comprise the sole cellular substrate for synaptic information storage leading to persistent memories. Certain components of spatial learning may, in fact, be associated with LTD. It is indeed becoming increasingly likely that LTP and LTD work together to encode different aspects of spatial information that are combined to generate a complete spatial representation of the environment (Kemp and Manahan-Vaughan 2007; Hagena and Manahan-Vaughan 2011). We do not purport to claim that plasticity is “triggered” by the learning event (this will require greater scrutiny and other methodological tools), however, our current data indicate that LTD is tightly associated with specific aspects of spatial learning. Indeed, other studies in rats have shown that preventing spatial learning by, for example, treatment with antagonists of either the N-methyl-d-aspartate receptor or of specific metabotropic glutamate, dopamine, or noradrenergic receptors also prevents the concurrent facilitation of LTD by the learning task (Lemon and Manahan-Vaughan 2006; Kemp and Manahan-Vaughan 2008b; Popkirov and Manahan-Vaughan 2011) thereby adding further support to this possibility.
In the rat hippocampus, learning-facilitated plasticity occurs at the different synapses of the trisynaptic circuit in close association with the experience of different kinds of spatial learning paradigms (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2008a; Hagena and Manahan-Vaughan 2011). LTP is facilitated at the perforant path—dentate gyrus synapse, the mossy fiber—CA3 synapse, the commissural associational—CA3 synapses, and the Schaffer collateral—CA1 synapses by exposure to novel space (Kemp and Manahan-Vaughan 2004, 2008a; Hagena and Manahan-Vaughan 2011). LTD on the other hand shows subregional differences: it is facilitated in the dentate gyrus and at mossy fiber—CA3 synapses by spatial configurations of large navigational cues (Kemp and Manahan-Vaughan 2008a; Hagena and Manahan-Vaughan 2011), whereas at commissural associational—CA3 synapses and Schaffer collateral—CA1 synapses by exposure to novel and more discrete object–place configurations (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004). Learning-facilitated plasticity is thus a very robust phenomenon that may reflect the differential contribution of LTP and LTD to different components of a spatial representation. LTD that occurs in association with spatial learning may reflect changes in signal-to-noise ratios, pruning of synapses that were previously potentiated by LTP, or information storage through synaptic depression (Hagena and Manahan-Vaughan 2011). It is important to note, however, that LTP may also enable nonspatial forms of memory storage in the hippocampus. In line with this, both fear conditioning (Whitlock et al. 2006) and trace conditioning (Gruart et al 2006) elicit potentiation of synaptic responses, suggesting that LTP and LTD can functionally dissociate depending on the information being processed.
In conclusion, our data support that intrinsic synaptic depression occurs in the mouse hippocampus in association with OR. This change is coupled to the spatial component of OR: a finding that supports that the hippocampus is responsible for the processing of the novel object–space presented in the task. Furthermore, this phenomenon is tightly related to LTD and not to LTP, as attempting to induce LTP during both object and SR result in the impairment of this type of synaptic plasticity. Taken together, these data support that learning-facilitated synaptic plasticity is a common property of mammalian systems, that the hippocampus recognizes and encodes the spatial component of OR, and that OR may be enabled by encoding through long-term synaptic depression in the hippocampus.
Funding
This work is support by a grant from the German research foundation (Deutsche Forschungsgemeinscaft, www.dfg.de) to D.M.-V. (SFB 874, TP B1).
Acknowledgments
We thank Jens Klausnitzer and Beate Krenzek for technical assistance and Nadine Gomell for animal care. We are grateful to Arne Buschler for methodological advice. Conflict of Interest : None declared.
References
- Aggleton JP, Brown MW. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q J Exp Psychol B. 2005;58:218–233. doi: 10.1080/02724990444000131. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001;11:61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Clark RE, Martin SJ. Interrogating rodents regarding their object and spatial memory. Curr Opin Neurobiol. 2005;15:593–598. doi: 10.1016/j.conb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-Garcia JM. Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci U S A. 2010;107:2652–2657. doi: 10.1073/pnas.0915059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav Brain Res. 1999a;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Conscious awareness, memory and the hippocampus. Nat Neurosci. 1999b;12:775–776. doi: 10.1038/12137. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Fagan JF., 3rd The paired-comparison paradigm and infant intelligence. Ann N Y Acad Sci. 1990;608:337–357. doi: 10.1111/j.1749-6632.1990.tb48902.x. ; discussion 358–364. [DOI] [PubMed] [Google Scholar]
- Farah MJ. Agnosia. Curr Opin Neurobiol. 1992;2:162–164. doi: 10.1016/0959-4388(92)90005-6. [DOI] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Eichenbaum H. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learn Mem. 2010;17:12–17. doi: 10.1101/lm.1616209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport SI. Altered brain functional connectivity and impaired short-term memory in Alzheimer's disease. Brain. 2001;124:739–756. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- Gruart A, Munoz MD, Delgado-Garcia JM. Involvement of CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26:1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses Reveals different roles in information processing. Cereb Cortex. 2011;21:2442–2449. doi: 10.1093/cercor/bhq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 2008a;18:968–977. doi: 10.1093/cercor/bhm136. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Beta-adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex. 2008b;18:1326–1334. doi: 10.1093/cercor/bhm164. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Passive spatial perception facilitates the expression of persistent hippocampal long-term depression. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr233. doi:10.1093/cercor/bhr233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatu S, Revonsuo A, Pihko L, Portin R, Rinne JO. Visual object recognition deficits in early Parkinson's disease. Parkinsonism Relat Disord. 2004;10:227–233. doi: 10.1016/j.parkreldis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Lee I, Hunsaker MR, Kesner RP. The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem. 2009;16:616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Pinel JP. Rhinal cortex lesions and object recognition in rats. Behav Neurosci. 1994;108:11–18. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. New York: Oxford University Press; 1978. [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb Cortex. 2011;21:501–509. doi: 10.1093/cercor/bhq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci. 1997;111:667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Straube T, Korz V, Balschun D, Frey JU. Requirement of beta-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J Physiol. 2003;552:953–960. doi: 10.1113/jphysiol.2003.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Korz V, Frey JU. Bidirectional modulation of long-term potentiation by novelty-exploration in rat dentate gyrus. Neurosci Lett. 2003;344:5–8. doi: 10.1016/s0304-3940(03)00349-5. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. Episodic memory signals in the rat hippocampus. Neuron. 2003;40:1055–1056. doi: 10.1016/s0896-6273(03)00806-7. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Alonso M, Viola H, Quevedo J, de Paris F, Furman M, de Stein ML, Medina JH, Izquierdo I. Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem. 2000;7:333–340. doi: 10.1101/lm.34600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall TR. Mental time travel in animals: a challenging question. Behav Process. 2006;72:173–183. doi: 10.1016/j.beproc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, McCabe BJ, Aggleton JP. Effects of the novelty or familiarity of visual stimuli on the expression of the immediate early gene c-fos in rat brain. Neuroscience. 1995;69:821–829. doi: 10.1016/0306-4522(95)00320-i. [DOI] [PubMed] [Google Scholar]
- Zhu XO, McCabe BJ, Aggleton JP, Brown MW. Mapping visual recognition memory through expression of the immediate early gene c-fos. Neuroreport. 1996;7:1871–1875. doi: 10.1097/00001756-199607290-00037. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]