Abstract
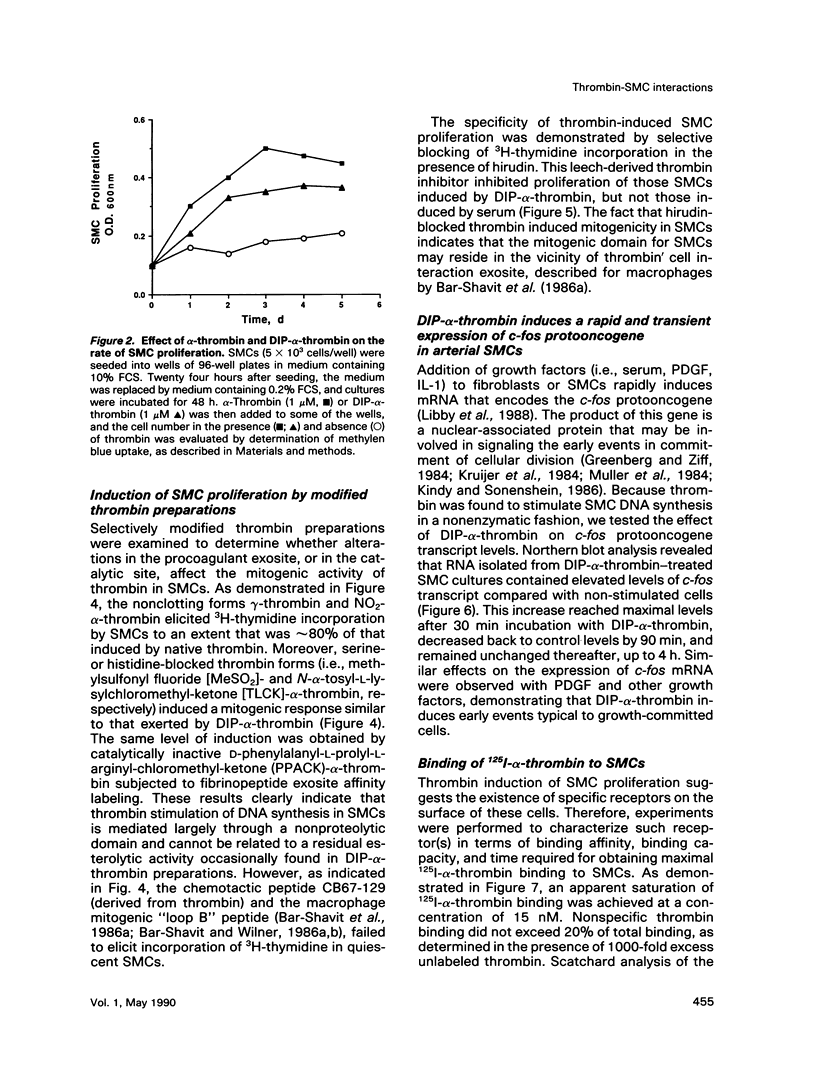
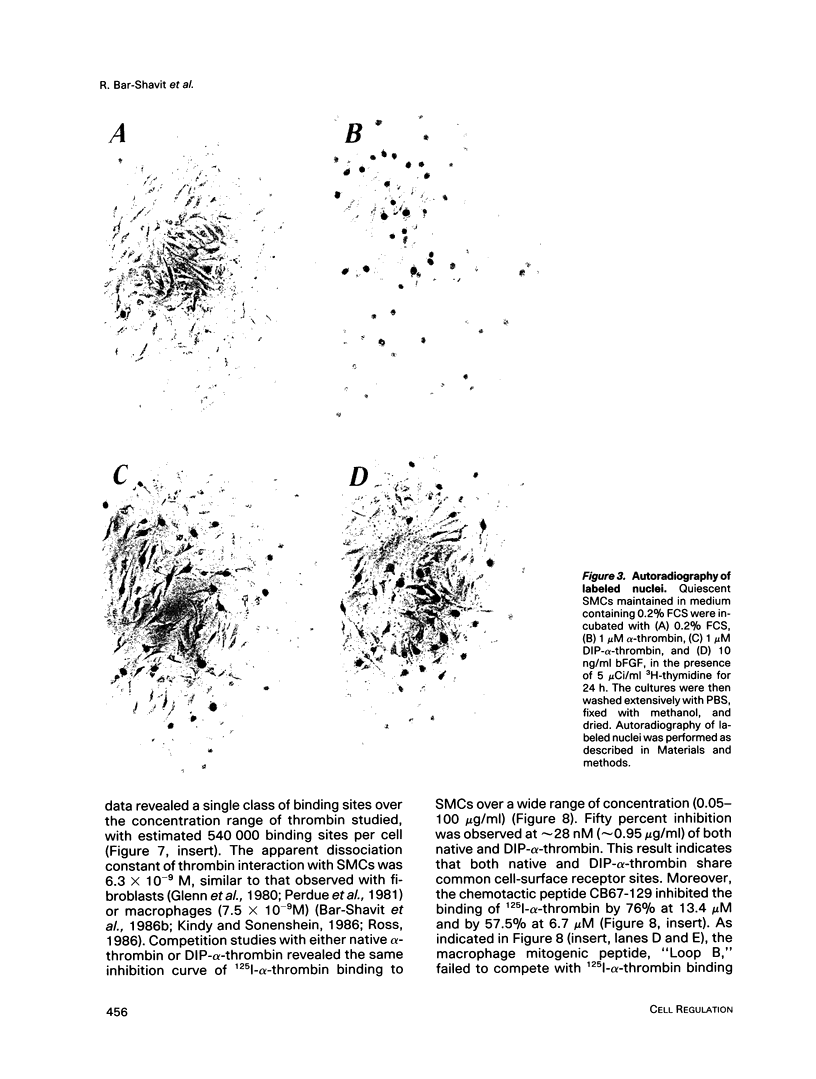
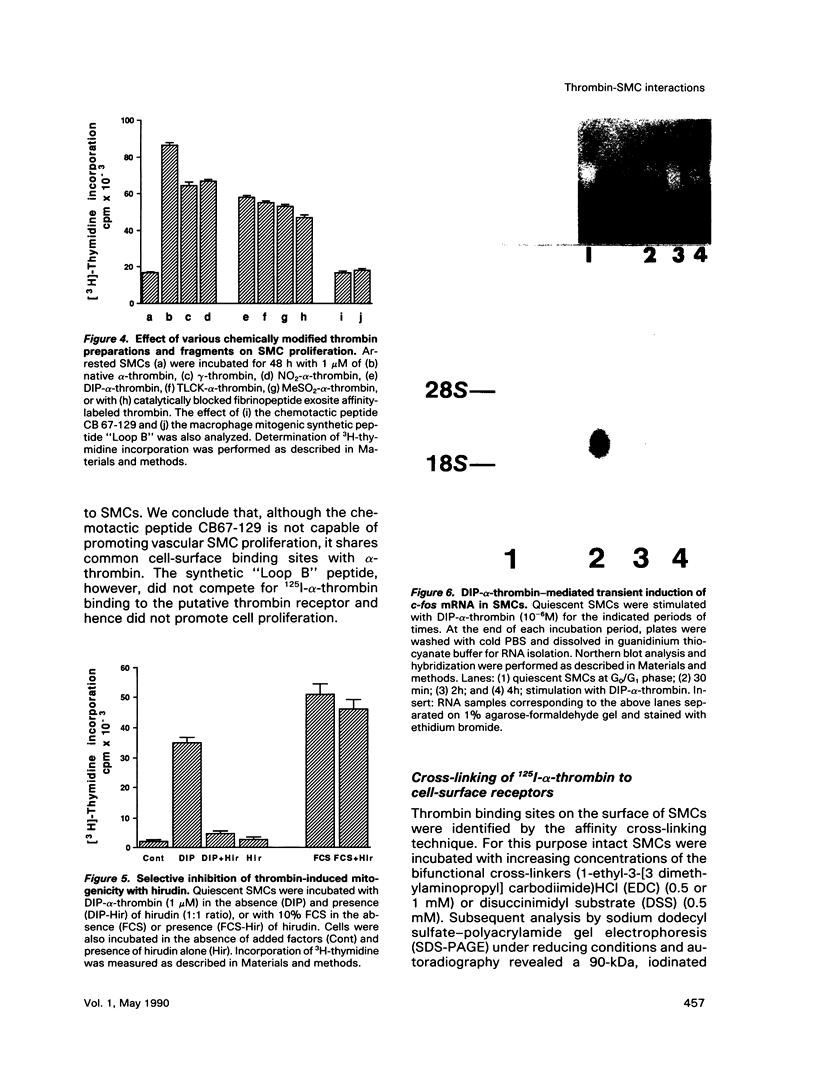
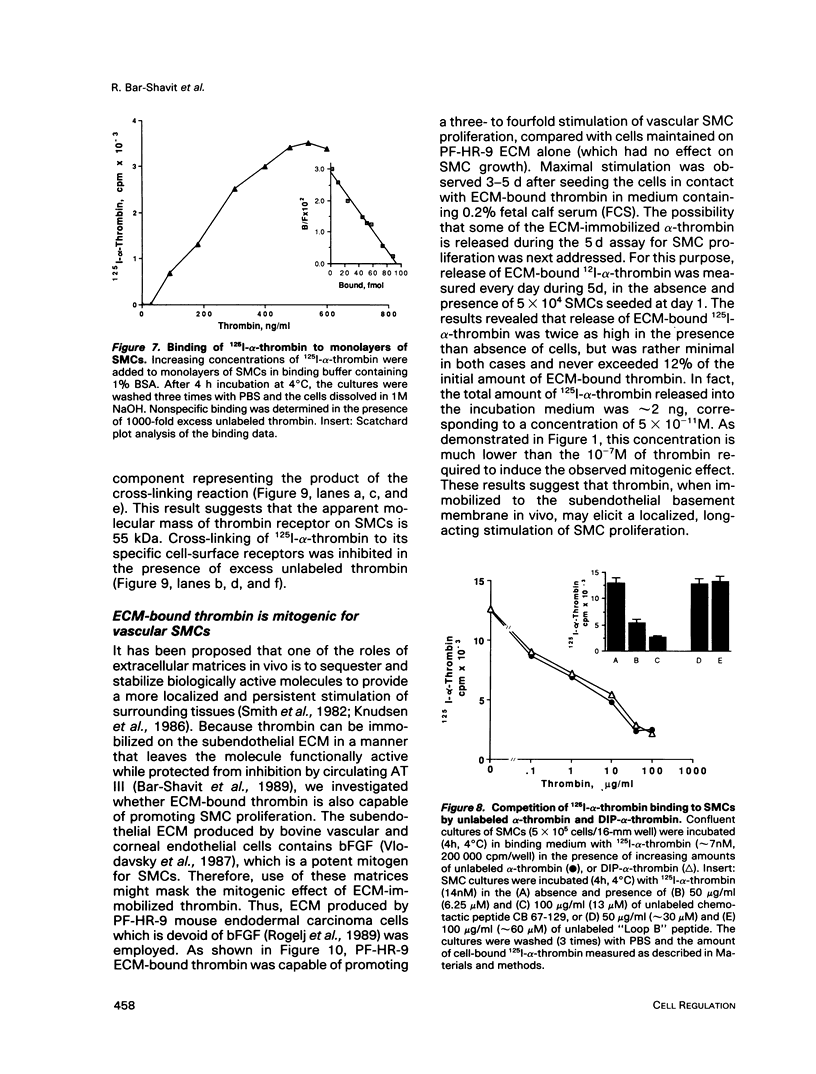
Esterolytically inactive diisopropyl fluorophosphate-conjugated thrombin (DIP-alpha-thrombin) stimulated 3H-thymidine incorporation and proliferation of growth-arrested vascular smooth muscle cells (SMCs), similar to native alpha-thrombin. Half-maximal mitogenic response of SMCs was obtained at 1 nM thrombin and was specifically blocked by the leech-derived, high-affinity thrombin inhibitor, hirudin. Native thrombin and a variety of thrombin species that were chemically modified to alter thrombin procoagulant or esterolytic functions were found to induce 3H-thymidine incorporation to a similar extent. Exposure of SMCs to DIP-alpha-thrombin caused a rapid and transient expression of the c-fos protooncogene, determined by Northern blot analysis. These results indicate that thrombin is a potent mitogen for SMCs through a distinct non-enzymatic domain. Binding of 125I-alpha-thrombin to SMC cultures revealed an apparent dissociation constant of 6 nM and an estimated 5.4 x 10(5) binding sites per cell. This binding was inhibited to the same extent by native thrombin and by its nonenzymatic form, DIP-alpha-thrombin. Moreover, the chemotactic fragment of thrombin (CB67-129), which failed to elicit a mitogenic response, competed for 125I-alpha-thrombin binding to SMCs. Cross-linking analysis of 125I-alpha-thrombin to SMCs revealed a specific cell-surface binding site 55 kDa in size. Finally, thrombin immobilized to a naturally produced extracellular matrix retained potent mitogenic activity toward SMCs. These observations lend support to the possibility that in vivo, subendothelial basement membranes sequester thrombin (as well as other bioactive molecules), which may stimulate localized and persistent growth of arterial SMCs. Thrombin may thus be involved directly in progression of atherosclerotic plaque formation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit R., Eldor A., Vlodavsky I. Binding of thrombin to subendothelial extracellular matrix. Protection and expression of functional properties. J Clin Invest. 1989 Oct;84(4):1096–1104. doi: 10.1172/JCI114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. H., Cunningham D. D. Cell surface action of thrombin is sufficient to initiate division of chick cells. Cell. 1978 Aug;14(4):811–823. doi: 10.1016/0092-8674(78)90337-9. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Favreau L. V., Karnovsky M. J., Rosenberg R. D. Inhibition of vascular smooth muscle cell growth by endothelial cell-derived heparin. Possible role of a platelet endoglycosidase. J Biol Chem. 1982 Oct 10;257(19):11256–11260. [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung A. E., Estes L. E., Shinozuka H., Braginski J., Lorz C., Chung C. A. Morphological and biochemical observations on cells derived from the in vitro differentiation of the embryonal carcinoma cell line PCC4-F. Cancer Res. 1977 Jul;37(7 Pt 1):2072–2081. [PubMed] [Google Scholar]
- Craik C. S., Sprang S., Fletterick R., Rutter W. J. Intron-exon splice junctions map at protein surfaces. Nature. 1982 Sep 9;299(5879):180–182. doi: 10.1038/299180a0. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Garcia J. G., Siflinger-Birnboim A., Bizios R., Del Vecchio P. J., Fenton J. W., 2nd, Malik A. B. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986 Jul;128(1):96–104. doi: 10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Carney D. H., Fenton J. W., 2nd, Cunningham D. D. Thrombin active site regions required for fibroblast receptor binding and initiation of cell division. J Biol Chem. 1980 Jul 25;255(14):6609–6616. [PubMed] [Google Scholar]
- Goldman R., Bar-Shavit Z. Dual effect of normal and stimulated macrophages and their conditioned media on target cell proliferation. J Natl Cancer Inst. 1979 Oct;63(4):1009–1016. [PubMed] [Google Scholar]
- Gospodarowicz D., Lepine J., Massoglia S., Wood I. Comparison of the ability of basement membranes produced by corneal endothelial and mouse-derived Endodermal PF-HR-9 cells to support the proliferation and differentiation of bovine kidney tubule epithelial cells in vitro. J Cell Biol. 1984 Sep;99(3):947–961. doi: 10.1083/jcb.99.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Kaminski M., McDonagh J. Studies on the mechanism of thrombin. Interaction with fibrin. J Biol Chem. 1983 Sep 10;258(17):10530–10535. [PubMed] [Google Scholar]
- Kindy M. S., Sonenshein G. E. Regulation of oncogene expression in cultured aortic smooth muscle cells. Post-transcriptional control of c-myc mRNA. J Biol Chem. 1986 Sep 25;261(27):12865–12868. [PubMed] [Google Scholar]
- Klagsbrun M., Edelman E. R. Biological and biochemical properties of fibroblast growth factors. Implications for the pathogenesis of atherosclerosis. Arteriosclerosis. 1989 May-Jun;9(3):269–278. doi: 10.1161/01.atv.9.3.269. [DOI] [PubMed] [Google Scholar]
- Knudsen B. S., Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Binding of plasminogen to extracellular matrix. J Biol Chem. 1986 Aug 15;261(23):10765–10771. [PubMed] [Google Scholar]
- Kramer R. H., Vogel K. G. Selective degradation of basement membrane macromolecules by metastatic melanoma cells. J Natl Cancer Inst. 1984 Apr;72(4):889–899. [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Laposata M., Dovnarsky D. K., Shin H. S. Thrombin-induced gap formation in confluent endothelial cell monolayers in vitro. Blood. 1983 Sep;62(3):549–556. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen H. R., Uytterhoeven M., Collen D. Inhibition of trypsin-like serine proteinases by tripeptide arginyl and lysyl chloromethylketones. Thromb Res. 1984 Jun 1;34(5):431–437. doi: 10.1016/0049-3848(84)90247-0. [DOI] [PubMed] [Google Scholar]
- MacGillivray R. T., Davie E. W. Characterization of bovine prothrombin mRNA and its translation product. Biochemistry. 1984 Apr 10;23(8):1626–1634. doi: 10.1021/bi00303a007. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Neurath H. Evolution of proteolytic enzymes. Science. 1984 Apr 27;224(4647):350–357. doi: 10.1126/science.6369538. [DOI] [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue J. F., Lubenskyi W., Kivity E., Sonder S. A., Fenton J. W., 2nd Protease mitogenic response of chick embryo fibroblasts and receptor binding/processing of human alpha-thrombin. J Biol Chem. 1981 Mar 25;256(6):2767–2776. [PubMed] [Google Scholar]
- Rogelj S., Klagsbrun M., Atzmon R., Kurokawa M., Haimovitz A., Fuks Z., Vlodavsky I. Basic fibroblast growth factor is an extracellular matrix component required for supporting the proliferation of vascular endothelial cells and the differentiation of PC12 cells. J Cell Biol. 1989 Aug;109(2):823–831. doi: 10.1083/jcb.109.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D. Biologic actions of heparin. Semin Hematol. 1977 Oct;14(4):427–440. [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Singh J. P., Lillquist J. S., Goon D. S., Stiles C. D. Growth factors adherent to cell substrate are mitogenically active in situ. Nature. 1982 Mar 11;296(5853):154–156. doi: 10.1038/296154a0. [DOI] [PubMed] [Google Scholar]
- Stern D., Nawroth P., Handley D., Kisiel W. An endothelial cell-dependent pathway of coagulation. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2523–2527. doi: 10.1073/pnas.82.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus D. W., Blank M. K. Heparin cofactor II. Purification and properties of a heparin-dependent inhibitor of thrombin in human plasma. J Biol Chem. 1982 Mar 10;257(5):2162–2169. [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilner G. D., Danitz M. P., Mudd M. S., Hsieh K. H., Fenton J. W., 2nd Selective immobilization of alpha-thrombin by surface-bound fibrin. J Lab Clin Med. 1981 Mar;97(3):403–411. [PubMed] [Google Scholar]