Abstract
Objectives
Despite many interventions that have been tried, controversy remains regarding the efficacy of interventions for contrast-induced nephropathy (CIN), so we aimed to evaluate the best evidence from recent meta-analyses.
Methods
We searched MEDLINE, EMBASE and the Cochrane library for interventions which have been used for CIN. We included only the most recent meta-analysis of each intervention. We extracted data on the methodology, quality and results of each meta-analysis. We performed narrative synthesis and adjusted indirect comparison of interventions that were shown to be statistically significant compared with a placebo.
Results
We included 7 systematic reviews and meta-analyses involving 9 different interventions for CIN, with a total of 15 976 participants. A significantly decreased risk of CIN was reported in meta-analysis of the following interventions: N-acetylcysteine [odds ratio (OR) 0.65, 95% confidence interval (CI) 0.48–0.88, I2=64%], theophylline [relative risk (RR) 0.48, 95% CI 0.26–0.89, I2=44%], statins (RR 0.51, 95% CI 0.34–0.77, I2=0%) and sodium bicarbonate (RR 0.62, 95% CI 0.45–0.86, I2=49%). Furosemide was shown to increase the risk of CIN (RR 3.27, 95% CI 1.48–7.26, I2=0%). Other interventions such as renal replacement therapy, angiotensin-converting enzyme inhibitors, dopamine and fenoldapam failed to show any significant difference from the control group.
Conclusion
Although there is some evidence to suggest that N-acetylcysteine, theophylline, sodium bicarbonate and statins may reduce incidence of CIN, limitations in the study quality and heterogeneity preclude any firm recommendations.
Advances in knowledge
N-acetylcysteine, theophylline, sodium bicarbonate and statins show some promise as potentially efficacious agents for preventing CIN, but more high-quality studies are needed before they can be recommended for use in routine practice.
Contrast-induced nephropathy (CIN) is a well-recognised complication of contrast administration and the third leading cause of hospital-acquired acute kidney injury [1]. Despite some heterogeneity in definition, CIN is generally defined as an increase in serum creatinine over 25% or 44 μmol l−1 from baseline value 48–72 h after contrast media administration in the absence of any other aetiology [2]. CIN occurs in 0–10% of patients with normal renal function and in up to 25% of patients with pre-existing renal disease or certain risk factors such as diabetes, advanced age or nephrotoxic drugs [3]. A recent meta-analysis of 40 studies found that the pooled incidence of CIN was 6.4% after CT scanning [4].
The pathophysiology of CIN is not completely understood but it is believed that contrast media causes vasoconstriction and renal medulla ischaemia, which leads to generation of free radicals and oxidative injury to tubular cells [5-8]. Studies have demonstrated that CIN is associated with increased in-hospital length of stay, morbidity and mortality and increased cost of medical care, especially in patients who require dialysis [9].
A number of measures (hydration methods and physical agents) have been tried to prevent or treat contrast nephropathy. Hydration methods include N-acetylcysteine (NAC) and sodium bicarbonate. Studies have explored the value of NAC for contrast nephropathy and some have found that its use is associated with clinical benefits [10-13], but a recent large randomised trial found no such benefit associated with NAC use [14]. A number of published studies have suggested that bicarbonate is superior to saline in contrast-induced nephropathy [15-18]. However, there are also a number of unpublished studies which suggest no benefit associated with sodium bicarbonate use [19,20]. Theophylline and other drugs are methods that have been tried to reduce CIN. Theophylline has been shown to be protective in CIN in a few studies [21,22] but other studies have found no difference between theophylline and a control [23,24]. In addition, many drugs have been tried to prevent CIN [25-27].
In response to the many studies, a number of meta-analyses have been published which have evaluated different measures used for CIN [28,29]. We reviewed the literature to identify these meta-analyses in order to determine the most up-to-date evidence on each intervention for CIN.
Methods
Systematic reviews and meta-analyses that evaluated interventions for CIN were included. There was no restriction on type of intervention but the review had to perform a meta-analysis of randomised controlled trials for risk of CIN or other surrogate outcomes such as need for renal replacement therapy. Studies were excluded if there were more recent meta-analyses reviewing the same topic. Reviews were identified by searching MEDLINE and EMBASE in July 2011. The full search string is shown in Appendix A. The search was limited to meta-analyses published in English and to human studies.
The studies retrieved were checked independently by all the reviewers and relevant reviews were selected according to the stated inclusion and exclusion criteria. Disagreements were resolved by consensus.
Data on methods in each meta-analysis, quality of meta-analysis and results were extracted by CSK, CLP and JKY. The methods used for the meta-analysis, including search date, databases searched, interventions, definition of CIN, extraction methods and outcomes, were collected. Extracted information on the control arm, types of procedure, items in assessment, use of quality score, limitations and statistical heterogeneity in analysis were used in the quality assessment. Results included the number of studies, number of patients/events and the risk of CIN; other outcomes were collected.
Data synthesis
Where the pooled data were not available, we used RevMan 5.1 (The Cochrane Collaboration; http://ims.cochrane.org/revman) to perform random effects meta-analysis from the reported raw study data. The data collected were presented in tables and compared graphically using informal indirect comparison of overlap of the risk estimates and 95% confidence intervals. Adjusted indirect comparison (Bucher's method) [30] was performed using ITC software (Canadian Agency for Drugs and Technologies in Health; http://www.cadth.ca/en/resources/about-this-guide) [31]. Here, pooled risk estimates for CIN from meta-analysis of interventions that were shown to be significant compared with a placebo were indirectly compared using a placebo or saline as the common control.
Results
Meta-analysis selection
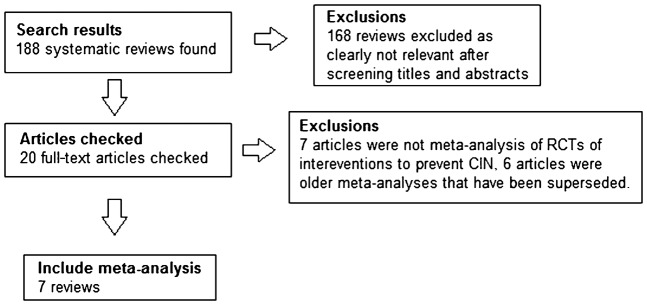
The studies identified by the search and the included meta-analyses are shown in Figure 1. We included seven systematic reviews and meta-analyses with nine different interventions for CIN.
Figure 1.
Flow diagram of study selection. CIN, contrast-induced nephropathy; RCT, randomised controlled trial.
Methods used in included meta-analyses
The methods used in the included meta-analyses are shown in Table 1. The review of theophylline had the most recent search date (July 2011) [29] while the oldest was conducted for the fenoldopam, dopamine, furosemide study [26]. All reviews were searched using MEDLINE or PUBMED and a combination of EMBASE, the Cochrane Library and unpublished literature via conference proceedings and clinicaltrials.gov. In general, most studies used similar definitions of CIN. All the studies had independent extractors and in four of the seven studies, authors were contacted for missing information. All seven studies evaluated CIN as an outcome, and four studies also considered other surrogate outcomes such as requirement of dialysis, heart failure, long-term changes in renal function and death.
Table 1. Methods used in included meta-analyses.
| Study | Search date | Databases searched | Intervention | Other inclusion criteria | Definition of CIN | Extraction methods | Missing information | Outcomes |
| ACT 2011 | April 2011 | MEDLINE | N-acetylcysteine vs control | RCT, placebo controlled | 25% elevation in serum creatinine above baseline between 48 and 96 h after angiography | Independent extraction | Not mentioned | Contrast-induced acute kidney injury defined as 25% elevation in serum creatinine above baseline between 48 and 96 h after angiography, death, need for dialysis, raised creatinine and other adverse events |
| Dai 2012 | July 2011 | MEDLINE, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials | Adenosine receptor anatagonist vs control | RCT, treatments with or without addition of N-acetylcysteine | Increase in baseline serum creatinine levels of 25% of an absolute increase of 0.5 mg dl−1 within 2–3 days after exposure to contrast medium | Independent extraction | Contacted authors | CIN, change in serum creatinine levels, requirement of dialysis and in-hospital mortality |
| Cruz 2012 | March 2011 | MEDLINE, EMBASE | Periprocedural renal replacement therapy vs standard medical care | >10 human subjects | Increase in serum creatinine ≥0.5 mg dl−1 | Independent extraction | Not mentioned | CIN, renal replacement therapy, long-term changes in renal function and death. |
| Kelly 2008 | November 2006 | MEDLINE, EMBASE, Web of Knowledge, Cochrane Library | One of treatment group received fenoldopam, dopamine, furosemide | RCT, compare treatment with control, use of intravenous iodinated contrast, defined CIN | Absolute increase in baseline serum creatinine >0.5 mg dl−1 or a relative increase of >25% at 48 h after contrast injection | Independent extraction | Contacted authors | CIN |
| Li 2012 | November 2011 | MEDLINE, Cochrane trial register | Angiotensin-converting enzyme inhibitor vs control | RCT, patients undergoing intravascular angiography, outcome of CIN | Change in serum creatinine pre and post procedure | Independent extraction | Not mentioned | CIN |
| Zhang 2011 | February 2011 | Pubmed, Cochrane library, China National Knowledge Infrastructure, conference abstracts, contacted authors. Data from non-peer reviewed abstracts were excluded | Short-term high-dose statin treatment vs low-dose statin treatment or placebo | None | 25% increase in baseline serum creatinine levels or absolute increase of 0.5 mg dl−1 at a 48 h time period and another time period | Independent extraction | Contacted authors | CIN |
| Zoungas 2009 | December 2008 | MEDLINE, EMBASE, Cochrane trial register, clinicaltrials.gov, conference proceedings and data requests from authors | Sodium bicarbonate vs control | RCT | 25% increase in baseline serum creatinine levels or an absolute increase of 0.5 mg day−1 2–5 days after radiocontrast administration | Independent extraction | Contacted authors | CIN, requirement of dialysis, heart failure and death |
CIN, contrast-induced nephropathy; RCT, randomised controlled trial.
Quality assessment of included meta-analyses
A variety of control groups were used in the included meta-analyses. Most had normal saline or standard treatment as the control. Different procedures were included, such as coronary or peripheral angiography, X-ray examinations with contrast or CT with contrast medium or intravenous pyelograms. All reviews had some form of quality assessment, and four of the seven studies [1,27-29] used the Jadad score for methodological quality [32]. The authors of the theophylline review conducted subgroup analysis and meta-regression based on the Jadad score, and demonstrated substantial differences between the results of lower quality and higher quality studies. One study used a score with 1 point for each outcome assessed (total out of four) [26]. Significant statistical heterogeneity was observed for the CIN analysis in two of the seven studies [1,14]. The possibility of publication bias was noted in the angiotensin-converting enzyme (ACE) inhibitor analysis [26] and our analysis of the NAC studies. Results are shown in Table 2.
Table 2. Quality assessment of include meta-analyses.
| Study | Control arm | Types of procedures | Items included in assessment | Average quality score | Limitation | Statistical heterogeneity in CIN analysis |
| ACT 2011 | Placebo controlled trials | Coronary or peripheral angiography | Allocation concealment, blinding of investigators, participants, outcome assessor, intention to treat | Score not used | Significant heterogeneity, variable methodological quality. We also found funnel plot asymmetry with potentially missing data from small studies that showed no benefit | I2=59%. |
| Dai 2012 | Control (n=14), combination treatment with NAC (n=2) | Coronary angiography (n=12), non-coronary angiography procedures [X-ray, CT (n=4)] | Blinding procedure, methods of randomisation, concealment of treatment allocation, reporting of losses to follow-up or missing outcome assessments, intention-to-treat analysis, evidence of important baseline differences between groups and eligibility criteria and Jadad score | Average Jadad score 1.9/5 (n=12) | Low to moderate quality of trials and presence of heterogeneity. Diverse theophylline regimens, Few trials evaluated clinical outcomes | I2=44.3% |
| Cruz 2012 | Normal saline (n=5), conservative treatment (n=2), standard treatment (n=1), non-haemodialysis (n=1) | Cardiovascular procedure (n=7), cardiovascular procedure or radiological investigation (n=1), radiocontrast procedure (n=1), not clear (n=1) | Jadad score | Average Jadad score 1.9/5 (n=9) | Use of creatinine as definition of contrast reaction as renal replacement therapy will remove creatinine | I2=84% |
| Kelly 2008 | Saline-only control group (n=6) | Coronary angioplasty (n=1), angiography study (n=2), cardiovascular procedure (n=2), radiological procedure (n=1) | Concealment of allocation, similarity of both groups at baseline, eligibility criteria, blinding of patients, blinding of care providers, blinding of outcome assessor, point estimates and measures of variability of primary outcome and inclusion of intention-to-treat analysis | Score not used | Exclusion of unpublished data risk of overestimate, poor-quality studies included | I2=0% |
| Li 2012 | All studies had placebo or routine without angiotensin converting enzyme inhibitor | Coronary angiography (n=4), coronary angioplasty (n=2), intravenous pyelography (n=1) | Appropriate randomisation, allocation concealment, blinded patients, blinded providers | Score out of 4 based on quality assessment items. Average score 1.7/4 | Publication bias from small studies with positive results, underpowered studies | I2=43% |
| Zhang 2011 | Statin and NAC vs NAC (n=1), placebo (n=3), low dose statin (n=4) | Coronary angiography/intervention (n=8) | Allocation of concealment, similarity of groups at baseline, specific inclusion criteria, placebo controlled, blinded care providers, blinded subjects, intention-to-treat analysis, estimated variability of outcome | Average Jadad score 3.3/5 (n=8) | Lack of patient specific data, inclusion of studies of variable quality, high dose of statin definition arbitrary, small sample size included | I2=0%. |
| Zoungas 2009 | Normal saline (n=12), normal saline and NAC (n=11) | Angiography or CT (n=1), coronary intervention (n=19), coronary or renal angiography (n=1), CT or intravenous pyelogram (n=1), scheduled radiocontrast (n=1) | Concealment of allocation, similarity of both groups at baseline, eligibility criteria, blinding of outcome assessment, care providers and patients, completeness of follow-up, intention-to-treat analysis | Average Jadad score 2.3/5 (n=9 published study only) | Uncertainty regarding search covering unpublished literature, few events limits statistical power, poor quality assessment, variability hydration protocols | I2=49.1% |
CIN, contrast-induced nephropathy; NAC, N-acetylcysteine.
Results of included meta-analyses
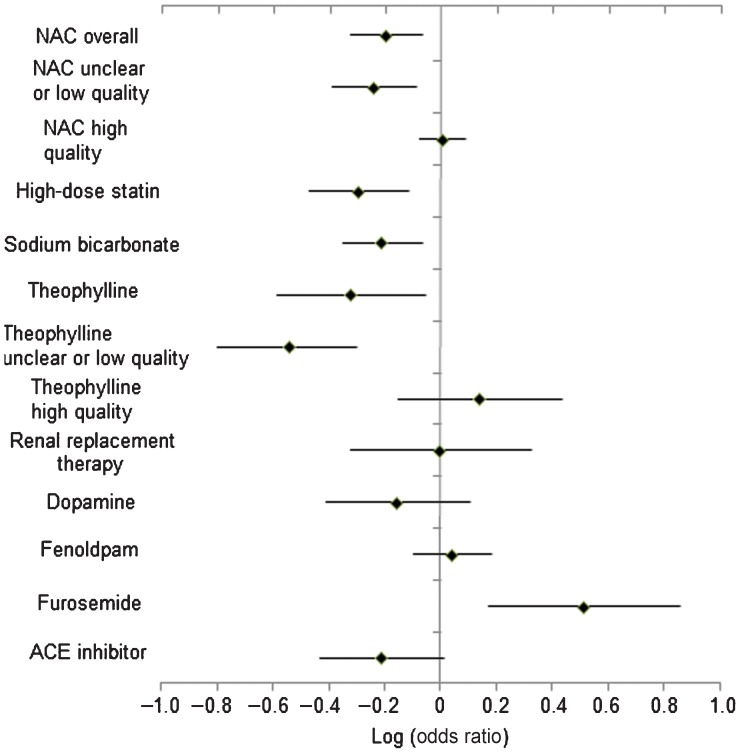
Results of the included meta-analyses are shown in Table 3. Each of the nine meta-analyses had a different number of randomised trials, which ranged from 2 [25] to 47 trials [14]. For the NAC analysis, we did our own analysis of the data presented in the article produced by the ACT collaborators. The number of participants and number of CIN events also varied: ranges were 90–7681 participants and 29–904 CIN events. A significantly decreased risk of CIN events was observed for the NAC analysis [odds ratio (OR) 0.65, 95% confidence interval (CI) 0.48–0.88, I2=64%], theophylline analysis [relative risk (RR) 0.48, 95% CI 0.26–0.89, I2=44%], statin analysis (RR 0.51, 95% CI 0.34–0.77, I2=0%) and sodium bicarbonate analysis (RR 0.62, 95% CI 0.45–0.86, I2=49.1%). The furosemide meta-analysis was the only study which showed a significantly increased risk of CIN using this intervention (RR 3.27, 95% CI 1.48–7.26, I2=0%). Renal replacement therapy was not associated with decreased risk of CIN, need for acute renal replacement therapy, chronic renal replacement therapy and mortality [1]. There was no difference for need for dialysis or mortality [28]. Results are shown in Figure 2.
Table 3. Results of included meta-analyses.
| Intervention | Number of RCTs | Number of patients | Number of events | Risk of CIN | Risk of other outcomes | Reference |
| N-acetylcysteine | 47 | 7681 (range 20–2272 in each study) | 904 CIN events (range 0–289 in each study) | Overall: CIN (n=47) RR 0.64 (0.48–0.86), I2=59%. Unclear or low quality: 0.58 (0.41–0.82) I2=59%. High quality, 1.02 (0.84–1.23), I2=0% | NA | ACT 2011 |
| Theophylline | 16 | 1412 (range 21–280 in each study) | 109 CIN events (n=13 RCTs) | CIN (n=13) RR 0.48 (0.26–0.89), I2=44.3%, p=0.043. Pooled RR of CIN with low-quality trials (score, 3) was 0.29 (95% CI, 0.16–0.50) but for higher quality trials was 1.39 (95% CI, 0.71–2.73) | Change in serum creatinine at 48 h (n=13) −27 (−44 to −10) μmol l−1. Overall incidence of dialysis was 3 out of 1222 patients. In-hospital mortality reported in 2 trials: 1 had no deaths, the other had 4 in treatment group and 3 in control group | Dai 2012 |
| Renal replacement therapy | 9 | 919 (range 15–424 in each study) | 167 CIN events (range 4–44 in each study) | CIN (n=7), RR 1.00 (0.48–2.11), I2=84% | Acute renal replacement therapy (n=5) RR 0.39 (0.09–1.74), I2=19%. Chronic renal replacement therapy (n=4) RR 0.66 (0.17–2.55), I2=31%. Mortality (n=4) RR 0.52 (0.12–2.30), I2=59% | Cruz 2012 |
| Dopamine | 2 | 90 (range 40–50 in each study) | 29 CIN events (range 12–17 in each study) | CIN (n=2), RR 0.70 (0.39–1.28), I2=0% | NA | Kelly 2008 |
| Fenoldapam | 2 | 361 (range 78–283 in each study) | 102 CIN events (range 12–90 in each study) | CIN (n=2), RR 1.11 (0.80–1.53), I2=0%. | NA | Kelly 2008 |
| Furosemide | 2 | 209 (range 53–156 in each study) | 29 CIN events (range 13–16 in each study) | CIN (n=2), RR 3.27 (1.48–7.26), I2=0% | NA | Kelly 2008 |
| Angiotensin-converting enzyme inhibitor | 7 | 792 (range 21–220 in each study) | 68 CIN events (range 1–19 in each study) | CIN (n=7), OR 0.65 (0.30–1.40), I2=43% | NA | Li 2012 |
| Statin | 8 | 1434 (range 92–304 in each study) | 97 CIN events (n=7 RCTs), (range 2–31 in each study) | CIN (n=7), RR 0.51 (0.34–0.77), I2=0% | Change in serum creatinine at 48 h (n=7) −6.2 (−9.7 to −3.5) μmol l−1, I2=16% | Zhang 2011 |
| Sodium bicarbonate | 23 | 3563 (range 18–502 in each study) | 396 CIN events (range 2–56 in each study) | CIN (n=23), RR 0.62 (0.45–0.86), I2=49.1% | Requirement of dialysis (n=8) RR 0.51 (0.17–1.51), I2=0%. Mortality (n=4) RR 0.83 (0.32–2.19), I2=0%. Heart failure, (n=5), RR 0.92 (0.49–1.74), I2=0% | Zoungas 2009 |
CIN, contrast-induced nephropathy; NA, not available; RCT, randomised controlled trial; RR, relative risk.
Figure 2.
Risk of contrast-induced nephropathy with different interventions. ACE, angiotensin-converting enzyme; NAC, N-acetylcysteine.
Other interventions such as renal replacement therapy, ACE inhibitors, dopamine and fenoldapam failed to show any significant difference from the control group.
Adjusted indirect comparison
NAC, sodium bicarbonate, theophylline and statin were all statistically better at reducing CIN than the placebo and these were compared using adjusted indirect comparison (Table 4). There was no difference between NAC and sodium bicarbonate (RR 1.05, 95% CI 0.53–2.09), theophylline (1.35, 95% CI 0.68–2.69) and statin therapy (RR 1.28, 95% CI 0.77–2.12). For the indirect comparison of sodium bicarbonate vs theophylline and statin therapy, there was no significant difference for both comparisons (RR 1.22, 95% CI 0.72–2.05; RR 1.29, 95% CI 0.64–2.59, respectively).
Table 4. Adjusted indirect comparison of interventions significantly better than placebo.
| Comparison | Relative risk (95% confidence interval) |
| N-acetylcysteine vs | |
| Sodium bicarbonate | 1.05 (95% CI 0.53–2.09) |
| Theophylline | 1.35 (95% CI 0.68–2.69) |
| Statin therapy | 1.28 (95% CI 0.77–2.12) |
| Sodium bicarbonate vs | |
| Theophylline | 1.29 (95% CI 0.64–2.59) |
| Statin therapy | 1.22 (95% CI 0.72–2.05) |
| Theophylline vs | |
| Statin therapy | 0.94 (95% CI 0.45–1.97) |
Discussion
Our review finds that the use of NAC, theophylline, statin and sodium bicarbonate may be useful in the prevention or management of CIN. However, we also found major weaknesses in these reviews that led us to downgrade the quality of evidence. The efficacy of other agents such as ACE inhibitors and dopamine are unproven, while furosemide appears harmful. As such, use of any of these agents to prevent CIN remains unproven.
NAC is the most studied agent for treatment of CIN. It is believed to reduce oxidative stress and improves renal haemodynamics, which may be beneficial in preventing CIN [33-35]. Our review found that overall pooling of all the evidence may suggest a benefit, but more recent evidence seems to suggest otherwise. A recent large and high-quality trial by the ACT investigators found no difference between the NAC and the control group, and they suggest that its use is not recommended. Compared with other NAC studies, this trial had a broader inclusion criterion; some trials included only patients with renal failure. However, subgroup analysis within the large cohort failed to define a subgroup which would benefit from this treatment. This trial had limitations, as there were relatively small numbers of adverse events (mortality and need for dialysis) and the contrast volume used in the trial was low. The ACT triallists also identified other studies and performed a meta-analysis of the high-quality studies (without including their own ACT trial data). The ACT investigators concluded that the results provide consistent evidence that NAC fails to prevent CIN. One interpretation of this could be that there may be a degree of publication bias where poorer quality trials which show a positive effect may explain the overall potential benefit. Therefore, at present more high-quality evidence is needed before NAC is used.
Recent evidence suggests that theophylline might be a promising agent in preventing CIN because it reduces both CIN and serum creatinine levels [29]. Adenosine is an important vasocontrictor which has been shown from animal studies to induce afferent arteriolar vasoconstriction and decrease the glomerular filtration rate [36]. It is believed that adenosine antagonists preserve kidney function by attenuating the vasoconstrictive response after contrast administration [37,38]. However, the meta-analysis found diametrically different effects between lower quality and higher quality studies, with the lower quality ones showing significant benefit with theophylline while the meta-analysis of higher quality studies showed no evidence of efficacy. At present, the effect of theophylline seems heterogeneous and relatively modest with uncertain clinical impact.
There has been recent interest that statins might be useful in preventing CIN. Although the exact mechanism is not understood, statins may be beneficial for several reasons. Statins could modulate the kidney hypoperfusion secondary to contrast media administration by downregulating angiotensin receptors and decreasing endothelin production [39]. The pleiotropic effects on the renal vasculature might provide clinical benefit [27]. Moreover, the antioxidant, anti-inflammatory and antithrombotic properties of statins might reduce injury due to CIN [40]. Compared with a previous meta-analysis presented by the same group which showed a non-significant trend, the most recent review included more studies, was able to stratify by renal failure and included consideration for differences in serum creatinine. One critical limitation of the statin data is that only three trials were placebo controlled [41-43], whereas four of the studies actually compared two different doses of statins (high dose vs low dose) [44-47], thus making the data very difficult to interpret. Moreover, the eight included studies are of variable quality, and, as such, the robustness of the evidence would be considered to be weak. Hence, a large high-quality trial may be needed to determine whether indeed statins should be used in those at risk of CIN.
Sodium bicarbonate is another agent which may be protective in CIN, by the mechanism of alkalinasation of renal tubular fluid and increased urinary flow [15,16,48]. While the pooling of all studies may suggest that there is a potential benefit, there is a major concern about publication bias. The review by Zoungas et al [28] revealed strong publication bias in the literature as small studies showing positive significant results are more likely to be published while studies which fail to show any difference are unpublished. This suggests that poor methodology may give rise to these discrepant results and that a large, high-quality study is still needed. Therefore, at present, a large, well-designed, multicentre randomised controlled study is needed before sodium bicarbonate should be used for CIN.
We found no studies that directly compared one intervention with another. As a result, we were able to compare studies directly only by adjusted indirect comparison. Comparing each intervention that was significantly better than the placebo against each other failed to demonstrate any significant difference among them.
Our review has a few strengths. We included only results from randomised trials which, if performed using a good methodology, had a high level of evidence. We were able to evaluate reviews for nine different interventions for CIN. Our review also identifies the most up-to-date meta-analyses for each intervention, and all studies included CIN as a primary outcome, with similar definitions for CIN across most studies.
There are a few limitations to our review. There was considerable heterogeneity in participants and methods in individual studies. We cannot exclude the possibility of publication bias in the studies which suggested positive results for each intervention. Aside from NAC and sodium bicarbonate, there were few trials in most reviews. Moreover, most reviews contained studies that were inadequately powered, as adverse reactions to contrast are infrequent. Other limitations include the observation that not all control groups were placebos, and the type of contrast (iodinated or not) was not considered.
We believe that more research is needed before we can conclude that there is evidence to choose one treatment over another for CIN. We recognise that the ACT investigators conducted a study of high methodology quality and more studies looking at different interventions should use it as a model. The sample size of the study was large enough to enable the analysis of subgroups, which is useful, but the only caveat was that there were few events, which may relate to the low dose of contrast used. More studies are needed to specifically investigate prophylaxis of CIN in endovascular abdominal aneurysm repair or embolisation because the protocols are designed based on expert opinion.
Conclusions
At present, there does not appear to be robust, conclusive evidence for any particular intervention for routine use in the prevention of CIN. While there is some evidence suggesting that NAC might be useful, the ACT investigators trial was an adequately powered high-quality study that showed no benefit compared with a placebo. Further research is needed on agents such as theophylline, statins and sodium bicarbonate, whereas furosemide should be avoided before and after the use of contrast.
Appendix A
Search strategy
[sb] = (systematic review [ti] OR meta-analysis [pt] OR meta-analysis [ti] OR systematic literature review [ti] OR
(systematic review [tiab] AND review [pt]) OR consensus development conference [pt] OR
practice guideline [pt] OR cochrane database syst rev [ta] OR acp journal club [ta] OR
health technol assess [ta] OR evid rep technol assess summ [ta])
OR
((evidence based[ti] OR evidence-based medicine [mh] OR best practice* [ti] OR evidence synthesis [tiab])
AND
(review [pt] OR diseases category[mh] OR behavior and behavior mechanisms [mh] OR therapeutics [mh] OR
evaluation studies[pt] OR validation studies[pt] OR guideline [pt]))
OR
((systematic [tw] OR systematically [tw] OR critical [tiab] OR (study selection [tw]) OR
(predetermined [tw] OR inclusion [tw] AND criteri* [tw]) OR exclusion criteri* [tw] OR main outcome measures [tw] OR
standard of care [tw] OR standards of care [tw])
AND
(survey [tiab] OR surveys [tiab] OR overview* [tw] OR review [tiab] OR reviews [tiab] OR search* [tw] OR
handsearch [tw] OR analysis [tiab] OR critique [tiab] OR appraisal [tw] OR
(reduction [tw]AND (risk [mh] OR risk [tw]) AND (death OR recurrence)))
AND
(literature [tiab] OR articles [tiab] OR publications [tiab] OR publication [tiab] OR
bibliography [tiab] OR bibliographies [tiab] OR published [tiab] OR
unpublished [tw] OR citation [tw] OR citations [tw] OR database [tiab] OR internet [tiab] OR textbooks [tiab] OR
references [tw] OR scales [tw] OR papers [tw] OR datasets [tw] OR trials [tiab] OR meta-analy* [tw] OR
(clinical [tiab] AND studies [tiab]) OR treatment outcome [mh] OR treatment outcome [tw]))
NOT
(letter [pt] OR newspaper article [pt] OR comment [pt])
References
- 1.Cruz DN, Goh CY, Marenzi G, Corradi V, Ronco C, Perazella MA. Renal replacement therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Med 2012;125:66–78. [DOI] [PubMed] [Google Scholar]
- 2.Morcos SK. Contrast media-induced nephrotoxicity-questions and answers. Br J Radiol 1998;71:357–85. [DOI] [PubMed] [Google Scholar]
- 3.Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol 1999;9:1602–13. [DOI] [PubMed] [Google Scholar]
- 4.Kooiman J, Pasha SM, Zondag W, Sijpkens YW, van derMolen AJ, Huisman MV, et al. Meta-analysis: Serum creatinine changes following contrast enhanced CT imaging. Eur J Radiol 2012;81:2554–61. [DOI] [PubMed] [Google Scholar]
- 5.Bakris G, Baber A, Jones J. Oxygen free radicals involvement in urinary Tamm-Horsfall protein excretion after intrarenal injection of contrast medium. Radiology 1990;175:57–60. [DOI] [PubMed] [Google Scholar]
- 6.Bakris G, Lass N, Gager A. Radiocontrast medium-induced declines in kidney function: a role for oxygen free radical. Am J Physiol 1990;258:F115–20. [DOI] [PubMed] [Google Scholar]
- 7.Bakris G, Lass N, Glock D. Renal hemodynamics in radiocontrast medium-induced renal dysfunction. Kidney Int 1999;56:206–10. [DOI] [PubMed] [Google Scholar]
- 8.Heyman S, Reichman J, Brezis M. Pathophysiology of radiocontrast nephropathy: a role for medullary hypoxia. Invest Radiol 1999;34:685–91. [DOI] [PubMed] [Google Scholar]
- 9.McCullough PA, Adam A, Becker CR, Davidson C, Lameire N, Stacul F, et al. Epidemiology and prognostic implications of contrast induced nephropathy. Am J Cardiol 2006;98:5K–13K. [DOI] [PubMed] [Google Scholar]
- 10.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 2006;354:2773–82. [DOI] [PubMed] [Google Scholar]
- 11.Carbonell N, Sanjuan R, Blasco M, Jorda A, Miguel A. N-acetylcysteine: short-term clinical benefits after coronary angiography in high-risk renal patients. Rev Esp Cardiol 2010;63:12–19. [DOI] [PubMed] [Google Scholar]
- 12.Lawlor DK, Moist L, DeRose G, Harris KA, Lovell MB, Kribs Sw , et al. Prevention of contrast-induced nephropathy in vascular surgery patients. Ann Vasc Surg 2007;21:593–7. [DOI] [PubMed] [Google Scholar]
- 13.Shy KG, Cheng JJ, Kuan P. Acetylecysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol 2002;40:1383–8. [DOI] [PubMed] [Google Scholar]
- 14.ACT Investigators. Acetylecysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography. Main results from the randomized acetylcysteine for constrast-induced nephropathy trial (ACT). Circulation 2011;124:1250–9. [DOI] [PubMed] [Google Scholar]
- 15.Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, et al. Prevention of constrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA 2004;291:2328–34. [DOI] [PubMed] [Google Scholar]
- 16.Recio-Mayoral A, Chaparro M, Prado B, Cozar R, Mendez I, Banderjee D, et al. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO study. J Am Coll Cardiol 2007;49:1283–8. [DOI] [PubMed] [Google Scholar]
- 17.Briguori C, Airoldi F, D'Andrea D, Bonizzoni E, Morici N, Focaccio A, et al. Renal insufficiency following contrast media administration trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation 2007;115:1211–17. [DOI] [PubMed] [Google Scholar]
- 18.Masuda M, Yamada T, Mine T, Morita T, Tamaki S, Tsukamoto Y, et al. Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. Am J Cardiol 2007;100:781–6. [DOI] [PubMed] [Google Scholar]
- 19.Saidin R, Zainudin S, Kong N, Maskon O, Saaidin N, Shah S. Intravenous sodium bicarbonate versus normal saline infusion as prophylaxis against contrast nephropathy in patients with chronic kidney disease undergoing coronary angiography or angioplasty [Abstract]. J Am Soc Nephrol 2006;17:766A. [Google Scholar]
- 20.Addad F, Gamra H, Jemmali M, Dridi Z, Ben Hamda K, Betbout F, et al. Acetylcysteine versus bicarbonate or combination to prevent contrast-induced nephropathy in patients with diabetes and chronic renal insufficiency: ABC contrast study. In. World Congress of Cardiology—ESC Congress 2006; 2–6 September 2006; Barcelona, Spain: P1544. Barcelona, Spain: European Society of Cardiology Congress; 2006. [Google Scholar]
- 21.Kapoor A, Kumar S, Gulati S, Gambhir S, Sethi RS, Sinha N. The role of theophylline in contrast-induced nephropathy: a case-control study. Nephrol Dial Transplant 2003;17:1936–41. [DOI] [PubMed] [Google Scholar]
- 22.Huber W, Schipek K, Ilgmann K, Page M, Hennig M, Wacker A, et al. Effectiveness of theophylline prophylaxis of renal impairment after coronary angiography in patients with chronic renal insufficiency. Am J Cardiol 2003;91:1157–62. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi M, Brown P, Romanowski C, Morcos S, Campbell S, El nahas A, et al. The use of theophylline, an adenosine receptor antagonist in the prevention of contrast media induced nephrotoxicity [letter]. Br J Radiol 1992;65:838. [DOI] [PubMed] [Google Scholar]
- 24.Erley CM, Duda SH, Rehfuss D, Scholtes B, Bock J, Muller C, et al. Prevention of radiocontrast-media-induced nephropathy in patients with pre-existing renal insufficiency by hydration in combination with the adenosine antagonist theophylline. Nephrol Dial Transplant 1999;14:1146–9. [DOI] [PubMed] [Google Scholar]
- 25.Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC. Meta-analysis: Effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med 2008;148:284–94. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Li T, Fu N, Hu Y, Cong H. Is angiotensin-converting enzyme inhibitor appropriate for contrast-induced nephropathy? A meta-analysis about this field. Int J Cardiol 2012:486–8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang B-C, Li W-M, Xu Y-W. High-dose statin pretreatment for the prevention of contrast-induced nephropathy a meta-analysis. Can J Cardiol 2011;27:851–8. [DOI] [PubMed] [Google Scholar]
- 28.Zoungas S, Ninomiya T, Huxley R, Cass A, Jardine M, Gallagher M, et al. Systematic review: Sodium bicarbonate treatment regimens for the prevention of contrast-induced nephropathy. Ann Intern Med 2009;151:631–8. [DOI] [PubMed] [Google Scholar]
- 29.Dai B, Liu Y, Fu L, Li Y, Zhang J, Mei C. Effect of theophylline on prevention of contrast-induced acute kidney injury: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2012;60:360–70. [DOI] [PubMed] [Google Scholar]
- 30.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683–91. [DOI] [PubMed] [Google Scholar]
- 31.Wells GA, Sultan SA, Chen L, Khan M, Coyle D. Indirect treatment comparison [computer program]. In: Indirect treatment comparison. 1.0 edn. Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 32.Jadad AR, Moore RA, Carrol D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 33.Drager LF, Andrade L, Barros deToledo JF, Laurindo FR, Machado CesarLamSeguro AC. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant 2004;19:1803–7. [DOI] [PubMed] [Google Scholar]
- 34.Lopez BL, Snyder JW, Birenbaum DS, Ma XI. N-Acetylcysteine enhances endothelium-dependent vasorelaxation in the isolated rat mesenteric artery. Ann Emerg Med 1998;32:405–10. [DOI] [PubMed] [Google Scholar]
- 35.Heyman SN, Rosen S, Khamaisi M, Idee JM, Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol 2010;45:188–95. [DOI] [PubMed] [Google Scholar]
- 36.Arend LJ, Barkris GL, Burnett JC, JR, Megerian C, SPielman WS. Role for intrarenal adenosine in the renal hemodynamic response to contrast media. J Lab Clin Med 1987;110:406–11. [PubMed] [Google Scholar]
- 37.Katholi RE, Taylor GJ, McCann WP, Woods WT, Jr, Womack KA, McCoy CD, et al. Nephrotoxicity from contrast media: attenuation with theophylline. Radiology 1995;195:17–22. [DOI] [PubMed] [Google Scholar]
- 38.Oldroyd SD, Fang L, Haylor JL, Yates, MS ElNahas AM, Morcos SK. Effects of adenosine receptor antagonists on the responses to contrast media in the isolated rate kidney. Clin Sci (Lond) 2000;98:303–11. [PubMed] [Google Scholar]
- 39.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering: are they clinically relevant? Eur Heart J 2003;24:225–48. [DOI] [PubMed] [Google Scholar]
- 40.Wierzbicki AS, Poston R, Ferro A. The lipid and non-lipid effects of statins. Pharmacol Ther 2003;99:95–112. [DOI] [PubMed] [Google Scholar]
- 41.Toso A, Maioli M, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, et al. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am J Cardiol 2010;105:288–92. [DOI] [PubMed] [Google Scholar]
- 42.Acikel S, Muderrisoglu H, Yidirir A, Aydinalp A, Sade E, Bayraktar N, et al. Prevention of contrast-induced impairment of renal function by short-term or long-term statin therapy in patients undergoing elective coronary angiography. Blood Coagul Fibrinolysis 2010;21:750–5. [DOI] [PubMed] [Google Scholar]
- 43.Jo SH, Koo BK, Park JS, Kang HJ, Cho YS, Kim YJ. Prevention of radiocontrast medium-inducted nephropathy using short-term high-dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial—a randomized controlled study. Am Heart J 2008;155:499.e1–8. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Jin YZZ, Wang Q. Efficacy of high dose atorvastatin on preventing contrast nephropathy in patients underwent coronary angiography. Zhonghua Xin Xue Guan Bing Za Zhi 2009;37:394–96. [PubMed] [Google Scholar]
- 45.Xinwei J, Xianghua F, Jing Z, Xinshun G, Ling X, Weize F, et al. Comparison of usefulness of simvastatin 20 mg versus 80 mg in preventing contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol 2009;104:519–24. [DOI] [PubMed] [Google Scholar]
- 46.Lin J, Hong L-R, Qiu Y. [Clinical research of atorvastatin on preventing contrast-induced nephropathy in patients underwent coronary artery CTA]. Clin Med Eng 2010;17:48–50. [Google Scholar]
- 47.Hua X-P, Wu R-X, Yang Y, Cao Z, Chen B. [Prevention of contrast-induced nephropathy using high-dose atorvastin in patients with coronary heart disease undergoing elective percutaneous coronary intervention] Milit Med J South China 2010;24:448–51. [Google Scholar]
- 48.Fischereder M. Use of intravenous sodium bicarbonate might increase the risk of contrast nephropathy. Nat Clin Pract Nephrol 2008;4:296–7. [DOI] [PubMed] [Google Scholar]