Abstract
In mouse heart, four connexins (Cxs), Cx30.2, Cx40, Cx43, and Cx45, form gap junction (GJ) channels for electric and metabolic cell-to-cell signaling. Extent and pattern of Cx isoform expression together with cytoarchitecture and excitability of cells determine the velocity of excitation spread in different regions of the heart. In the SA node, cell– cell coupling is mediated by Cx30.2 and Cx45, which form lowconductance (approximately 9 and 32 pS, respectively) GJ channels. In contrast, the working cardiomyocytes of atria and ventricles express mainly Cx40 and Cx43, which form GJ channels of high conductance (approximately 180 and 115 pS, respectively) that facilitate the fast conduction necessary for efficient mechanical contraction. In the AV node, cell–cell coupling is mediated by abundantly expressed Cx30.2 and Cx45 and Cx40, which is expressed to a lesser extent. Cx30.2 and Cx45 may determine higher intercellular resistance and slower conduction in the SA- and AV-nodal regions than in the ventricular conduction system or the atrial and ventricular working myocardium. Cx30.2 and its putative human ortholog, Cx31.9, under physiologic conditions form unapposed hemichannels in nonjunctional plasma membrane; these hemichannels have a conductance of approximately 20 pS and are permeable to cationic dyes up to approximately 400 Da in molecular mass. Genetic ablation of Cxs confirmed that Cx40 and Cx43 are important in determining the high conduction velocities in atria and ventricles, whereas the deletion of the Cx30.2 complementary DNA led to accelerated conduction in the AV node and reduced the Wenckebach period. We suggest that these effects are caused by (1) a dominant-negative effect of Cx30.2 on junctional conductance via formation of low-conductance homotypic and heterotypic GJ channels, and (2) open Cx30.2 hemichannels in non-junctional membranes, which shorten the space constant and depolarize the excitable membrane.
In the mammalian heart, gap junction (GJ) channels mediate electric and metabolic communication between cardiac myocytes, thereby allowing cardiac impulse propagation and coordinated contraction of the chambers. Gap junction channels, which connect the cytosplasms of two adjacent cells and enable the diffusion of molecules up to approximately 1 kDa molecular mass, are composed of two hemichannels or connexons (Kumar and Gilula 1996). Each hemichannel consists of six connexin (Cx) protein subunits that are encoded by a multigene family comprising at least 20 members in the mouse and human genome (Söhl and Willecke 2004). Different Cxs coexpressed in the same tissue can form homotypic (same Cx isotype in both hemichannels), heterotypic (two Cx isotypes form cell–cell channels, but each hemichannel is assembled from one isotype), and heteromeric (different Cx isotypes in the single hemichannel) GJ channels, which display distinct electrophysiologic properties as well as different permeabilities to ions and second messengers. Recently, mouse Cx30.2 was characterized as a novel cardiac Cx, which, like its human ortholog Cx31.9, forms gap junctional channels of low unitary conductance and low sensitivity of gating to transjunctional voltage in transfected cells (Nielsen et al. 2002, White et al. 2002, Kreuzberg et al. 2005).
Expression of Cxs in the Heart of Rodents
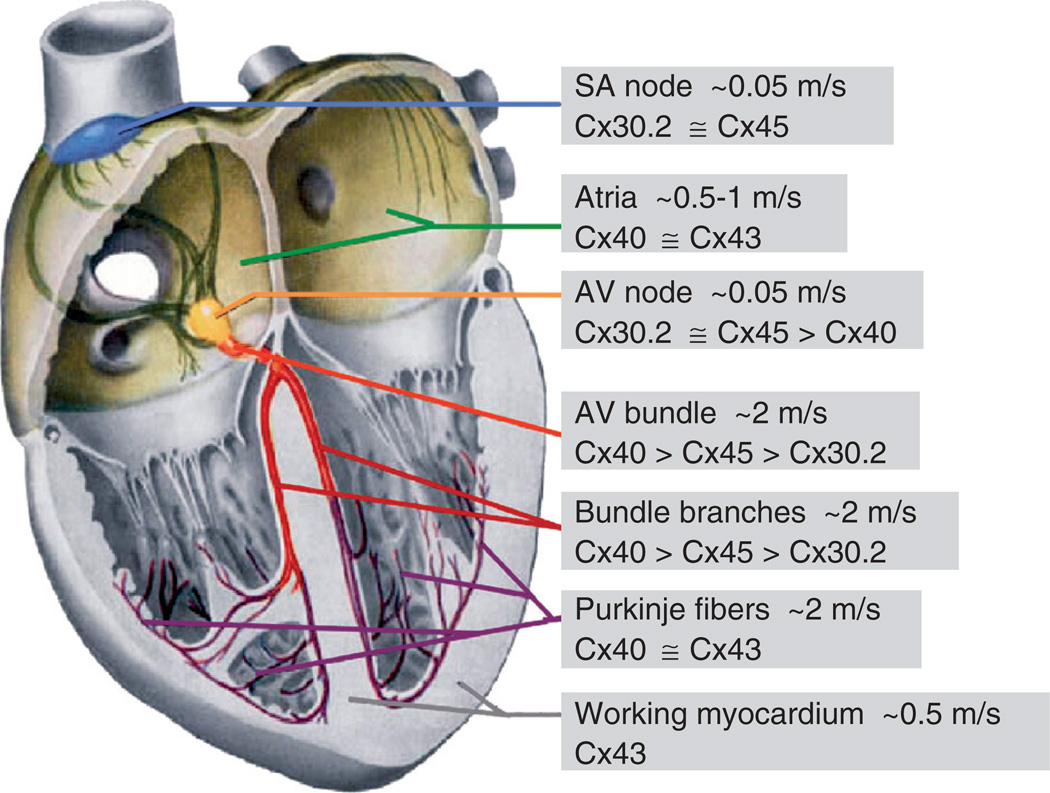
For more than a decade, it was assumed that Cx40, Cx43, and Cx45 determine the propagation of excitation in the heart. Cx30.2 was recently characterized as a fourth cardiac Cx, which is expressed preferentially in the SA- and AV-nodal regions of the mouse heart (Kreuzberg et al. 2005). Expression patterns of all cardiac Cxs are shown in Figure 1. Cx43, the major Cx forming GJ channels between working cardiomyocytes of atria and ventricles, is also expressed in the network of Purkinje fibers forming the distal part of the ventricular conduction system (Beyer et al. 1989, Gourdie et al. 1993, Coppen et al. 1999a). Cx40 colocalizes with Cx43 in the atrial working myocytes and is abundantly expressed in the His bundle, bundle branches, and subendocardial network of Purkinje fibers, that is, structures with high conduction velocities. Furthermore, Cx40 is expressed in the central region of the AV node (Kirchhoff et al. 1998, Coppen et al. 1999b). Cx45 is abundantly expressed in the SA node and in the AV node with its posterior extension. In the His bundle and branches, Cx40 and Cx45 expression overlaps, and at the transition from bundle branches to subendocardial network of Purkinje fibers, the expression of Cx45 decreases, whereas expression of Cx43 increases (reviewed by Lo 2000) (Figure 1). Our recent data show that expression of Cx30.2 in mice is mainly restricted to the cardiac conduction system, with the most abundant expression in the SA node, in the atrial vestibule, and in the AV node with its posterior extension. Only low levels of Cx30.2 protein were found in the His bundle and bundle branches (Kreuzberg et al. 2005). Cx30.2 expression was largely colocalized with Cx45 expression throughout the cardiac conduction system (Figure 2). An overlapping expression pattern of Cx30.2 and Cx40 was found only in part of the AV node, the His bundle, and the bundle branches (Figure 1).
Figure 1.
Schematic of the heart (modified from Netter 1993) illustrating the spread of excitation and Cxs expression patterns. Boxed areas connected with different regions of the heart indicate conduction velocities and Cxs expressed.
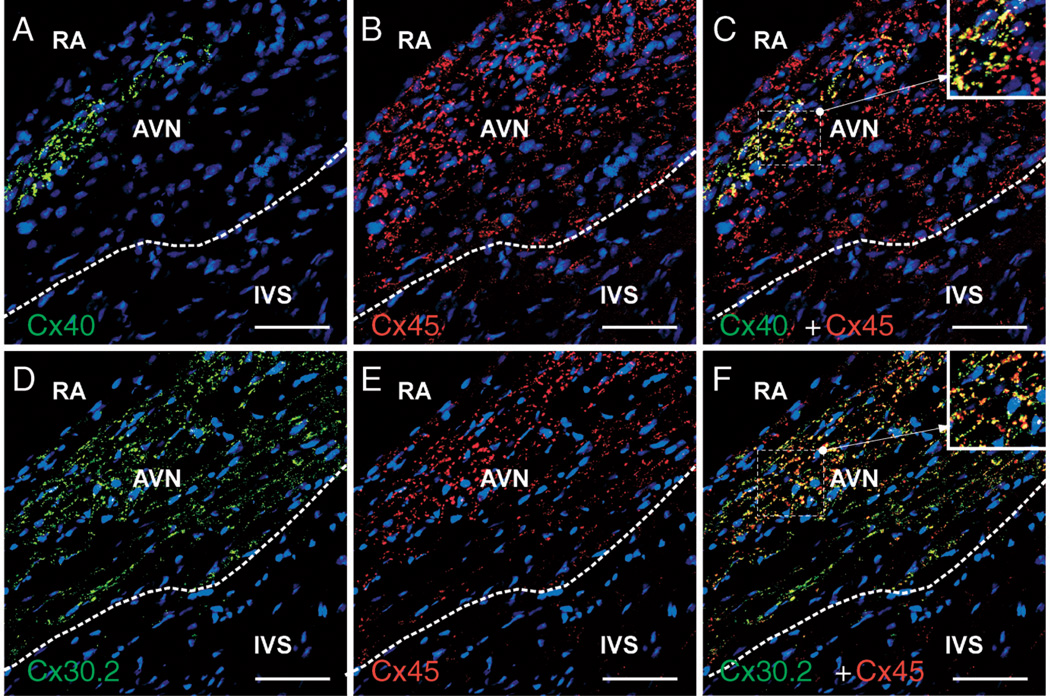
Figure 2.
Coexpression of Cx30.2, Cx40, and Cx45 in AV-nodal regions of the mouse heart revealed by immunohistochemical labeling of Cx pairs. Image (C) is a merge of images (A and B). Image (F) is a merge of images (D and E). It is evident that Cx30.2 (D) and Cx45 (E) are more intensely and broadly coexpressed than are Cx30.2 and Cx40. Nuclei are stained in blue. AVN indicates atrioventricular node; IVS, interventricular septum; RA, right atrium. Scale bars, 20 µm.
Impulse Propagation in the Heart
In the mammalian heart, the cardiac impulse is initiated in the spontaneously active pacemaker cells of the SA node. Electric signals then propagate toward the crista terminalis, and excitation from there spreads with the velocity of 0.5 to 1 m/s (in the human heart) through the right and left atria. The excitation wave enters the AV node by passing the transitional A–N zone, and its velocity of propagation gradually decreases to approximately 0.05 m/s in the central region called compact node or N region (Figure 1, which shows velocities of excitation propagation in different regions of the human heart). After passing the transitional N–H region and entering the His bundle, the propagation of excitation quickly accelerates, reaching approximately 2 m/s, and remains at this level in the subendocardial network of Purkinje fibers, resulting in coordinated contraction of both ventricles.
In the normal heart, the AV node is the sole connection between atria and ventricles for excitation transfer, and the delay of conduction appears to be its main function. The AV-nodal delay is necessary for the sequential contraction of atria and ventricles, which is important for the optimal hemodynamics (Mazgalev and Tchou 2000). In addition, the relatively long refractory period of the AV-nodal cells and the dependence of the refractory period and of the conduction velocity on the frequency of excitation limit the maximum number of impulses within a given period that can be transmitted to the ventricles (Meijler and Janse 1988). Thus, the AV node filters high atrial frequencies during atrial fibrillation and therefore protects the ventricular myocardium against the transmission of atrial tachyarrhythmias (Dobrzynski et al. 2003). Slow AV-nodal conduction under pathophysiologic conditions can also lead to the generation of life-threatening reentry tachycardias.
Correlation Between Cx Expression and Propagation of Excitation
The spread of excitation in different parts of the heart is determined by the tissue geometry, the excitability of the cells, and the extent of gap junctional coupling. The number, size, and distribution of GJ plaques (clusters of GJ channels in the junctional membrane), as well as the expression pattern of Cx isoforms, determine the electrophysiologic properties of assembled GJ channels and cell-to-cell coupling. In the SA node, Cx30.2 and Cx45 channels integrate thousands of pacemaker cells with various intrinsic frequencies of excitation into one functional unit, which defines the heart rhythm. These Cxs can form low-conductance homotypic GJ channels of 9 and 32 pS, respectively, as well as Cx30.2/Cx45 heterotypic GJs with the single-channel conductance of approximately 17 pS (Bukauskas and Verselis 2004, Kreuzberg et al. 2005). Electric coupling in the transitional region between the SA node and the right atrium should be such that the functional identity of pacemaker cells from surrounding cells of the atrium is maintained (Verheijck et al. 1998). In this region, expression of all Cx isoforms typical for both atria and the SA node, that is, Cx40, Cx43, Cx30.2, and Cx45, is likely to occur, which may result in intercellular communication through a high variety of homotypic, heterotypic, and even heteromeric channels. Cx30.2 protein can form low-conductance heterotypic junctions with all other cardiac Cxs with conductances of approximately 18 pS or less and may have a dominant-negative effect on intercellular coupling in the transitional region. Cx45 can form heterotypic junctions with all other cardiac Cxs with single-channel conductances in the range of approximately 17 to 50 pS (Table 1), but a substantial fraction of these channels (>0.5) are closed in the absence of transjunctional voltage, that is, at Vj = 0 mV (Bukauskas and Verselis 2004). Heteromeric junctions can be formed from Cx45 and Cx40 proteins (Martinez et al. 2002), but there are no data yet demonstrating the formation of heteromeric channels containing Cx30.2.
Table 1.
Single-channel conductances of mouse cardiac Cxs analyzed in transfected HeLa cells
| Single-channel conductance of homotypic and heterotypic GJ channels (pS) | ||||
|---|---|---|---|---|
| Cx43 | Cx40 | Cx45 | Cx30.2 | |
| Cx43 | 115 | – | – | – |
| Cx40 | No function | 180 | – | – |
| Cx45 | 55 | 40a | 32 | – |
| Cx30.2 | 18 | 18 | 17 | 9 |
Unless otherwise indicated, data were taken from Haubrich et al. (1996), Bukauskas and Verselis (2004), and Kreuzberg et al. (2005).
Hayrapetyan and Moreno (2003) and our unpublished data.
Within atria, gap junctional channels formed of Cx40 and Cx43 provide fast spread of excitation, assuring almost synchronous contraction of both atria. We assume that cell–cell coupling in atria is maintained by homotypic Cx40 and Cx43 GJ channels exhibiting high single-channel conductances of the open state (180 and 115 pS, respectively) (Bukauskas et al. 1995, Bukauskas and Verselis 2004). The occurrence of functional Cx40/Cx43 heterotypic junctions has been disputed (Bruzzone et al. 1993, Haubrich et al. 1996, Valiunas et al. 2000, Cottrell et al. 2002), and Cx40/Cx43 heteromeric connexons do not yield functional gap junctional channels (Valiunas et al. 2001). The original studies performed in Xenopus oocytes and HeLa cells expressing Cx40 and Cx43 (Bruzzone et al. 1993, Haubrich et al. 1996) showed that Cx40 and Cx43 are not compatible to form heterotypic junctions, which is in accord with our latest unpublished data, and we assume that excitation transfer between working cardiomyocytes of atria occurs through homotypic Cx40 and Cx43 GJ channels.
Conduction in the AV node may be slow for several reasons, which include reduced cellular excitability, by, for example, decreased number of Na+ channels, reduced resting potential that partially inactivates inward currents, branching of fibers, and lower cell–cell coupling (Spach et al. 2000, Kléber and Rudy 2004). Electric intercellular coupling in SA- and AV-nodal regions is several times lower than in working myocardium (Bukauskas and Veteikis 1977, Bukauskas et al. 1982). Like the SA node, cells of the AV node express Cx30.2 and Cx45 and, in addition, Cx40, although only in restricted areas (Figure 2). There are reports that Cx43 is also expressed in the transitional regions of the AV node in rabbits, and Cx expression patterns can be species dependent (reviewed by Efimov et al. 2004). Heterotypic channels, formed by docking of Cx30.2 hemichannel with hemichannels of other cardiac Cxs, exhibit low unitary conductances (approximately 17–18 pS) (Kreuzberg et al. 2005). Low conductance of homotypic and heterotypic GJ channels formed of Cx30.2 may result in a dominant-negative effect of Cx30.2 on junctional conductance between nodal cells and may explain the low conduction velocity in the wild-type mice and enhanced AV conduction in the Cx30.2-deficient mice (Kreuzberg et al. 2006).
As shown in Figure 1, the expression pattern of Cxs changes along the pathway in which the His bundle ramifies into right and left branches and Purkinje terminals. Cx40 is expressed along this pathway, whereas the expression of Cx30.2 and Cx45 gradually decreases and Cx43 appears to be expressed in the Purkinje terminals (van Rijen et al. 2001, Miquerol et al. 2004). From the Purkinje terminals, the excitation is transferred to the ventricular working myocytes presumably through Cx43 homotypic GJs.
In recent years, it has become evident that permeability of unapposed Cx-based hemichannels to ions, metabolites, and signaling molecules is important to cellular functions under normal and pathologic conditions (Bennett et al. 2003). Two cardiac Cxs, Cx43 (Kondo et al. 2000, Bennett et al. 2003) and Cx45 (Valiunas 2002), have been shown to form functional hemichannels, which are preferentially in the closed state at negative resting potentials and tend to open during depolarization. Presumably, open channel probability of these hemichannels increases during action potentials and to a greater extent during tachycardia. In one report, Cx40 hemichannels were found not to be functional (Beahm and Hall 2004). We recently reported that both Cx30.2 and Cx31.9 hemichannels residing in the plasma membrane could open under physiologic conditions allowing uptake of cationic fluorescent dyes up to approximately 400 Da in molecular mass (Bukauskas et al. 2006). Unitary conductance of mouse Cx30.2 hemichannels is approximately 20 pS, about twice the cell–cell channel conductance. We hypothesize that a relatively high fraction of Cx30.2 or Cx31.9 hemichannels are open under normal conditions because of their low-voltage gating sensitivity, whereas gating mechanisms of Cx43 and Cx45 hemichannels are substantially more sensitive to voltage, and more of these hemichannels are closed at the inside negative resting potential. Opening of hemichannels in nodal cells, which exhibit very high membrane resistance, would depolarize them, shorten the space constant, and increase locally extracellular concentration of K+ ions. Depolarization could inactivate inward currents driving excitability and contribute to the reduction in conduction velocity.
Alterations of Cardiac Conduction in Cx-Deficient Mice
Unrestricted and cardiomyocyte-specific null mutant mice of different cardiac Cx genes were generated during the last decade to investigate the role of Cxs in cardiac impulse propagation.
Deletion of Cx43 gene resulted in postnatal death because of malformation of the right ventricular outflow tract (Reaume et al. 1995). The postnatal lethality of Cx43-deficient mice can be circumvented by cardiomyocyte-directed deletion. The resulting mice exhibit a reduced conduction velocity in the ventricles, thus, demonstrating the importance of Cx43 in the maintenance of ventricular conduction (Gutstein et al. 2001, van Rijen et al. 2004).
Cx40-deficient mice exhibit several conduction abnormalities. Ectopic breakthrough points revealed impaired SA-nodal function (Bagwe et al. 2005). In addition, Cx40-deficient mice show slower conduction velocity in the atria and the AV-nodal region and impaired conduction in the bundle branches (Kirchhoff et al. 1998, Hagendorff et al. 1999, van Rijen et al. 2001). Furthermore, impaired AV-nodal conduction is reflected by a prolonged Wenckebach period and AV-nodal refractory period in these mice (VanderBrink et al. 2000).
The impact of Cx45 on cardiac impulse propagation remains elusive because unrestricted and cardiac-actin promoter-Cre-mediated deletion of Cx45 resulted in embryonic lethality because of endocardial cushion defects and impaired vascularization (Kumai et al. 2000, Krüger et al. 2000, Nishii et al. 2001). The embryonic lethality can be circumvented by α myosin heavy chain promoter-Cre-mediated deletion (Agah et al. 1997) of Cx45 in conditional Cx45- deficient mice (Maxeiner et al. 2005; S. Maxeiner and K. Willecke, unpublished observations). These mice may therefore be useful for further study of the function of Cx45 in the mammalian heart.
The Cx30.2-deficient mice show no obvious morphologic heart abnormalities but exhibit alterations of impulse propagation in the cardiac conduction system (Kreuzberg et al. 2006). In contrast to the findings in Cx40-deficient mice, the ablation of Cx30.2 results in accelerated impulse propagation through the AV node (Kreuzberg et al. 2006). Alterations in AV-nodal function are also observed during induced episodes of atrial fibrillation, which result in faster ventricular response rates. In addition, the Wenckebach period is shortened in these mice. Thus, Cx30.2 contributes to slow AV-nodal conduction and protection of ventricles from atrial tachyarrhythmias. No alterations of impulse propagation were found in atria, ventricles, His bundle, or bundle branches where Cx30.2 expression is absent or low.
Conclusions and Open Questions
The AV node plays a critical role in cardiac impulse propagation under physiologic as well as pathophysiologic conditions. In human hearts, the formation of dual pathways with different conduction velocities (slow and fast) has been described as facilitating the development of local reentry circuits that can result in AV-nodal reentry tachycardia (Efimov et al. 2004). In rabbit, dual-pathway conduction in the AV node was observed, and the slow pathway was thought to be located in the posterior nodal extension and Cx45 positive (Dobrzynski et al. 2003). So far, there are no reports regarding slow and fast pathways in the mouse heart. Because Cx30.2 and Cx45 are both expressed in the AV node and its posterior extension, these Cxs are likely to provide the molecular substrate for the formation of a slow pathway. However, electrophysiologic investigations of the dual pathway in the AV node and of the susceptibility to reentry tachycardias in Cx30.2-deficient mice have not been carried out because of its small size.
The expression pattern of the rabbit and human orthologs of Cx30.2 has not yet been studied, but they can be expected to be found in the AV node and its posterior extension in these species. The generation of specific antibodies directed to Cx31.9, the putative human ortholog of Cx30.2, and their application on human cardiac tissue should help to answer the question whether Cx31.9 is also expressed preferentially in SA- and AV-nodal regions. We demonstrated that Cx30.2 expression is associated with slow conduction in the AV node in mice (Kreuzberg et al. 2006) and thus might be relevant for the formation of a slow AV-nodal pathway, which can play a role in AV-nodal reentry tachycardia. Cx31.9, similar to Cx30.2, forms homotypic GJ channels exhibiting low gating sensitivity to transjunctional voltage (Nielsen et al. 2002) and the lowest single-channel conductance among known members of the Cx family (White et al. 2002). Cx31.9 also forms unapposed hemichannels that open with relatively high efficiency under normal perfusion conditions (Bukauskas et al. 2006). Thus, Cx31.9 might have a function in the human AV node similar to that of Cx30.2 in mice.
During atrial tachyarrhythmias such as atrial fibrillation, the slow AV-nodal conduction velocity and long refractory period serve as a protection mechanism against extension of the high atrial excitation rates to the ventricles. Mice lacking Cx30.2 during short periods of induced atrial fibrillation exhibited higher ventricular response rates than wild-type mice (Kreuzberg et al. 2006).
The mechanism by which Cx30.2 slows down AV-nodal conduction remains unproven. The unitary conductances of Cx30.2 homotypic gap junctional channels were shown to be very low in HeLa transfectants (Table 1). In addition, Cx30.2 forms functional heterotypic channels with all other cardiac Cxs, and these channels also exhibited low unitary conductances (Kreuzberg et al. 2005). It remains essential to examine whether heteromeric hemichannels and cell–cell channels containing Cx30.2 can be formed and function. Therefore, it is likely that Cx30.2 has a dominant-negative effect on junctional conductance when it forms heterotypic and heteromeric gap junctional channels with other cardiac Cxs; a similar action was recently hypothesized for Cx45 when it was overexpressed in the ventricular myocardium (Betsuyaku et al. 2006). This action could explain the higher conduction velocity within the AV node in Cx30.2-deficient mice, which do not show obvious up-regulation of the other cardiac Cxs. We assume that Cx45 and Cx40 in the absence of Cx30.2 form homotypic, heterotypic, and heteromeric GJ channels of higher single-channel conductance resulting in lower intracellular axial resistance of the nodal tissue and accelerated conduction. Lower conduction velocity in the wild-type animals could be contributed to by open Cx30.2 hemichannels in the surface membrane, which would reduce the space constant and depolarize the cells. Besides the unitary conductances, the permeability of GJ channels to specific ions or second messengers could also influence the conduction and thus should be investigated. Recently, it was shown that Cx43 and Cx45 homotypic gap junctional channels in transfected HeLa cells were both permeable to the second messenger cyclic adenosine monophosphate, but with a fivefold lower efficacy of Cx45 GJ channel (Bedner et al. 2006).
In the heart of patients suffering from the Wolff–Parkinson–White syndrome, the AV-nodal conduction is bypassed by an accessory pathway (Kent’s bundle), which is not anatomically associated with the AV node. The presence of two AV connections greatly facilitates macro reentry formation in the human heart, leading to life-threatening tachycardias that are highly resistant to chemical therapy owing to their anatomic origin. Other structural abnormalities of cardiac conduction system related to formation of James and Mahaim accessory pathways bypassing the atrioventricular node and/or the His bundle also result in shorter latency for ventricular excitation compared with normal AV conduction and in sudden death due to arrhythmias (Aliot et al. 1998). However, expression and distribution of Cxs in such accessory pathways have not yet been investigated in the human heart.
Some forms of arrhythmias appear to be genetically determined by associated mutations that lead to decreased expression of Cx40 and modification (D1275N) of the sodium channel (SCN5A) (Groenewegen et al. 2003). Some patients with oculodentodigital dysplasia, which is caused by mutations in the Cx43 gene, were reported to suffer from cardiac dysfunction (Paznekas et al. 2003). So far, no congenital heart diseases have been linked to the lack or mutation of Cx45 in humans, although Cx45-deficient mice show severe heart abnormalities and embryonic death. Alterations in distribution and expression levels of Cx43, Cx40, and Cx45 have been described in several cardiac diseases. Reduced levels of Cx43 and up-regulation of Cx45 were observed in congestive heart failure (Dupont et al. 2001, Yamada et al. 2003).
Numerous studies highlight the importance of GJs in the genesis of cardiac arrhythmias, which has stimulated the search for antiarrhythmics targeting GJ channels (Eloff et al. 2003). Several reports show that antiarrhythmic properties of the peptide AAP10 and its analogue ZP123, which is more resistant to proteolytic degradation in the digestive tract, are determined by decreased dispersion of activation and increased velocity of impulse conduction in the heart (Haugan et al. 2005). In addition, ZP123 reduced the incidents of spontaneous ventricular arrhythmias of ischemic origin and size of infarct scars (Hennan et al. 2006). It was suggested that these effects resulted from enhancement of intercellular communication, and it was shown that ZP123 does not affect the normal functioning myocardium but is protective during acidosis and metabolic stress (Eloff et al. 2003, Haugan et al. 2005). Thus, a new class of antiarrhythmic peptides that act on gap junctional communication may emerge.
Acknowledgments
Work in the Bonn laboratory was supported by grants of the German Research Association (Wi270/25-1.2 and Wi 270/29-1) and the Bonner Forum Biomedizin to KW. Work in the New York laboratory was funded by National Institutes of Health, RO1 NS036706, to FFB.
Contributor Information
Maria M. Kreuzberg, Institut für Genetik, Abteilung Molekulargenetik, Universität Bonn, 53117 Bonn, Germany
Klaus Willecke, Institut für Genetik, Abteilung Molekulargenetik, Universität Bonn, 53117 Bonn, Germany.
Feliksas F. Bukauskas, Department of Neuroscience, Albert Einstein College of Medicine, Bronx, NY 10461, USA
References
- Agah R, Frenkel PA, French BA, et al. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliot E, De Chillou C, Revault d’Allones G, et al. Mahaim tachycardias. Eur Heart J. 1998;19:E25–E31. [PubMed] [Google Scholar]
- Bagwe S, Berenfeld O, Vaidya D, et al. Altered right atrial excitation and propagation in connexin40 knockout mice. Circulation. 2005;112:2245–2253. doi: 10.1161/CIRCULATIONAHA.104.527325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahm DL, Hall JE. Opening hemi-channels in nonjunctional membrane stimulates gap junction formation. Biophys J. 2004;86:781–796. doi: 10.1016/S0006-3495(04)74154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedner P, Niessen H, Odermatt B, et al. Selective permeability of different connexin channels to the second messenger cyclic AMP. J Biol Chem. 2006;28:6673–6681. doi: 10.1074/jbc.M511235200. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsuyaku T, Nnebe NS, Sundset R, et al. Overexpression of cardiac connexin45 increases susceptibility to ventricular tachyarrhythmias in vivo. Am J Physiol Heart Circ Physiol. 2006;290:H163–H171. doi: 10.1152/ajpheart.01308.2004. [DOI] [PubMed] [Google Scholar]
- Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, Haefliger JA, Gimlich RL, Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell. 1993;4:7–20. doi: 10.1091/mbc.4.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Veteikis RP. Passive electrical properties of the atrioventricular region of the rabbit heart. Biofizika. 1977;22:499–504. [PubMed] [Google Scholar]
- Bukauskas FF, Gutman AM, Kisunas KJ, Veteikis RR. Electrical cell coupling in rabbit sinoatrial node and atrium. Experimental and theoretical evaluation. In: Bouman LN, Jongsma HJ, editors. Cardiac rate and rhythm. Physiological, morphological and developmental aspects. Hague: Martinus Nijhoff Publishers; 1982. pp. 195–216. [Google Scholar]
- Bukauskas FF, Elfgang C, Willecke K, Weingart R. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys J. 1995;68:2289–2298. doi: 10.1016/S0006-3495(95)80411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Kreuzberg MM, Rackauskas M, et al. Functional properties of mouse connexin 30.2 and human connexin 31.9 hemichannels; implications for atrioventricular conduction in the heart. Proc Natl Acad Sci U S A. 2006;103:9726–9731. doi: 10.1073/pnas.0603372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppen SR, Kodama I, Boyett MR, et al. Connexin45, a major connexin of the rabbit sinoatrial node, is co-expressed with connexin43 in a restricted zone at the nodal-crista terminalis border. J Histochem Cytochem. 1999a;47:907–918. doi: 10.1177/002215549904700708. [DOI] [PubMed] [Google Scholar]
- Coppen SR, Severs NJ, Gourdie RG. Connexin45 (alpha 6) expression delineates an extended conduction system in the embryonic and mature rodent heart. Dev Genet. 1999b;24:82–90. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<82::AID-DVG9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Wu Y, Burt JM. Cx40 and Cx43 expression ratio influences heteromeric/heterotypic gap junction channel properties. Am J Physiol. 2002;282:1469–1482. doi: 10.1152/ajpcell.00484.2001. [DOI] [PubMed] [Google Scholar]
- Dobrzynski H, Nikolski VP, Sambelashvili AT, et al. Site of origin and molecular substrate of atrioventricular junctional rhythm in the rabbit heart. Circ Res. 2003;93:1102–1110. doi: 10.1161/01.RES.0000101913.95604.B9. [DOI] [PubMed] [Google Scholar]
- Dupont E, Matsushita T, Kaba RA, et al. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- Efimov IR, Nikolski VP, Rothenberg F, et al. Structure–function relationship in the AV junction. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:952–965. doi: 10.1002/ar.a.20108. [DOI] [PubMed] [Google Scholar]
- Eloff BC, Gilat E, Wan X, Rosenbaum DS. Pharmacological modulation of cardiac gap junctions to enhance cardiac conduction: evidence supporting a novel target for antiarrhythmic therapy. Circulation. 2003;108:3157–3163. doi: 10.1161/01.CIR.0000101926.43759.10. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Severs NJ, Green CR, et al. The spatial distribution and relative abundance of gap-junctional connexin40 and connexin43 correlate to functional properties of components of the cardiac atrioventricular conduction system. J Cell Sci. 1993;105:985–991. doi: 10.1242/jcs.105.4.985. [DOI] [PubMed] [Google Scholar]
- Groenewegen WA, Firouzi M, Bezzina CR, et al. A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res. 2003;92:14–22. doi: 10.1161/01.res.0000050585.07097.d7. [DOI] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Tamaddon H, et al. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagendorff A, Schumacher B, Kirchhoff S, et al. Conduction disturbances and increased atrial vulnerability in connexin40-deficient mice analyzed by transesophageal stimulation. Circulation. 1999;99:1508–1515. doi: 10.1161/01.cir.99.11.1508. [DOI] [PubMed] [Google Scholar]
- Haubrich S, Schwarz HJ, Bukauskas F, et al. Incompatibility of connexin 40 and 43 hemichannels in gap junctions between mammalian cells is determined by intracellular domains. Mol Biol Cell. 1996;7:1995–2006. doi: 10.1091/mbc.7.12.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugan K, Olsen KB, Hartvig L. The antiarrhythmic peptide analog ZP123 prevents atrial conduction slowing during metabolic stress. J Cardiovasc Electrophysiol. 2005;16:537–545. doi: 10.1111/j.1540-8167.2005.40687.x. [DOI] [PubMed] [Google Scholar]
- Hayrapetyan V, Moreno AP. Gating of heterotypic connexin40 and connexin45 gap junction channels (abstract). Biophys J; 47th Meeting of Biophysics; Washington, D.C.. 2003. p. 526a. #2583. [Google Scholar]
- Hennan JK, Swillo RE, Morgan GA, et al. Rotigaptide (ZP123) prevents spontaneous ventricular arrhythmias and reduces infarct size during myocardial ischemia/reperfusion injury in open-chest dogs. J Pharmacol Exp Ther. 2006;317:236–243. doi: 10.1124/jpet.105.096933. [DOI] [PubMed] [Google Scholar]
- Kirchhoff S, Nelles E, Hagendorff A, et al. Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr Biol. 1998;8:299–302. doi: 10.1016/s0960-9822(98)70114-9. [DOI] [PubMed] [Google Scholar]
- Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- Kondo RP, Wang SY, John SA, et al. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- Kreuzberg MM, Söhl G, Kim JS, et al. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ Res. 2005;96:1169–1177. doi: 10.1161/01.RES.0000169271.33675.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzberg MM, Schrickel JW, Ghanem A, et al. Connexin30.2 containing gap junction channels decelerate impulse propagation through the atrioventricularnode. Proc Natl Acad Sci U S A. 2006;103:5959–5964. doi: 10.1073/pnas.0508512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger O, Plum A, Kim JS, et al. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- Kumai M, Nishii K, Nakamura K, et al. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501–3512. doi: 10.1242/dev.127.16.3501. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Lo CW. Role of gap junctions in cardiac conduction and development: insights from the connexin knockout mice. Circ Res. 2000;87:346–348. doi: 10.1161/01.res.87.5.346. [DOI] [PubMed] [Google Scholar]
- Martinez AD, Hayrapetyan V, Moreno AP, Beyer EC. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ Res. 2002;90:1100–1107. doi: 10.1161/01.res.0000019580.64013.31. [DOI] [PubMed] [Google Scholar]
- Maxeiner S, Dedek K, Janssen-Bienhold U, et al. Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. J Neurosci. 2005;25:566–576. doi: 10.1523/JNEUROSCI.3232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazgalev TN, Tchou PJ. Surface potentials from the region of the atrioventricular node and their relation to dual pathway electrophysiology. Circulation. 2000;101:2110–2117. doi: 10.1161/01.cir.101.17.2110. [DOI] [PubMed] [Google Scholar]
- Meijler FL, Janse MJ. Morphology and electrophysiology of the mammalian atrioventricular node. Physiol Rev. 1988;68:608–647. doi: 10.1152/physrev.1988.68.2.608. [DOI] [PubMed] [Google Scholar]
- Miquerol L, Meysen S, Mangoni M, et al. Architectural and functional asymmetry of the His–Purkinje system of the murine heart. Cardiovasc Res. 2004;63:77–86. doi: 10.1016/j.cardiores.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Netter FH. Atlas der Anatomie des Menschen. 4th ed. New York: Ciba Geigy Corporation; 1993. [Google Scholar]
- Nielsen PA, Beahm DL, Giepmans BN, et al. Molecular cloning, functional expression, and tissue distribution of a novel human gap junction-forming protein, connexin-31.9. Interaction with zona occludens protein-1. J Biol Chem. 2002;277:38272–38283. doi: 10.1074/jbc.M205348200. [DOI] [PubMed] [Google Scholar]
- Nishii K, Kumai M, Shibata Y. Regulation of the epithelial–mesenchymal transformation through gap junction channels in heart development. Trends Cardiovasc Med. 2001;11:213–218. doi: 10.1016/s1050-1738(01)00103-7. [DOI] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, De Sousa PA, Kulkarni S, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Söhl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Spach MS, Heidlage JF, Dolber PC, Barr RC. Electrophysiological effects of remodeling cardiac gap junctions and cell size: experimental and model studies of normal cardiac growth. Circ Res. 2000;86:302–311. doi: 10.1161/01.res.86.3.302. [DOI] [PubMed] [Google Scholar]
- Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J Gen Physiol. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V, Weingart R, Brink PR. Formation of heterotypic gap junction channels by connexins 40 and 43. Circ Res. 2000;86:E42–E49. doi: 10.1161/01.res.86.2.e42. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Gemel J, Brink PR, Beyer EC. Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ. 2001;281:H1675–H1689. doi: 10.1152/ajpheart.2001.281.4.H1675. [DOI] [PubMed] [Google Scholar]
- van Rijen HV, Van Veen TA, van Kempen MJ, et al. Impaired conduction in the bundle branches of mouse hearts lacking the gap junction protein connexin40. Circulation. 2001;103:1591–1598. doi: 10.1161/01.cir.103.11.1591. [DOI] [PubMed] [Google Scholar]
- van Rijen HV, Eckardt D, Degen J, et al. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- VanderBrink BA, Sellitto C, Saba S, et al. Connexin40-deficient mice exhibit atrioventricular nodal and infra-Hisian conduction abnormalities. J Cardiovasc Electrophysiol. 2000;11:1270–1276. doi: 10.1046/j.1540-8167.2000.01270.x. [DOI] [PubMed] [Google Scholar]
- Verheijck EE, Wilders R, Joyner RW, et al. Pacemaker synchronization of electrically coupled rabbit sinoatrial node cells. J Gen Physiol. 1998;111:95–112. doi: 10.1085/jgp.111.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Srinivas M, Ripps H, et al. Virtual cloning, functional expression, and gating analysis of human connexin31.9. Am J Physiol Cell Physiol. 2002;283:C960–C970. doi: 10.1152/ajpcell.00163.2002. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Rogers JG, Sundset R, et al. Up-regulation of connexin45 in heart failure. J Cardiovasc Electrophysiol. 2003;14:1205–1212. doi: 10.1046/j.1540-8167.2003.03276.x. [DOI] [PubMed] [Google Scholar]