Abstract
Background
Metastatic thyroid cancers that are refractory to radioiodine (iodine-131) are associated with a poor prognosis. In mouse models of thyroid cancer, selective mitogen-activated protein kinase (MAPK) pathway antagonists increase the expression of the sodium–iodide symporter and uptake of iodine. Their effects in humans are not known.
Methods
We conducted a study to determine whether the MAPK kinase (MEK) 1 and MEK2 inhibitor selumetinib (AZD6244, ARRY-142886) could reverse refractoriness to radioiodine in patients with metastatic thyroid cancer. After stimulation with thyrotropin alfa, dosimetry with iodine-124 positron-emission tomography (PET) was performed before and 4 weeks after treatment with selumetinib (75 mg twice daily). If the second iodine-124 PET study indicated that a dose of iodine-131 of 2000 cGy or more could be delivered to the metastatic lesion or lesions, therapeutic radioiodine was administered while the patient was receiving selumetinib.
Results
Of 24 patients screened for the study, 20 could be evaluated. The median age was 61 years (range, 44 to 77), and 11 patients were men. Nine patients had tumors with BRAF mutations, and 5 patients had tumors with mutations of NRAS. Selumetinib increased the uptake of iodine-124 in 12 of the 20 patients (4 of 9 patients with BRAF mutations and 5 of 5 patients with NRAS mutations). Eight of these 12 patients reached the dosimetry threshold for radioiodine therapy, including all 5 patients with NRAS mutations. Of the 8 patients treated with radioiodine, 5 had confirmed partial responses and 3 had stable disease; all patients had decreases in serum thyroglobulin levels (mean reduction, 89%). No toxic effects of grade 3 or higher attributable by the investigators to selumetinib were observed. One patient received a diagnosis of myelodysplastic syndrome more than 51 weeks after radioiodine treatment, with progression to acute leukemia.
Conclusions
Selumetinib produces clinically meaningful increases in iodine uptake and retention in a subgroup of patients with thyroid cancer that is refractory to radioiodine; the effectiveness may be greater in patients with RAS-mutant disease. (Funded by the American Thyroid Association and others; ClinicalTrials.gov number, NCT00970359.)
Metastatic disease is the most frequent cause of death related to thyroid Mcancer.1 Radioiodine (iodine-131) remains a mainstay of therapy for patients with metastatic thyroid cancer of follicular origin (i.e., papillary thyroid cancer or follicular thyroid cancer). Unfortunately, many patients have tumors that do not concentrate iodine, resulting in radioiodine resistance and a poor prognosis (the 10-year survival rate among patients with metastatic thyroid cancer that retains radioiodine avidity is approximately 60%, whereas it is only 10% if the metastases are refractory to radioiodine therapy).2 Several trials have evaluated strategies to “redifferentiate” metastatic thyroid cancers and render them responsive to radioiodine, including trials evaluating retinoids3–5 and lithium,6 which only yielded a modest clinical benefit. These studies were also limited by methodologic issues such as the inability to quantify radioiodine uptake and retention in tumors, so the effect of the interventions was difficult to assess.
Approximately 70% of papillary thyroid cancers have mutually exclusive gene mutations encoding the growth factor receptors RET or NTRK1, the three isoforms of RAS (N, H, K), and BRAF.7–10 Constitutive activation of these proteins stimulates mitogen-activated protein kinase (MAPK) signaling, which inhibits the expression of thyroid hormone biosynthesis genes, including the sodium–iodide symporter and thyroid peroxidase, which facilitate iodine uptake and organification, respectively.11–15 Cancers that do not concentrate radioiodine develop in transgenic mice in which mutant BRAF is expressed in thyroid cells.16 When BRAF activation is switched off genetically or its downstream signaling is inhibited with kinase inhibitors targeting either MAPK kinase (MEK) or BRAF, the tumors regain the ability to trap radioiodine.
These preclinical observations provided the rationale for our pilot clinical study, in which patients who were found to have metastases that were refractory to radioiodine were treated with the selective, allosteric MEK 1 and MEK 2 inhibitor selumetinib (AZD6244, ARRY-142886),17 and changes in iodine uptake were assessed by means of serial iodine-124 positron-emission tomography (PET)–computed tomography (CT). The use of iodine-124 PET-CT rather than traditional whole-body iodine-131 scintigraphy allowed for precise quantification of iodine uptake before and after selumetinib treatment in individual metastatic lesions (“lesional dosimetry”) and prediction of the dose of radiation that could be delivered with iodine-131.18,19
METHODS
STUDY CONDUCT
The trial was conducted in accordance with the study protocol, available with the full text of this article at NEJM.org. All patients provided written informed consent. The study was approved by the research committees of the Departments of Medicine, Radiology, and Medical Physics at Memorial Sloan-Kettering Cancer Center (MSKCC) and by the center’s institutional review board. All authors vouch for the data, the fidelity of the study to the protocol, and the analysis. No one who is not listed as an author contributed to the manuscript.
PATIENTS
Patients were required to have differentiated thyroid carcinoma of follicular-cell origin, or its respective variants, histopathologically confirmed at the MSKCC. Patients also had to meet at least one of the following criteria for radioiodine-refractory disease: an index metastatic lesion that was not radioiodine-avid on diagnostic radioiodine scanning performed up to 2 years before enrollment; a radioiodine-avid metastatic lesion that remained stable in size or progressed despite radioiodine treatment 6 months or more before entry into the study; and 18F-fluorodeoxy-glucose (FDG)–avid lesions on PET scanning (FDG avidity is indicative of less differentiated thyroid tumors with impaired iodine uptake20 and resistance to radioiodine,21 which are associated with a poor prognosis22). (For additional inclusion and exclusion criteria, see the Supplementary Methods section in the Supplementary Appendix, available at NEJM.org.) Thyrotropin alfa (Thyrogen) was provided by Genzyme, and selumetinib was provided by AstraZeneca. IBA Molecular provided the iodine-124 for the study. These companies did not participate in any aspect of the study design, data accrual, data analysis, or manuscript preparation. The investigational new drug application for selumetinib was held by MSKCC.
STUDY DESIGN
After adhering to a low-iodine diet for 5 days, patients underwent a thyrotropin alfa–stimulated iodine-124 PET-CT study, followed by treatment with selumetinib at a dose of 75 mg given orally twice daily for 4 weeks. In the fourth week of selumetinib treatment, patients underwent a second iodine-124 PET-CT study. Spot urinary iodine measurements were performed before each iodine-124 PET evaluation (median urinary iodine value during the study, 99 μg per liter; range, 0 to 374) to rule out clinically significant iodine contamination before each scan. Patients discontinued the study if the second iodine-124 PET evaluation did not show an increase in iodine uptake to a pre-specified dosimetry threshold. If this dosimetry threshold was met, then patients continued to receive selumetinib (and a low-iodine diet) as thyrotropin alfa–stimulated whole-body and blood dosimetry studies were performed to determine the maximum tolerable activity pursuant to the standard of care at MSKCC.23 After determination of the maximum tolerable activity, a therapeutic dose of iodine-131 was administered after stimulation with thyrotropin alfa.24 Selumetinib was continued until 2 days after ingestion of therapeutic iodine-131. Toxic effects were monitored for 30 days after the last dose of selumetinib. Patients continued to receive suppressive thyroid hormone treatment throughout the protocol. The second iodine-124 PET-CT study in one patient was delayed by a week because of a temporary shortage of the iodine-124 radionuclide; this study showed no appreciable change in iodine uptake.
In patients who received iodine-131, CT imaging, magnetic resonance imaging, or both was performed 2 and 6 months after radioiodine therapy. One radiologist assessed the radiologic response in all patients, as compared with prestudy scans, according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Levels of serum thyrotropin, free thyroxine, thyroglobulin, and thyroglobulin antibodies were measured 1, 2, and 6 months after administration of radioiodine.
IODINE-124 PET-CT STUDIES
Patients received 0.9 mg of thyrotropin alfa intra-muscularly once daily for 2 consecutive days, and then 6 mCi of iodine-124 orally the next day. Two days later, imaging was performed with the use of a PET-CT scanner (Discovery STE, GE Healthcare), without the administration of contrast material. The patient was scanned from the canthomeatal line (the line between the center of the ear canal and the junction of the upper and lower eyelids) to the midthigh with the use of seven to nine bed positions, with scans of 6 minutes per bed position. The images in all the patients were interpreted in a blinded fashion by the same board-certified nuclear-medicine physician, and the number, size, and maximal standardized uptake value of the lesions in each body region were recorded.
If the second iodine-124 PET-CT study indicated increased iodine uptake in the index lesion (or lesions), an estimate was made of the administered activity of iodine-131 required to deliver a projected absorbed dose of 2000 cGy or more to the lesion. This estimate was based on the lesion activity concentration measured on PET multiplied by a recovery coefficient based on the lesion dimensions measured on CT and on an assumed biologic half-life of at least 2 days. If it appeared that one or more lesions could be treated with a dose of 2000 cGy or more with an iodine-131 administered activity of up to 300 mCi, the patient was then eligible for the standard iodine-131 dosimetry protocol to determine the actual activity to be administered. In addition, as previously described, an imaging analysis tool was used with the GE PET volume computer-assisted reading system to localize individual iodine-124 uptake to a specific, corresponding lesion on CT.25
TISSUE GENOTYPING
Mutation detection in DNA isolated from formalin-fixed, paraffin-embedded archival samples was performed with the use of the mass-spectrometry genotyping assay (MassArray, Sequenom), as previously described.26 This assay interrogated for mutations in some of the most common thyroid oncogenes, including BRAF, NRAS, KRAS, PIK3CA, and AKT1. Since the mass-spectrometry assays for codons 12 and 13 of HRAS were not informative, these sites were evaluated by means of Sanger sequencing. If mass spectrometry analysis failed, samples were assayed for BRAF, NRAS, KRAS, and HRAS mutations by means of Sanger sequencing. Samples that were wild-type for oncogene point mutations were screened for RET and PAX8–peroxisome proliferator–activated receptor gamma (PPARG) rearrangements. Tumor complementary DNA (cDNA) was used as a template for a quantitative polymerase-chain-reaction assay to identify unbalanced expression of exons 10 and 11 relative to 12 and 13 of RET, which flank the rearrangement site in intron 11 (Table S1 in the Supplementary Appendix). Samples with greater expression of exons 12 and 13 than of exons 10 and 11 were considered to be positive for an RET/PTC translocation. Cell-line cDNA from medullary thyroid cancers (TT cells) and papillary thyroid cancers (TPC1 cells) were used as positive controls for expression of full-length RET and RET fusion messenger RNA, respectively.
STATISTICAL ANALYSIS
The primary end point was the percentage of patients with selumetinib-induced increases in iodine uptake in the index tumor (or tumors), as quantified by iodine-124 PET at baseline and after 4 weeks of selumetinib. We adopted as the null hypothesis an increase in iodine-124 PET–quantified iodine uptake in 5% of patients, with increased uptake in 25% of patients considered to be desirable. With a type 1 error of 5% and a power of 85%, increased iodine uptake in the second iodine-124 PET scan would need to be observed in at least three patients for the study to be considered positive. A second primary end point was the tumor response at approximately 2 and 6 months after iodine-131 treatment according to RECIST, version 1.1.
A secondary end point was an assessment of whether treatment with iodine-131 after selumetinib was associated with decreased serum thyroglobulin levels at 2 and 6 months. A Wilcoxon signed-rank test for paired samples was used for this landmark comparison at each follow-up assessment. Undetectable thyroglobulin values clinically reported as less than 0.2 ng per milliliter were assigned a value of 0.2 ng per milliliter for calculations. An additional, exploratory end point was an assessment of differences in selumetinib efficacy for enhancing radioiodine uptake between patients with BRAF mutations and patients with wild-type BRAF (additional details of the statistical design are included in the Supplementary Appendix). We used descriptive statistics for this assessment.
RESULTS
STUDY POPULATION
Between August 2010 and December 2011, a total of 24 patients were referred and screened for the study. Two patients were ineligible because of baseline measurements of the QT interval corrected for heart rate which were outside the study range. Two patients were enrolled but discontinued the study before selumetinib was administered: 1 in whom a new brain metastasis was diagnosed and 1 in whom iodinated contrast material was used for a diagnostic scan that revealed a pulmonary embolism.
Baseline clinical characteristics of the 20 patients who could be evaluated are listed in Table 1. Five patients (25%) had classic papillary thyroid cancer, 8 (40%) had tall-cell–variant papillary thyroid cancer, and 7 (35%) had poorly differentiated carcinoma. Nine patients had a BRAF V600E mutation, 5 had an NRAS mutation at codon 61 (Q→R or K), 3 had RET/PTC rearrangements, and 3 had tumors that were wild-type for all genes examined. No PAX8-PPARG rearrangements were detected. One BRAF-mutant tumor and one RET/PTC tumor were poorly differentiated carcinomas; the remaining tumors of these genotypes were papillary thyroid cancers. All five NRAS-mutant tumors were poorly differentiated carcinomas.
Table 1.
Baseline Characteristics of the 20 Patients.
| Characteristic | Value |
|---|---|
| Age — yr | |
| Median | 61 |
| Range | 44–77 |
| Sex — no. | |
| Male | 11 |
| Female | 9 |
| Type of tumor — no./total no. (%) | |
| Classic papillary | 5/20 (25) |
| Tall-cell papillary | 8/20 (40) |
| Poorly differentiated | 7/20 (35) |
| Tumor genotype — no./total no. (%) | |
| BRAF V600E | 9/20 (45) |
| NRAS Q61R and Q61K | 5/20 (25) |
| RET/PTC | 3/20 (15) |
| Wild type | 3/20 (15) |
| Median no. of prior radioiodine treatments per patient | 2.1 |
| Other treatments for thyroid cancer — no. of patients/total no. (%) | |
| External-beam radiation therapy | 7/20 (35) |
| Targeted therapies* | 2/20 (10) |
These patients received sorafenib in combination with an mTORC1 (mammalian target of rapamycin complex 1) inhibitor before enrollment in the study.
EFFICACY
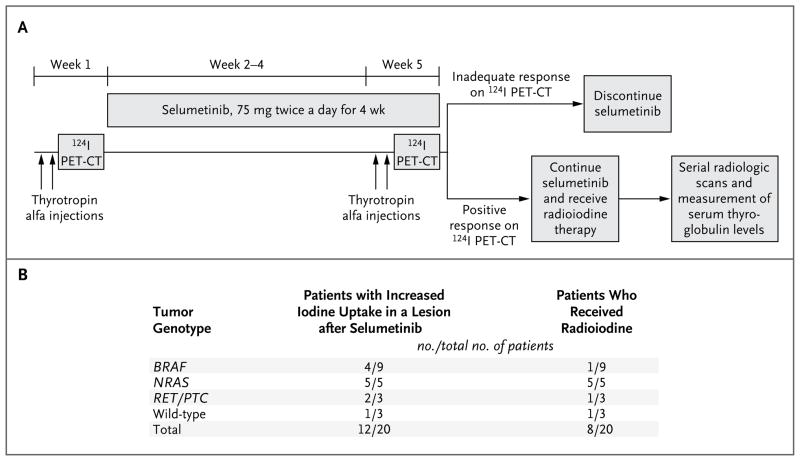
Figure 1A shows the protocol design. Of the 20 patients who could be evaluated, 12 (60%) had iodine-124 uptake that was new, increased, or both after selumetinib (Fig. 1B). In 8 patients (40%), the second iodine-124 PET study indicated that the absorbed radiation dose in the lesion would equal or exceed 2000 cGy with 300 mCi of radioiodine or less; these patients continued to receive selumetinib, and they received therapeutic radioiodine. In all 5 patients with NRAS-mutant tumors, this dosimetry threshold was exceeded, and these patients were treated with radioiodine. In contrast, 4 of 9 patients with BRAF mutations had selumetinib-induced increases in iodine-124 uptake, but only 1 had an increase that exceeded the threshold for radioiodine treatment. Two of 3 patients with RET/PTC and 1 of 3 patients with wild-type tumors had greater iodine uptake on the second iodine-124 PET study; 1 patient in each of those genotype groups went on to be treated with iodine-131. Increases in iodine uptake were achieved in patients with papillary thyroid cancers and those with poorly differentiated carcinomas (Table S2 in the Supplementary Appendix), as well as in patients in each protocol-specified category of radioiodine refractoriness (Table S3 in the Supplementary Appendix). Of the 10 patients with no detectable iodine-124 uptake at baseline, 2 had increased uptake after receiving selumetinib, 1 of whom went on to receive iodine-131.
Figure 1. Protocol Design and Changes in Iodine Uptake.
Panel A shows the protocol design. Baseline iodine avidity in the lesion was first assessed with thyrotropin alfa–stimulated iodine-124 positron-emission tomographic–computed tomographic (PET-CT) scanning. Patients were then treated with selumetinib at a dose of 75 mg given orally twice a day for 4 weeks. In the final week of treatment, a second thyrotropin alfa–stimulated 124I PET-CT study was performed. The double arrows indicate the two thyrotropin alfa injections. Patients with 124I dosimetry that predicted tumor uptake of less than 2000 cGy discontinued the study. If the absorbed dose of radioiodine in the lesion was predicted to be 2000 cGy or greater, full dosimetry with iodine-131 was performed to calculate the maximum tolerable activity that could be administered safely. Patients then received a therapeutic dose of radioiodine the next week after preparation with thyrotropin alfa. Selumetinib was continued until 2 days after the administration of therapeutic radioiodine. Thyroglobulin levels and the radiographic response were assessed at 2 and 6 months after radioiodine administration. Panel B shows a summary of the changes in iodine uptake quantified by 124I PET-CT and the number of patients who met the criteria for treatment with iodine-131.
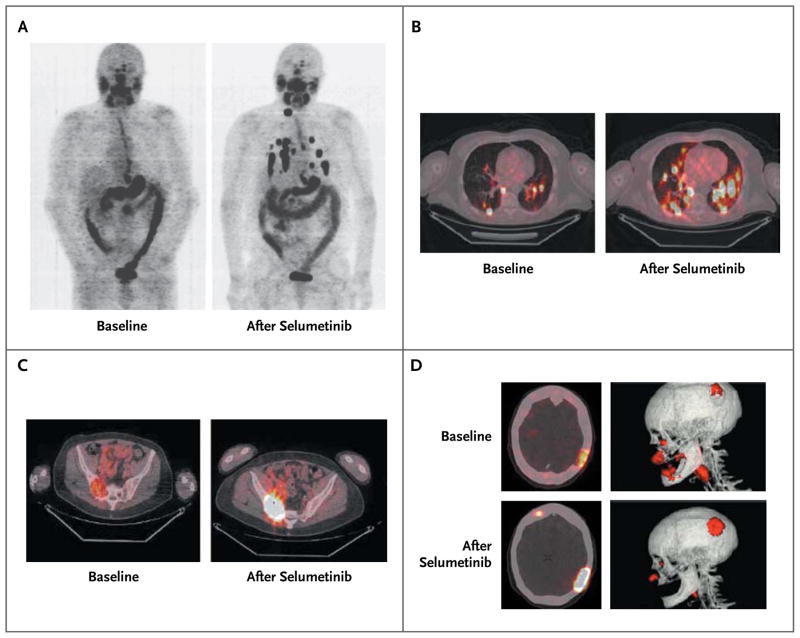
The selumetinib-induced changes in the iodine-124 PET-CT scans are shown in Figure 2. The only patient with a BRAF mutation who qualified for radioiodine therapy had dramatic increases in iodine uptake in a right cervical lymph node and lung metastases that showed no uptake at baseline (Fig. 2A). In other patients, iodine uptake was enhanced both in lesions with low-level iodine avidity and those with no avidity at baseline (Fig. 2B). Striking increases in selumetinib-induced iodine-124 uptake were also observed in bone metastases (Fig. 2C and 2D).
Figure 2. Iodine-124 PET-CT Scans Obtained before and after Selumetinib Treatment in Selected Patients with Positive Responses.
Panel A shows whole-body maximum-intensity projection images of a patient with a BRAF-mutant papillary thyroid cancer. New iodine uptake is shown in nearly all previously negative lung and neck metastases. Panel B shows fused axial PET-CT images of a patient with an NRAS-mutant, poorly differentiated thyroid cancer. Both new and significantly increased iodine uptake in lung metastases is shown. Panels C and D show PET-CT images from another patient with an NRAS-mutant, poorly differentiated thyroid cancer. In Panel C, fused axial PET-CT images show significantly increased iodine uptake in a sacroiliac bone metastasis after administration of selumetinib (right). In Panel D, fused axial images (top and bottom left) show new iodine uptake in a previously negative site as well as increased avidity in a large left parietal skull metastasis. Three-dimensional rendering highlights changes in the left parietal skull metastasis before and after selumetinib (top and bottom right).
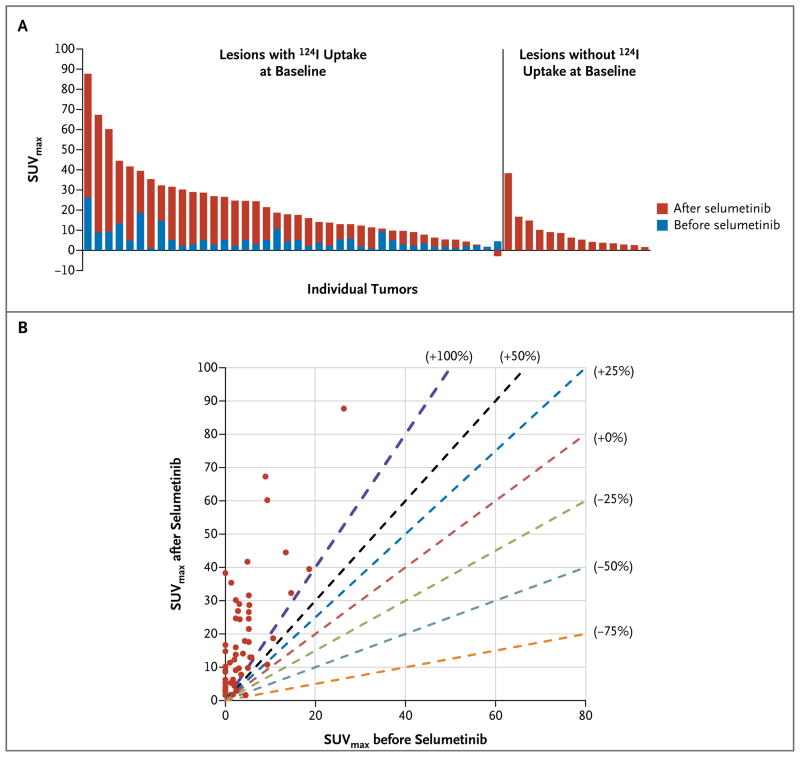
Quantification of baseline and post-selumetinib iodine-124 in each tumor in a patient with an NRAS mutation who qualified for radioiodine therapy revealed either new iodine uptake or more than a 100% increase in uptake in nearly every lesion (Fig. 3). Analysis of every lesion in all eight patients treated with radioiodine showed that selumetinib increased iodine-124 uptake in nearly all lesions, with more than a 100% increase in uptake in most lesions (Fig. S1 in the Supplementary Appendix). Iodine uptake in salivary glands was not significantly affected by selumetinib (Fig. S2 in the Supplementary Appendix); this suggests that the effect was restricted to thyroid tumors.
Figure 3. Quantification of Iodine-124 PET Uptake in a Lesion in a Patient with an NRAS Mutation Who Later Received Radioiodine.
Panel A shows the maximal standardized uptake value (SUVmax) for iodine in all tumors in a patient with an NRAS-mutant, poorly differentiated thyroid cancer. Each bar represents one malignant lesion identified on the iodine-124 PET-CT scan. The bars to the left indicate the increases in iodine-124 avidity achieved after selumetinib administration in lesions that absorbed some iodine at baseline. The bars on the right indicate selumetinib-induced changes in lesions that were negative for iodine at baseline. Panel B shows the SUVmax in every metastatic lesion identified in the same patient before and after the administration of selumetinib. The dashed lines mark points on the graph corresponding to different degrees of change in the SUVmax in the lesion after the administration of selumetinib. The red dashed line demarcates no change in iodine uptake after the administration of selumetinib (0%). Dashed lines to the left of the red dashed line represent graded percentage increases in iodine-124 uptake (+25%, +50%, and +100%), whereas the lines to the right represent graded percentage decreases (−25%, −50%, and −75%). Nearly all the metastatic lesions in this patient (circles) had more than a 100% increase in iodine uptake after administration of selumetinib. The SUVmax for a sternal metastasis was off the scale (it increased from 220 to 599 with selumetinib) and thus could not be included in these graphs without obscuring the data for the other 54 lesions analyzed.
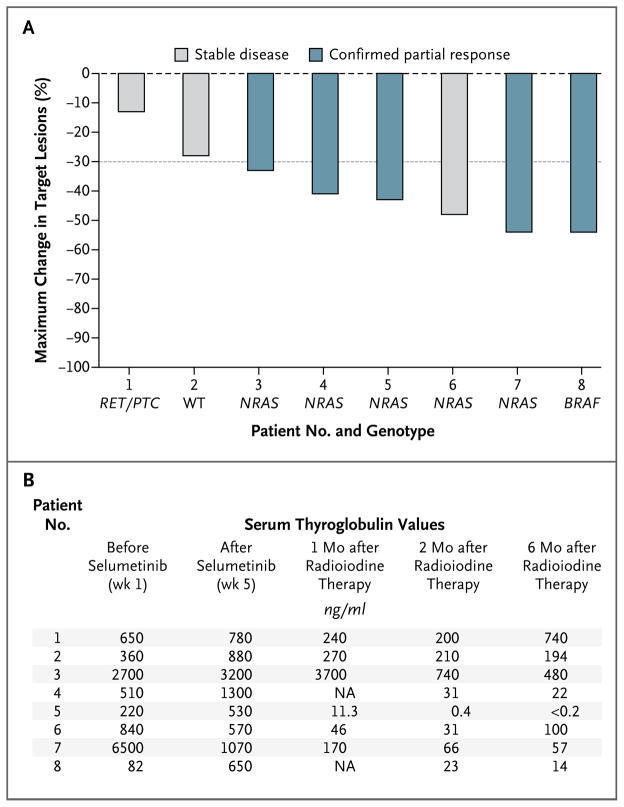
During 6 months of follow-up, a reduction in the size of target lesions was observed in all patients after the administration of radioiodine, with confirmed partial responses in five patients and stable disease in three as the best overall response (Fig. 4A, and Fig. S3 in the Supplementary Appendix). Significant decreases in thyrotropin-suppressed serum thyroglobulin levels after radioiodine administration were achieved in all eight patients at 2 months and 6 months after radioiodine administration, with mean reductions of 89% (P = 0.004) and 80% (P = 0.004), respectively, as compared with the value measured within 3 weeks before radioiodine administration (Fig. 4B). In seven of the eight patients, confirmed partial responses and stable disease outcomes were durable over the 6-month period of follow-up after radioiodine administration. The remaining patient had a 48% reduction in tumor dimensions 2 months after receiving radioiodine but then had tumor progression at 6 months, representing a best overall response of stable disease.
Figure 4. Response to Iodine-131 Therapy with Selumetinib Treatment.
Panel A shows a waterfall plot of the maximum change in target lesions (relative to a prestudy scan) in the eight patients who received therapeutic radioiodine. The best overall response in each patient according to the Response Evaluation Criteria in Solid Tumors, version 1.1, is also shown. The dashed line indicates a 30% reduction in tumor dimensions. WT denotes wild-type. Panel B shows serum thyroglobulin values in the eight patients treated with radioiodine. NA denotes not available.
SAFETY
All patients who could be evaluated completed the full course of selumetinib without a dose reduction or delay in administration. All toxic effects attributed to selumetinib were grade 1 or 2 and were consistent with adverse events reported in larger studies of selumetinib,27,28 including fatigue (in 80% of patients), maculopapular rash (70%), and acneiform rash (25%). Fourteen patients (70%) had grade 1 elevations in liver aminotransferase levels, possibly related to selumetinib; these levels reverted to baseline values after drug discontinuation. (Tables S4 and S5 in the Supplementary Appendix list all the toxic effects reported during the study.) One patient who was treated with 139 mCi of radioiodine during the study received a diagnosis of the myelodysplastic syndrome more than 51 weeks later; the disease eventually progressed to acute leukemia. Before enrollment, this patient had received three courses of radioiodine (a cumulative prestudy dose of 976.2 mCi), as well as 8640 cGy of external-beam radiation therapy, for prostate cancer, and thrombocytopenia developed when the patient was treated with sorafenib and temsirolimus in a clinical trial.
DISCUSSION
Previous studies using various compounds to promote radioiodine uptake in refractory metastatic thyroid cancers have not shown a clinically significant benefit.3,6 The approach undertaken in this study was facilitated by several developments: the discovery that genetic alterations that constitutively activate MAPK signaling can promote the dedifferentiation of thyroid-cancer cells,12,29,30 the clinical availability of a selective MEK inhibitor,17 and the development of iodine-124 PET-CT technology to quantify the uptake of iodine in a lesion.18
Our results show that inhibition of the MAPK pathway with selumetinib can renew the therapeutic efficacy of radioiodine by enhancing uptake in patients with thyroid cancer that is refractory to radioiodine. All five patients with NRAS-mutant tumors had increased iodine uptake in response to selumetinib; four had confirmed partial responses and one had stable disease after radioiodine administration. This finding is of particular interest, given the well-documented challenges of developing therapeutic approaches for RAS-driven cancers, and it is consistent with preclinical data showing that a RAS mutation can suppress thyroid-specific gene expression in a MEK-dependent manner.12,31 Only one of nine patients with BRAF mutations received radioiodine therapy. That patient had a particularly striking conversion of lesions from negative to positive on iodine-124 PET after treatment with selumetinib and had a confirmed partial response. Three other patients with BRAF mutations also had increased uptake on the post-selumetinib iodine-124 PET study, even though the threshold required for therapy was not reached. The differences observed between RAS-mutant and BRAF-mutant tumors remain to be explained, but it is possible that MAPK signaling is incompletely inhibited in some BRAF-mutant tumors because of higher flux through the pathway.
These results provide a proof of principle that MEK inhibitors can induce iodine uptake and retention in thyroid tumors. An advantage of this therapeutic strategy over long-term treatment with small-molecule kinase inhibitors alone32 is that only a short course of drug therapy is required to elicit a durable clinical effect. Enhanced iodine uptake was also observed in bone and nodal metastases, niches that have been comparatively refractory to treatment with kinase inhibitors.
Supplementary Material
Acknowledgments
Supported by grants from the American Thyroid Association, the Society of Memorial Sloan-Kettering Cancer Center, the National Institutes of Health (RO1-CA50706 and RO1-CA72598), AstraZeneca, and Genzyme, as well as funding from the Lefkofsky Family, Margot Rosenberg Pulitzer, Byrne, and J. Randolph Hearst foundations. The iodine-124 PET studies were supported in part by a grant from the In-Vivo Cellular and Molecular Imaging Center (P50 086438-10). Dr. Domínguez was supported in part by a grant from Pontificia Universidad Católica de Chile and Becas Chile (76100021), and Dr. Deandreis by a grant from Fondation de France (2010-12521).
We thank the research study staff, particularly Susan Korte, Alex F. Mak, Brynna Lipson, Donna Lisa, and Lisa Cox, R.N.
Footnotes
Disclosure: nothing to disclose
References
- 1.Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–63. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 2.Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–9. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 3.Coelho SM, Corbo R, Buescu A, Carvalho DP, Vaisman M. Retinoic acid in patients with radioiodine non-responsive thyroid carcinoma. J Endocrinol Invest. 2004;27:334–9. doi: 10.1007/BF03351058. [DOI] [PubMed] [Google Scholar]
- 4.Handkiewicz-Junak D, Roskosz J, Hasse-Lazar K, et al. 13-cis-Retinoic acid re-differentiation therapy and recombinant human thyrotropin-aided radioio-dine treatment of non-functional metastatic thyroid cancer: a single-center, 53-patient phase 2 study. Thyroid Res. 2009;2:8. doi: 10.1186/1756-6614-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon D, Körber C, Krausch M, et al. Clinical impact of retinoids in redifferentiation therapy of advanced thyroid cancer: final results of a pilot study. Eur J Nucl Med Mol Imaging. 2002;29:775–82. doi: 10.1007/s00259-001-0737-6. [DOI] [PubMed] [Google Scholar]
- 6.Liu YY, van der Pluijm G, Karperien M, et al. Lithium as adjuvant to radioiodine therapy in differentiated thyroid carcinoma: clinical and in vitro studies. Clin Endocrinol (Oxf) 2006;64:617–24. doi: 10.1111/j.1365-2265.2006.02515.x. [DOI] [PubMed] [Google Scholar]
- 7.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–7. [PubMed] [Google Scholar]
- 8.Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–7. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 9.Knauf JA, Fagin JA. Role of MAPK pathway oncoproteins in thyroid cancer pathogenesis and as drug targets. Curr Opin Cell Biol. 2009;21:296–303. doi: 10.1016/j.ceb.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etio-pathogenesis of PTC. Oncogene. 2003;22:4578–80. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 11.Durante C, Puxeddu E, Ferretti E, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;92:2840–3. doi: 10.1210/jc.2006-2707. [DOI] [PubMed] [Google Scholar]
- 12.Knauf JA, Kuroda H, Basu S, Fagin JA. RET/PTC-induced dedifferentiation of thyroid cells is mediated through Y1062 signaling through SHC-RAS-MAP kinase. Oncogene. 2003;22:4406–12. doi: 10.1038/sj.onc.1206602. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Hu S, Hou P, Jiang D, Condouris S, Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. 2007;13:1341–9. doi: 10.1158/1078-0432.CCR-06-1753. [DOI] [PubMed] [Google Scholar]
- 14.Mitsutake N, Knauf JA, Mitsutake S, Mesa C, Jr, Zhang L, Fagin JA. Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res. 2005;65:2465–73. doi: 10.1158/0008-5472.CAN-04-3314. [DOI] [PubMed] [Google Scholar]
- 15.Riesco-Eizaguirre G, Gutiérrez-Martínez P, García-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer. 2006;13:257–69. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–11. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerji U, Camidge DR, Verheul HM, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–23. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 18.Pentlow KS, Graham MC, Lambrecht RM, et al. Quantitative imaging of iodine-124 with PET. J Nucl Med. 1996;37:1557–62. [PubMed] [Google Scholar]
- 19.Sgouros G, Kolbert KS, Sheikh A, et al. Patient-specific dosimetry for 131I thyroid cancer therapy using 124I PET and 3-dimensional-internal dosimetry (3D-ID) software. J Nucl Med. 2004;45:1366–72. [PubMed] [Google Scholar]
- 20.Feine U, Lietzenmayer R, Hanke JP, Held J, Wöhrle H, Müller-Schauenburg W. Fluorine-18-FDG and iodine-131-iodide uptake in thyroid cancer. J Nucl Med. 1996;37:1468–72. [PubMed] [Google Scholar]
- 21.Wang W, Larson SM, Tuttle RM, et al. Resistance of [18f]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to treatment with high-dose radioactive iodine. Thyroid. 2001;11:1169–75. doi: 10.1089/10507250152741028. [DOI] [PubMed] [Google Scholar]
- 22.Robbins RJ, Wan Q, Grewal RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505. doi: 10.1210/jc.2005-1534. [DOI] [PubMed] [Google Scholar]
- 23.Robbins RJ, Larson SM, Sinha N, et al. A retrospective review of the effectiveness of recombinant human TSH as a preparation for radioiodine thyroid remnant ablation. J Nucl Med. 2002;43:1482–8. [PubMed] [Google Scholar]
- 24.Tala H, Robbins R, Fagin JA, Larson SM, Tuttle RM. Five-year survival is similar in thyroid cancer patients with distant metastases prepared for radioactive iodine therapy with either thyroid hormone withdrawal or recombinant human TSH. J Clin Endocrinol Metab. 2011;96:2105–11. doi: 10.1210/jc.2011-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox JJ, Autran-Blanc E, Morris MJ, et al. Practical approach for comparative analysis of multilesion molecular imaging using a semiautomated program for PET/CT. J Nucl Med. 2011;52:1727–32. doi: 10.2967/jnumed.111.089326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–93. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes DN, Lucas AS, Tanvetyanon T, et al. Phase II efficacy and pharmacogenomic study of selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res. 2012;18:2056–65. doi: 10.1158/1078-0432.CCR-11-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res. 2012;18:555–67. doi: 10.1158/1078-0432.CCR-11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Vita G, Zannini M, Cirafici AM, et al. Expression of the RET/PTC1 oncogene impairs the activity of TTF-1 and Pax-8 thyroid transcription factors. Cell Growth Differ. 1998;9:97–103. [PubMed] [Google Scholar]
- 30.Francis-Lang H, Zannini M, De Felice M, Berlingieri MT, Fusco A, Di Lauro R. Multiple mechanisms of interference between transformation and differentiation in thyroid cells. Mol Cell Biol. 1992;12:5793–800. doi: 10.1128/mcb.12.12.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vita G, Bauer L, da Costa VM, et al. Dose-dependent inhibition of thyroid differentiation by RAS oncogenes. Mol Endocrinol. 2005;19:76–89. doi: 10.1210/me.2004-0172. [DOI] [PubMed] [Google Scholar]
- 32.Ho AL, Sherman E. Clinical development of kinase inhibitors for the treatment of differentiated thyroid cancer. Clin Adv Hematol Oncol. 2011;9:32–41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.