Abstract
Objective
Glioblastomas (GBMs) are lethal cancers that display cellular hierarchies parallel to normal brain. At the apex are GBM stem cells (GSCs), which are relatively resistant to conventional therapy. An important driver of malignancy and self-renewal in GSCs are interactions with the adjacent perivascular niche. Extracellular matrix (ECM) cues instruct neural stem/progenitor cell-niche interactions and the objective of our study was to elucidate its composition and contribution to GSC maintenance in the perivascular niche.
Methods
We interrogated human tumor tissue for immunofluorescence analysis and derived GSC from tumor tissues for functional studies. Bioinformatics analyses were conducted by mining publicly available databases.
Results
We find that laminin ECM proteins are localized to the perivascular GBM niche and inform negative patient prognosis. To identify the source of laminins, we characterized cellular elements within the niche and found that laminin α chains were expressed by non-stem tumor cells and tumor associated endothelial cells (ECs). RNA interference targeting laminin α2 inhibited GSC growth and self-renewal. In co-culture studies of GSCs and ECs, laminin α2 knockdown in ECs resulted in decreased tumor growth.
Interpretation
Our studies highlight the contribution of non-stem tumor cell-derived laminin juxtracrine signaling. As laminin α2 has recently been identified as a molecular marker of aggressive ependymoma, we propose that the brain vascular ECM promotes tumor malignancy through maintenance of the GSC compartment providing not only a molecular fingerprint but also a possible therapeutic target.
Introduction
Among primary intrinsic brain tumors, GBMs [World Health Organization Grade IV] are the most prevalent and most lethal. Despite aggressive therapies including surgical resection, radiation, and chemotherapy, GBM patients have a median survival of 15 months [1]. GBMs are angiogenic, refractory to many therapies, and characterized by a high degree of cellular heterogeneity and a propensity to invade surrounding tissue [2]. Recent work has demonstrated that GBM has a defined hierarchy with a self-renewing cancer stem cell (CSC) at the apex [3]. Although the CSC hypothesis remains controversial due to unresolved issues in identifying the CSC and the cell-of-origin, numerous studies have shown that GBM contain cells functionally validated as CSCs through secondary transplantation assays and are key contributors to therapeutic resistance, angiogenesis, and invasion into surrounding tissues [4–11]. It is notable that many of these characteristics are shared with neural stem cells. Hence, a better understanding of the biology of GSCs may impact the development of more effective GBM therapies with possible extension to many other nervous system diseases in which neural stem and progenitor cells may be involved.
Within a tumor, the stem cell-like tumor cells are preferentially located in defined anatomical locations and are regulated by extrinsic contributions of their microenvironment or niche [12]. For brain tumors, the most well defined niche is adjacent to blood vessels, known as the perivascular niche [13], an area enriched in GSCs and distinct from the blood brain barrier and neurovascular unit. A variety of cell types, including GSCs, non-stem tumor cells, and vascular components (ECs, pericytes) are present in this niche. Within this microenvironment, GSC maintenance is facilitated through a variety of mechanisms including growth factor signaling, cell-to-cell communication, and cell-to-ECM interactions [14]. Recent work from our research group and others has elucidated the contributions of growth factors (including vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGF-β)) and cell-to-cell communication (e.g. Notch signaling) to niche maintenance [9, 15, 16]. Because of its importance, substantial efforts have been made to target the perivascular niche with the anti-VEGF antibody, bevacizumab [17]. However, clinical efficacy of bevacizumab has been mixed, underscoring the complexity of niche targeting and demonstrating the need to better define the biology of the microenvironment [18].
The contributions of ECM interactions to GSC maintenance still remain poorly understood. It has recently become appreciated that the ECM is more than a structural component and, for stem cells in particular, is pivotal in instructing cell fate choices and concentrating growth factors to defined niches. In the developing and adult brains, neural progenitor cells (NPCs) rely on ECM components for growth and survival [19–21]. Many of these interactions are guided by the laminin family of proteins, each consisting of an α, β, and γ subunit, and their receptors that include specific integrins, dystroglycan, and syndecans. While there are 15 known isoforms, the key distinguishing features between laminins are their structure, binding partners, and tissue specific expression. The α chain, in particular, mediates most of the receptor binding and hence is responsible for generating different cellular responses [22]. The use of laminin α chain mutant mice to examine nervous system disorders highlights the diversity of phenotypes and underscores the specific role of each laminin isoform [23]. Further highlighting the differences between laminin isoforms is the specific expression in NPC niches. These niches are enriched in several members of the laminin family (including α2, α4, and α5 chain) and the laminin-specific integrin (namely α6 and β1) are highly expressed on NPCs [24–27]. Mice with targeted disruption of either integrins α6 or β1 or laminin α2 have profound NPC defects underscoring their pivotal role in neural development [24, 27–29]. Building on this developmental relationship, we previously evaluated integrin expression on GSCs and found elevated levels of integrin α6 [30]. Importantly, integrin α6 enriches for GSCs and targeting integrin α6 attenuates self-renewal and tumor formation. The laminin α5 and laminin-411 (composed of α4, β1, and γ1 chains) expression informs glioma grade and poor patient survival [31, 32]. The importance of this relationship is further highlighted by adherent GSC culturing methods that rely on laminin [33, 34].
Understanding the repertoire of signals present in the perivascular niche which orchestrate GSC maintenance represents an immediate priority as it has direct relevance to therapeutic approaches for GBM and will provide insights into GSC biology. Here we have utilized human patient specimens to evaluate the laminins present on GSCs within the perivascular niche and to show that laminin α2, which is provided by non-GSCs and ECs, is critical for GSC maintenance. Our results also demonstrate that ECM components protect GSCs from radiation-induced damage, which highlights the contribution of the niche to therapeutic resistance, and must be considered in the design of next generation GBM therapies.
Methods
Genomic data and processing
Custom Agilent 244,000 feature Gene Expression Microarray and Affymetrix-HT-HG-U133A GeneChip gene expression data for 354 and 246 glioblastomas, respectively, and associated clinical data were obtained from the Open-Access and Controlled-Access Data Tiers Portal (http://tcga-data.nci.nih.gov/tcga/findArchives.htm) of The Cancer Genome Atlas (TCGA) Pilot Project (http://tcga-data.nci.nih.gov/tcga/tcgaHome2.jsp) upon National Human Genome Research Institute (NHGRI) approval. Gene expression data were background corrected and normalized through the Robust Multigene Average (RMA) algorithm [35] as described by TCGA [36]. Furthermore, a unified and integrated gene expression data set created by combining individual platform (Affymetrix-HT-HG-U133A GeneChip, Affymetrix HuEx GeneChip, and Custom Agilent 244,000 feature Gene Expression Microarray) gene level expression measurements for 202 TCGA glioblastoma samples and associated molecular subtype [37] and clinical data were retrieved from http://tcga-data.nci.nih.gov/docs/publications/gbm_exp/. The assumption of proportional hazards was tested using interactions of the predictor variable (laminin α2 expression) with time based on the Cox proportional-hazards regression model. Cumulative hazard functions were estimated at the mean of laminin α2 expression. P-values for the association of laminin α2, integrin α6, and integrin β1 expression with molecular subtypes of GBM were computed with the use of the Wilcoxon-rank sum test. To determine the correlation of laminin α2 chain with brain tumor expression a microarray database (Oncomine, Compendia Bioscience, Ann Arbor, MI http://www.oncomine.org) was queried. To determine the correlation between laminin chains and patient survival, the NCI REpository for Molecular BRAin Neoplasia DaTa bioinformatics database (REMBRANDT, https://caintegrator.nci.nih.gov/rembrandt/) was queried. To determine imaging differences between laminin α2 high and low patients, two expression probes were used to assess LAMA2 mRNA expression from a previously published dataset of 53 glioblastomas which included both imaging and gene expression analysis [38]. Values were expressed as ratios of red-to-green fluorescence dye intensity (log2R/G) estimated by the robust multigene average preprocessing algorithm. Linear regression confirmed a significant correlation of expression values between both probes (p < 0.000001). Locally weighted least squares (LOWESS) smooth fit confirmed the appropriateness of a linear regression analysis. Patients in the top and bottom quartile were then evaluated for edema based on MRI evaluation (scored 0 – 2).
Glioblastoma stem cell derivation, culture, and analysis
Human GBM cells were derived under written informed consent and approved IRB protocols from Cleveland Clinic, University Hospitals-Seidman Cancer Center at Case Western Reserve University, and Duke University to establish xenografts in BalbC nu/nu (athymic nude) mice for maintenance of the tumor hierarchy as previously published [30, 39, 40]. GBM xenografts for 3 tumors (556, 1966, and 387) were used for experimental studies. Only low (<5) passage cells were used for analysis to minimize cellular drift [41] and the majority of cells were used immediately after dissociation. Unenriched glioma cells were derived from tumor xenografts using a papain dissociation kit (Worthingon) and cultured overnight in neurobasal media supplemented with B-27 (Life Technologies, 1x), EGF (R&D Systems, 20ng/ml), and FGF-2 (R&D Systems 20ng/ml). For RNA studies, GSCs were enriched based on previously validated cell surface marker expression by flow cytometry (CD133/2-APC, Miltenyi, or integrin α6-FITC, BD). For functional studies, GSCs were enriched using CD133/2 magnetic beads and cultured in supplemental neurobasal media as described above. GSCs were functionally assayed for self renewal, multi-lineage differentiation, stem cell marker expression and tumor propagation as previously described. Non-stem tumor cells were processed in parallel and cultured as we previously described [30, 39, 40].
Brain tumor endothelial cell derivation, culture, and analysis
For isolation of brain tumor endothelial cells (BTECs), established protocols were used as previous described [42] for 3 separate primary GBM specimens. Purity was confirmed by immunofluorescence staining with anti-CD31 (Sigma, 1:200) and anti-von Willibrand factor (Abcam, 1:200) antibodies. Normal brain endothelial cells were purchased Cell Systems and cultured in conditions similar to BTECs prior to in vivo injection.
Immunostaining analysis
Immunostaining analysis on human GBM specimens or tumorspheres was done as previously described using 10 µm frozen sections obtained from the Duke University Brain Tumor Center Tissue Bank and histologically confirmed by a neuropathologist (R.E.M.) [30] using antibodies against integrin α6 (Millipore, 1:100), CD31 (Dako (mouse) or Abcam (rabbit), 1:200), laminin α2 (Millipore, 1:100), laminin α3 (Millipore, 1:100), laminin α5 (Dako, 1:50), pan-laminin (laminin α1, β1, γ1, Sigma, 1:100), fibronectin (Sigma, 1:100), collagen IV (Abcam, 1:100) and species appropriate secondary antibodies (Alexa 488, 568, or 633, Invitrogen, 1:200). Nuclei were counterstained with Hoechst 33342 (1:1000 dilution from a 5 mg/ml stock solution, Invitrogen). All imaging was done using a Leica SP-5 confocal microscope as described previously [30] and images were processed and assembled in Photoshop (Adobe). Tissue microarray analysis was performed as previously described [43] using the laminin α2 antibody (Millipore).
Quantitative real time PCR (qPCR)
qPCR analysis was done as previously described using an ABI 7900HT system with using SYBR-Green Mastermix (SA Biosciences). For analysis, fractions of marker positive and negative tumor cells (i.e. integrin α6 or CD133) cells were sorted directly into lysis buffer and RNA was extracted using the RNAeasy kit (Qiagen) and cDNA was synthesized using the Superscript III kit (Invitrogen). BTECs were expanded in vitro as described above and processed using the same methodology as the marker positive and negative tumor cells. For qPCR analysis, the threshold cycle (CT) values for each gene were normalized to expression levels of β-Actin. Dissociation curves were evaluated for primer fidelity and only threshold cycles below 35 cycles were reported.
The following primers were used:
| β-actin | Forward | 5’-AGAAAATCTGGCACCACACC-3’ |
| Reverse | 5’-AGAGGCGTACAGGGATAGCA-3’ | |
| Laminin α1 | Forward | 5’-AGGTCAGTGCCCATGTAAGG-3’ |
| Reverse | 5’-ACCCACAGGAGACACAGGTC-3’ | |
| Laminin α2 | Forward | 5’-GGGTGACTCTGAAGGCTGAG-3’ |
| Reverse | 5’-GGGCAACAATCTCTGGATGT-3’ | |
| Laminin α3 | Forward | 5’-CATTTCTACGCCTGCTTTCC-3’ |
| Reverse | 5’-GCACAAGCTTCCAGTCTTCC-3’ | |
| Laminin α4 | Forward | 5’-CCTCCTCAATCAAGCCAGAG-3’ |
| Reverse | 5’-TGCTTAACGGCATCACTGAG-3’ | |
| Laminin α5 | Forward | 5’-GAGAGCCCTTTGTGCTGAAC-3’ |
| Reverse | 5’-GCCTCGTAGTATGCGCTAGG-3’ |
Laminin α2 lentiviral short hairpin RNA (shRNA) construct production
Lentiviral shRNA constructs were prepared as we previously reported [30]. In short, using lipofectamine 2000 (Invitrogen), 293FT cells were co-transfected with packaging vectors psPAX2 and pCI-VSVG (Addgene) and lentiviral vectors directing expression of shRNA (Sigma) specific to laminin α2 (TRCN0000083857 (KD1) and TRCN0000083853 (KD2)) or a non-targeting control (NT) shRNA (SHC002) to produce virus. Media on the 293FT cell cultures were changed 18 hours after transfection and viral supernatants were collected 24 and 48 hours later, filtered, and concentrated for immediate use or stored at −80°C for future use.
Cell proliferation and tumorsphere formation assays
For cell proliferation assessments, cell populations of interest were plated at a density of 1000 cells/well in a 96-well plate in quadruplicate as previously described [30] using the CellTiter-Glo assay kit (Promega). Cell number was measured every other day and normalized to the initial reading. For Caspase 3/7 analysis, parallel wells were evaluated using the Caspase 3/7 substrate (Promega) and normalized to cell growth values. For tumorsphere formation assessments, 96-well plates were seeded at a density of 1 or 10 cells per well per condition as previously described [30]. Reported numbers represent a minimum of 16 wells per condition and were calculated ten days after plating. Tumorspheres containing more than 20 cells were scored.
Immunoblotting analysis
Protein analysis was done by immunoblotting as previously described [40]. Briefly, 10 µg of total protein was loaded per condition and probed using an anti-laminin α2 antibody (Millipore, 1:500). Immunoblotting with an α-Tubulin antibody (Sigma, 1:500) was used as a loading control and 250 ng of purified merosin (containing laminin α2, Millipore) was used as a positive control. Species specific horseradish peroxidase (HRP) conjugated secondary antibodies were used for detection (Invitrogen, 1:2000).
Cell mixing studies
For co-culture studies, ECs were infected with either a non-targeting control or a laminin α2 knock down construct (KD1) as described above in non-GSCs. In vivo mixing studies were done by labeling GSCs with a yellow fluorescent protein reporter virus (Sigma) for identification and 1,000 GSCs were mixed with 100,000 ECs were transplanted into the cortex of immune compromised mice as previously described with 5 mice per group [44].
DNA damage assessments
DNA damage was induced by exposing the cells to the indicated dose of ionizing radiation (IR; J.L. Shepherd Mark 1 Model 30, 137 Cesium Irradiator; J.L. Shepherd and Associates). Relative survival was measured using CellTiter-Glo (Promega) at 48 or 72 hours following irradiation. GSCs (specimen 387) were grown either on purified laminin α2 (Sigma) or with the equivalent protein concentration (10 µg/ml) of bovine serum albumin (BSA) as a control. Resolution of DNA damage was evaluated through staining for phospho-H2AX (S139) (γH2AX; Millipore, 1:1000) 24 hours after irradiation using methods described above. A minimum of 50 cells per condition were quantified from four independent trials with cells bearing greater than ten γH2AX foci and graphed as a percentage of total cells counted.
Statistical analysis
Reported values are mean values +/− standard error from studies done in at least triplicate. Unless otherwise stated, one-way ANOVA was used to calculate statistical significance with p-values are detailed in the text and figure legends.
Results
Laminin chain expression inversely correlates to patient prognosis in malignant gliomas
As laminins may promote GSC maintenance in culture [33, 34], we evaluated their expression with regards to tumor malignancy in human patient populations. We queried a microarray database (Oncomine) to compare the relative expression levels of laminin α2 between NPCs and GBM, and found significantly elevated levels of laminin α2 in GBM tissue compared to human NPCs (Fig. 1A). Laminin α2 was also significantly higher in GBM compared to other less malignant tumors (Astrocytoma, Oligodendroglioma, Mixed Glioma) suggesting a correlation to tumor grade (Fig. 1B–E). We assessed the association of laminin α2 expression in 354 glioblastoma patients of the TCGA (University of North Carolina dataset) and Cox proportional hazards regression modeling revealed a significant association between LAMA2 expression and poor patient survival (Fig. 2A, p = 0.03 by Cox model, hazard ratio for death: 1.11; 95% CI: 1.01–1.23). This association was confirmed in a second data set of 246 TCGA glioblastoma patients profiled independently on a different genomics platform (Broad Institute; p = 0.04 by Cox model, hazard ratio for death: 1.16; 95% CI: 1.00–1.34). The genomic pre-processing algorithms for the microarray data yielded estimates of laminin α2 gene expression whose units of measurement complicate interpretation of Cox model hazard ratios for continuous versions of these predictors. For easier interpretation of hazard ratios, we have estimated cumulative hazard curves for the minimum, median, and maximum expression of laminin α2 in each of the two data sets, which demonstrated similar cumulative hazard functions (Fig 2A). We found a strong negative correlation between laminin α2 RNA levels and patient survival (Figure 2B). A statistically significant negative correlation was also seen with key laminin receptors (integrin α6 and integrin β1), laminin α4, and laminin α5 but not with laminins α1 or α3 (Table 2). Additionally, only laminin α2 among the alpha-laminins was informative of extended survival in patients with low expression (Figure 2B, Table 2). To confirm the difference in laminin α2 expression between tumor types at the protein level, we evaluated laminin α2 immunoreactivity on a tissue microrarray containing non-tumor neural tissue and varying grades of gliomas (Grade II, Grade III, GBM). The specificity of the laminin α2 chain specific antibody was validated by probing for purified laminin α2, which was only detected by the laminin α2 but not laminin α3 and α5 antibodies (Supplementary Fig. 1). The tissue microarray analysis confirmed that laminin α2 was increased in GBM specimens as compared to Grades II and III (Supplementary Fig. 2). Statistical analysis revealed that there was significantly higher expression of laminin α2 in GBM versus lower grade tumors and a significant difference in survival between GBM and lower grade tumors (p<0.05, data not shown).
Figure 1. Laminin α2 chain is elevated in GBM.
Box and whisker plot (A) of expression level for laminin α2 using Oncomine (https://www.oncomine.org/) demonstrates significantly higher expression (p = 2.08 × 10−5 as assessed by one-way ANOVA) of laminin α2 in GBM tissue as compared with neural progenitor cells (NPCs). Box and whisker plot (B–E) of expression level for laminin α2 using Oncomine (https://www.oncomine.org/) demonstrates significantly higher expression (p = 5.92 × 10−6 (B) p = 0.011 (C), p = 7.87 × 10−14 (D), p = 6.89 × 10−6 (E) as assessed by one-way ANOVA) of laminin α2 in GBM tissue as compared with tissue from Oligodendroglia (Oligo) tumors, Astrocytoma, or mixed glioma (Oligodendroglia and Astrocytoma).
Figure 2. Laminin α2 chain expression correlates with glioma patient survival, and is differentially expressed in GBM molecular subtypes.
Cumulative hazard curves (A) plotted to estimate the risk of death according to time at the minimum (Low, green), median (blue), and maximum (High, red) of laminin α2 expression in two independent data sets of the TCGA using two different genomic platforms (University of North Carolina, left; Broad Institute, right), demonstrates similar cumulative hazard functions. 354 patient samples profiled by the University of North Carolina; p = 0.03 by Cox model for laminin α2 expression as a continuous variable, hazard ratio for death: 1.11 (95% CI, 1.01–1.23). 246 patient samples profiled by the Broad Institute; p = 0.04 by continuous Cox model, hazard ratio for death: 1.16 (95% CI, 1.00–1.34). Kaplan-Meier survival plot (B) for glioma patients with differential tumor laminin α2 expression calculated by NCI REpository for Molecular BRAin Neoplasia DaTa (REMBRANDT) bioinformatics database (https://caintegrator.nci.nih.gov/rembrandt/) at a level of two-fold upregulation indicates that laminin α2 levels inversely correlates with patient survival (red line indicates two-fold upregulation, green line indicates two-fold downregulation, and yellow line represents intermediate expression with number of patients per group indicated in legend). The log-rank p-value for significance of difference of survival between up-regulated group and intermediate group is 0.0007, difference of survival between up-regulated group and down-regulated group is 0.004, and difference of survival between down-regulated group and intermediate group is 0.04. Box plots (C) of association of laminin α2 with molecular subtype in 188 GBMs. Values for gene dosage are presented as log2R/G ratios as estimated by the robust multigene average preprocessing (RMA) algorithm. The box plots show the smallest and largest observations (upper and lower whiskers, respectively), the interquartile range (box), and the median (black line). Data points that are more than 1.5 times the interquartile range lower than the first quartile or 1.5 times the interquartile range higher than the third quartile were considered to be outliers. The p value was calculated with the use of the Wilcoxon rank-sum test and displayed in Table 1.
Table 2. Correlation between integrin and laminin chain expression and glioma patient survival.
Table summarizing survival results for glioma patients with differential tumor integrin α6, integrin β1, or laminin a chain expression calculated by NCI REMBRANDT bioinformatics database at a level of two-fold upregulation or downregulation. Integrins α6, β1, and laminin α2 expression levels inversely correlate with patient survival. The log-rank p-value for significance of difference of survival between up-regulated group and all other groups is given in p-value column and the numbers of patients per given condition is indicated in the n column. ND indicates not determined.
| 2 fold upregulated | 2 fold downregulated | |||
|---|---|---|---|---|
| Integrin/ Laminin |
n | p-value | n | p-value |
| α6 | 62 | 0.023 | 7 | 0.29 |
| β1 | 158 | 0.000003 | ND | - |
| Ln α1 | 130 | 0.06 | 29 | 0.84 |
| Ln α2 | 77 | 0.00007 | 7 | 0.047 |
| Ln α3 | 2 | 0.98 | 4 | 0.36 |
| Ln α4 | 208 | 0.0014 | 1 | - |
| Ln α5 | 215 | 0.00006 | 3 | 0.72 |
The recent identification by Philips [45] and the TCGA [45] of GBM subgroups distinguished by specific gene expression profiles and genetic alterations suggests that intertumoral heterogeneity of GBMs may be phenotypically important. To evaluate if laminin α2 was associated with a glioma molecular subtype, we interrogated the TCGA dataset and found that laminin α2 was significantly higher in the Mesenchymal and Classical subtypes as compared with the Neural and Proneural subtypes (Fig. 2C). To evaluate if laminin receptor expression had a similar trend, we evaluated integrins α6 and β1 expression in TCGA and found a similar increase in the Mesenchymal and Classical subtypes as compared with the Neural and Proneural subtypes (Supplementary Fig. 3). The laminin α2 molecular subclass results and survival differences were also confirmed in a recently published dataset [38] (data not shown). Collectively, these results emphasize the correlation between laminin chain expression and patient prognosis and are suggestive of a role for laminins in tumor progression.
Laminins are expressed in the perivascular glioblastoma stem cell niche
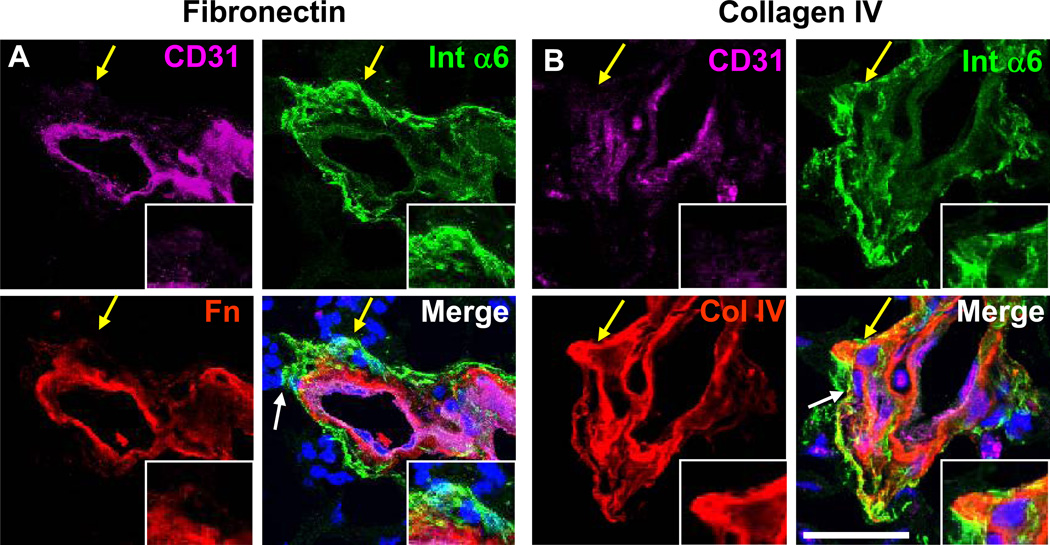
As the perivascular niche is a reservoir of GSCs and previous studies of normal brain have identified laminins as resident ECM on blood vessels [31, 32], we explored the expression of laminins in the GSC microenvironment. Using sections from human GBM tumors, we confirmed the presence of laminins in the perivascular niche using a pan-laminin antibody (recognizing the α1, β1, and γ1 subunits, Figure 3A), in conjunction with integrin α6 and CD31 to mark GSCs and blood vessels, respectively. As we previously reported, integrin α6 was present on both vascular and non-vascular cells in the perivascular niche with high selectivity (Fig. 3) [30]. To evaluate laminin chain specific expression patterns, we utilized chain specific antibodies against laminins α2, α3, and α5. Laminin α2 (Fig. 3B), α3 (Fig. 3C), and α5 (Fig. 3D) co-labeled with integrin α6 on both vascular and non-vascular cells in the area adjacent to the vasculature. Previous work in NPCs [28, 46] and our results on the expression pattern of laminin α2 in the perivascular niche suggest that GSCs may bind laminin α2. To test this hypothesis, we evaluated GSCs cultured from xenografted tumors grown as tumorspheres, which are derived from GSCs and contain a heterogeneous mixture of tumor cells including GSCs and non-GSCs. In tumorsphere cultures, we found elevated levels of laminin α2 in conjunction with integrin α6 expression, suggesting that laminin α2 is important for GSC growth in vitro (Fig. 3E). Evaluation of additional ECM ligands determined fibronectin and collagen IV were expressed on the vasculature (Fig. 4). While fibronectin expression was limited to the vascular compartment (Fig. 4A), collagen IV was detected on non-vascular cells adjacent to the blood vessels (Fig. 4B), suggesting the presence of basement membrane structures containing both laminins and collagen IV. We also evaluated tenascin-C, another ECM protein expressed in GSCs [6], upregulated in GBM tumors [47, 48], and linked with NPC migration [49]. Expression of tenascin-C was detected in the perivascular compartment and throughout the tumor (Supplementary Fig. 4A). Additionally, while elevated in glioma patients, tenascin-C itself was not informative for patient survival as analyzed by bioinformatics (Supplementary Fig. 4B), suggesting that the specific laminin chain expression and link to patient survival is unique to laminin chains and not a general ECM characteristic.
Figure 3. Unique laminin chain expression in the perivascular niche.
Confocal micrographs of immunostaining analysis from GBM patient specimens demonstrate that laminins (detected using a pan-laminin antibody reacting with α1, β1, and γ1 chains, red, A) are co-expressed with a laminin receptor, integrin α6 (Int α6, green), in the perivascular niche (marked by an antibody against CD31, purple). Chain specific antibodies demonstrate that laminin α2 (Ln α2, B), α3 (Ln α3, C), α5 (Ln α5, D) are co-expressed with integrin α6 (green) in the perivascular niche. Confocal micrographs of immunostaining analysis from GSCs derived from a xenografted tumor (tumor 387) grown as spheres (E) show expression of laminin α2 (red) in conjunction with integrin α6 (green). Yellow arrow indicates area of interest, inset in bottom right. Nuclei counterstained with Hoechst 33342, scale bar = 25 µm
Figure 4. Differential non-laminin ECM expression in the perivascular niche.
Confocal micrographs of immunostaining analysis from GBM patient specimens demonstrate that fibronectin (Fn, red, A) expression was limited to the vascular compartment while Collagen IV (Col IV, red, B) showed perivascular co-expression with a laminin receptor, integrin α6. White arrow indicates area of interest, yellow arrow indicates area shown in inset. Nuclei counterstained with Hoechst 33342. Scale bar = 25 µm
Non-stem cell tumor cells and endothelial cells provide niche laminins
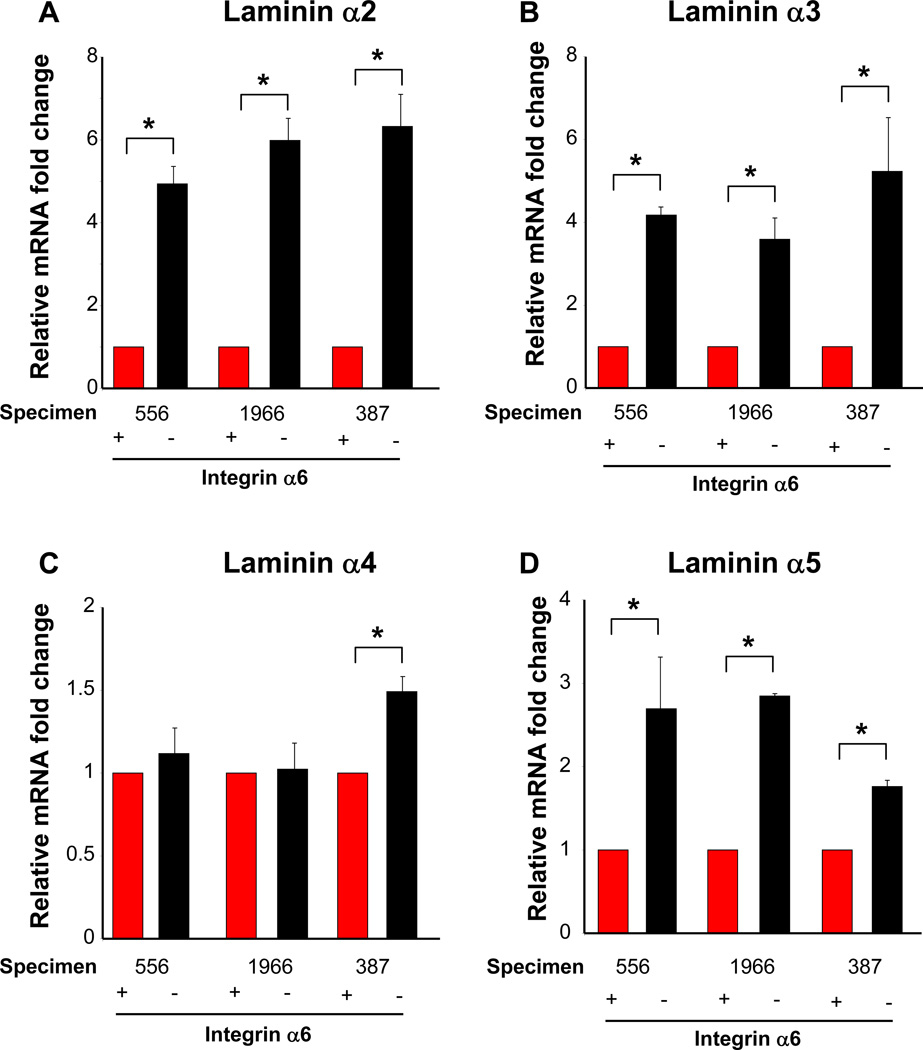
Having identified laminins as highly expressed in the perivascular niche, we interrogated the cellular source for the laminins to determine paracrine, juxtracrine, or autocrine communication. By comparing GSC marker positive (integrin α6) and negative cells from three xenografted tumors (propagated in nude mice), we found that non-GSCs had elevated RNA levels of laminin α2, α3, and α5 as compared to GSCs (Fig. 5). In particular, laminin α2, which had a negative correlation with patient survival, was preferentially expressed in the non-GSC population (Fig. 5A). Laminin α3 also had an elevated expression in non-GSCs (Fig. 5B). There were limited differences in laminin α4 expression between GSCs and non-GSCs (Fig. 5C). However there was significant enrichment of laminin α5 in non-GSCs (Fig. 5A). Analysis of GSCs enriched on CD133 expression showed similar results (Supplementary Fig. 5).
Figure 5. Laminins produced by non-GSCs in vivo.
Summary of quantitative real time PCR results from xenografted GBM tumors (556, 1966, 387) enriched based on integrin α6 expression and analyzed without cell culture expansion demonstrates that GSC marker negative cells (non-GSCs, black bars) have elevated levels of laminin chain transcripts when compared to GSC marker positive cells (GSCs, red bars). Bar graph of quantitative real time PCR results for laminin α2 (A), laminin α3 (B), laminin α4 (C), and laminin α5 (D) demonstrates that non-GSCs consistently contain significantly higher levels of laminin α2, α2, and α5 mRNA. *, p < 0.001 as assessed by one-way ANOVA for all groups except laminin α3 specimen 387 and laminin α5 specimen 556. *, p < 0.01.
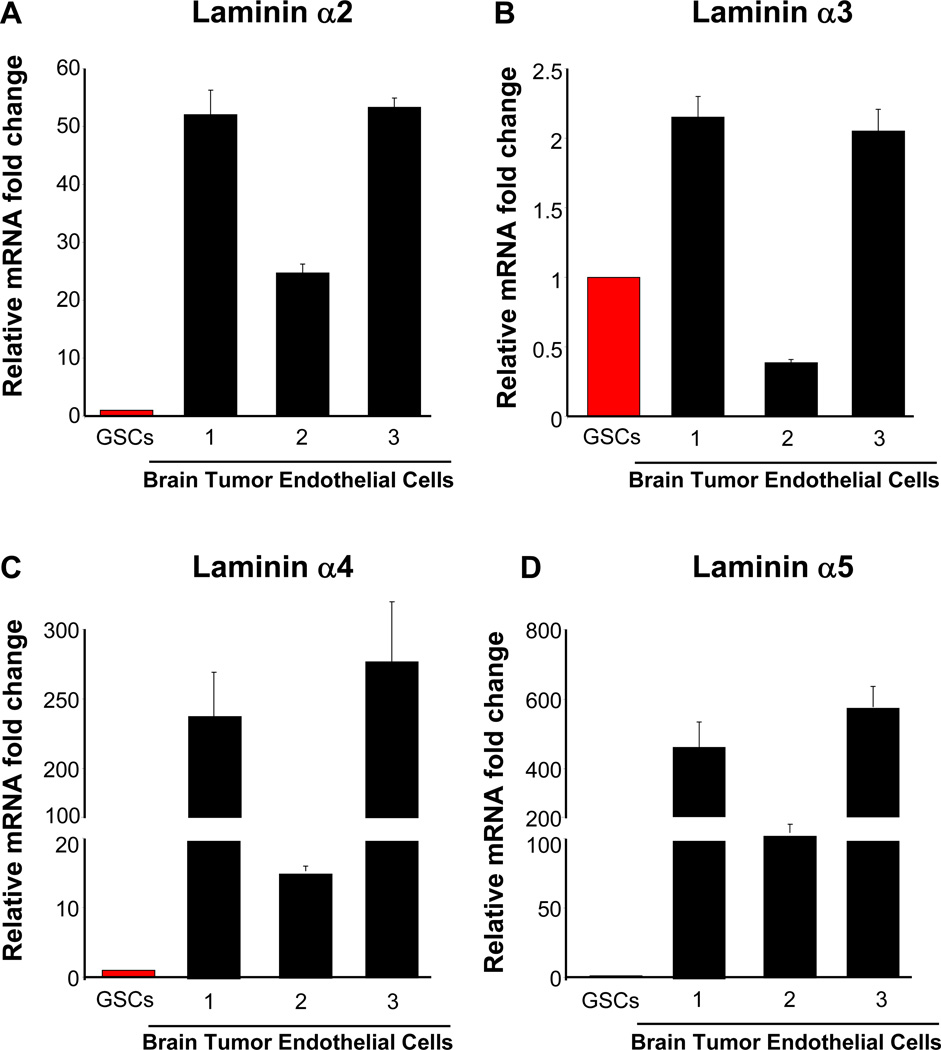
To evaluate laminin expression levels of endothelial cells, we compared the qPCR results from the GSCs in the above-mentioned analysis to brain tumor associated endothelial cells (BTECs). We found limited expression of laminin α1. Laminin α2 was greatly elevated in BTECs as compared to GSCs (Fig. 6A), however laminin α3 expression was not as elevated in BTEC as compared with GSCs (Fig. 6B). Both laminins α4 (Fig. 6C) and α5 (Fig. 6A) were also elevated in BTECs as compared with GSCs. These results demonstrate that GSCs express the least amount of several laminin chains (α2, α3, α5) and suggest a juxtacrine relationship, where GSCs receive ECM ligands from their microenvironment.
Figure 6. Laminins produced by ECs.
Summary of quantitative real time PCR results from primary brain tumor endothelial cells (BTEC, red bars) indicate consistently elevated laminin chains α2 (A), α4 (C), α5 (D) and decreased laminin α3 (B) expression when compared to GSCs (black bars). Prior to calculation of ratios between GSCs and BTECs, all data were first normalized to actin cycle number.
Laminin α2 is essential for glioblastoma stem cell maintenance
To disrupt laminin α2 function, we utilized two non-overlapping short hairpin RNA (shRNA) sequences against laminin α2 compared to a control non-targeting shRNA sequence not found in the human genome transduced by lentivirus (validated in Fig. 7A). As non-GSCs contained higher levels of laminin α2, validation of knockdown for laminin α2 was performed on non-GSCs derived from a xenografted tumor and laminin α2 knockdown did not results in substantial changes in laminins α3 and α5, confirming specificity for laminin α2 (Supplementary Fig. 6). A similar reduction in laminin α2 levels was observed in GSCs by immunoblotting (data not shown). The impact of disrupting laminin α2 function on GSCs was evaluated with several validated in vitro assays to measure cell proliferation, survival, and self-renewal, key aspects of the GSC phenotype. GSCs with attenuated laminin α2 had significantly decreased growth (Fig. 7B) and increased cell death (Fig. 7C) when compared to a non-targeting control. Additionally, tumorsphere formation was reduced in GSCs with attenuated laminin α2 (Fig. 7D). To determine if addition of exogenous laminin α2 could rescue the growth phenotype, we added purified laminin α2 (10 µg/ml) to GSC cultures with attenuated laminin α2 and observed a partial rescue in Caspase 3/7 activity (Fig. 7E). The introduction of shRNAs into GSCs was also controlled for by comparing uninfected GSCs. While non-targeting controls showed reduced growth and increased cell death as compared with uninfected GSCs, laminin α2 attenuation greatly decreased growth and increased cell death substantially as compared with both controls (Supplementary Fig. 7). Taken together, these results demonstrate that laminin α2 is pivotal for GSC maintenance (which includes survival, proliferation, and self-renewal).
Figure 7. Laminin α2 is required for GSC growth.
For functional studies, laminin α2 shRNA were generated and validated by immunoblotting in non-GSCs, with purified laminin α2 used as a positive control (A). Growth and self-renewal of GSCs (derived from tumor 387) was compromised upon laminin α2 shRNA mediated knockdown (KD) as shown in tumorsphere formation assay (B) and ATP based growth assay (C) graphs. Laminin α2 knockdown also resulted in increased cell death as shown by Caspase 3/7 activity assay graphs (D). Addition of exogenous purified laminin α2 provided a partial rescue as shown by Caspase 3/7 activity assay graphs (E). In vivo studies where 1000 GSCs were co-transplanted with 100,000 ECs with NT or laminin α2 knock down (KD1) demonstrate that EC derived laminin α2 contributes to tumor growth as shown in Kaplan-Meier survival graph (F). *, p < 0.001 as assessed by one-way ANOVA for sphere formation assays; *, p < 0.001 for NT vs both KD1 and KD2. †, p < 0.001 for KD1 vs KD2 as assessed by one-way ANOVA for growth assays; *, p < 0.01 and **, p < 0.001 by one-way ANOVA for rescue studies; p<0.01 as assessed by long-rank analysis for in vivo studies.
To determine the in vivo significance of EC laminin α2 contribution to tumor growth, we utilized a co-culture system in which GSCs were labeled and cultured with an equal number of control or shRNA laminin α2 targeted ECs. GSC only controls were used to verify strong Carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling (data not shown). Intracranial transplantation was done with 1000 GSCs (labeled with a constitutively active yellow fluorescent protein reporter) and 100,000 ECs with control or laminin α2 shRNA. Attenuation of laminin α2 resulted in significantly increased tumor latency (Fig. 7F), confirming that laminin α2 is critical for the tumorigenic process. Taken together, these results demonstrate that laminin α2 is important for GSC maintenance and that laminin α2 from other cells (ECs, non-GSCs) can impact GSC growth.
Laminin α2 promotes glioblastoma stem cell survival and DNA repair in response to radiation
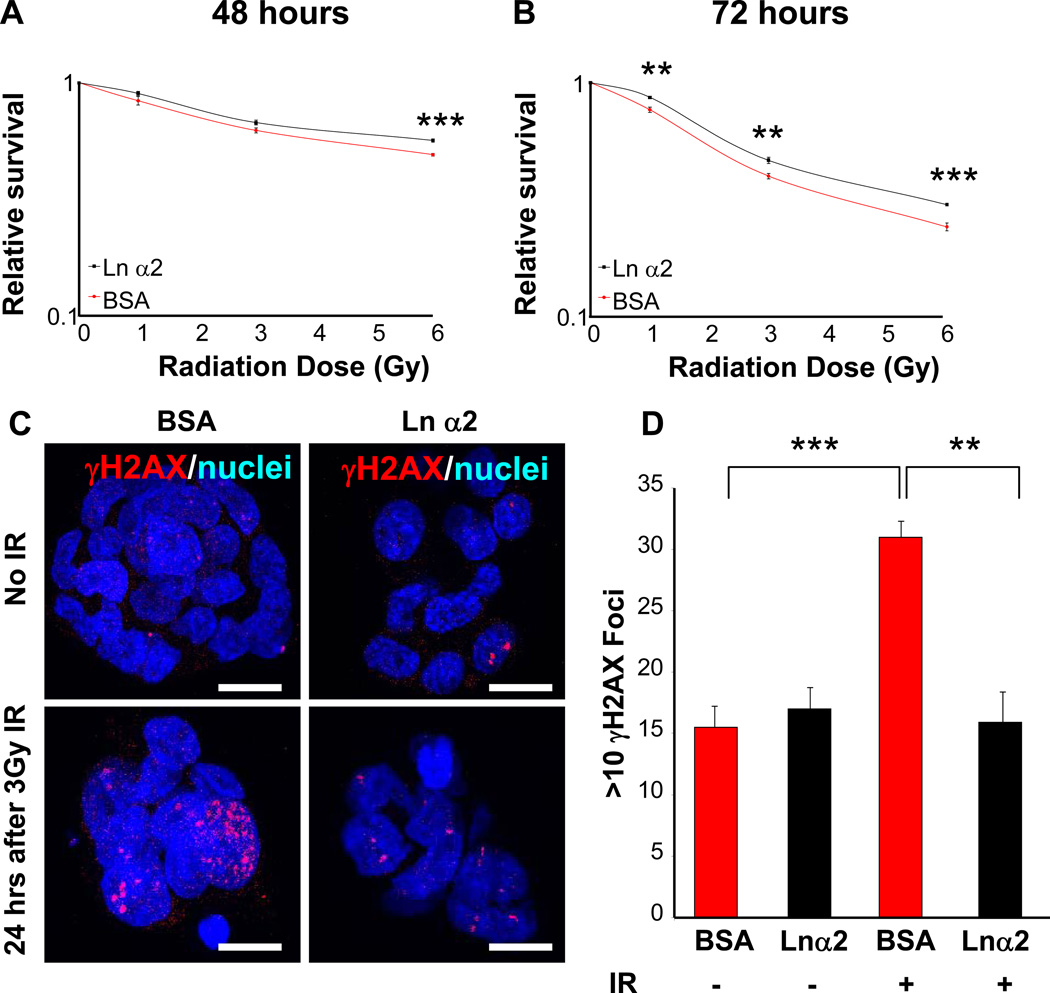
From a clinical standpoint, radiation is the most effective non-surgical therapy for GBM patients but remains palliative [1]. We previously demonstrated that GSCs were preferentially resistant to radiation-induced damage and contributed to recurrence after radiation treatment in laboratory models [10]. In a medulloblastoma mouse model, perivascular cells expressing a stem cell marker (Nestin) have an increased incidence of survival [50]. Based on these findings, we hypothesized that ECM interactions may increase GSC survival after radiation. To test this directly, GSCs were cultured with purified laminin α2 or a protein control (bovine serum albumin, BSA) and we observed a significant increase in survival 48 and 72 hours after single doses (1, 3, or 6 Gy) of ionizing radiation (Fig. 8A, B). To evaluate if exposure to laminin α2 facilitated increased DNA repair, γH2AX foci was assessed and found to be significantly reduced 24 hours after exposure to 3 Gy of ionizing radiation when GSCs were cultured in the presence of laminin α2 (Fig. 8C, D). No significant difference was detected between the groups with no ionizing radiation (Fig. 8C, D). These results confirm the importance of laminin α2 meditated cell survival after radiation and provide a mechanism by which the perivascular niche promotes GSC survival after irradiation.
Figure 8. Laminin α2 exposure attenuates radiation induced GSC cell death and increases DNA repair.
GSC survival is increased after exposure to purified laminin α2 as compared to protein control (0.5% bovine serum albumin, BSA) as shown with ATP-based cell survival assays 48 (A) and 72 (B) hours after single dose ionizing radiation (IR) of 0, 1, 3, or 6 Gy. Analysis of γH2aX 24 hours after exposure to 0 or 3 Gy IR shows increase foci (>10 foci) in BSA cultures as compared with laminin α2 at 3 Gy IR as shown in representative micrographs (C) and summary bar graph (D). Scale bar represents 10 µm. **, p < 0.01, ***, p < 0.001 as assessed by one-way ANOVA.
Discussion
Despite advances in the diagnosis and treatment of GBMs, these cancers remain universally fatal. Most systemic cancers are lethal due to metastases but GBMs cause death due to invasion into normal brain preventing surgical resection and striking therapeutic resistance [2] supporting the brain as a unique environment for cancer. The laminins are likely to be a biomarker of more aggressive molecular subgroups (in the case of GBM, the Classical and Mesenchymal subtypes). It is notable that another group using gene expression studies of ependymomas found that laminin α2 levels informed a poor prognosis [51], suggesting that this specific laminin may be particularly influential in tumor growth. From a clinical perspective, the role of laminins in brain tumors is central to the difficulty of effective treatment, namely therapeutic resistance. Previous work has demonstrated a protective role for the perivascular niche from ionizing radiation in a mouse model [50] and cell culture studies demonstrated the increased expression of integrin β1 after radiation [52]. Our study demonstrated that the ECM, a key component of the vasculature, increased survival and resulted in a more rapid resolution of DNA damage after radiation. Extending these findings, GBM patients with the highest quartile of laminin α2 expression had a higher incidence of edema as assessed by imaging as compared with the lowest quartile of laminin α2 patients (Supplementary Fig. 8). Taken together, our findings suggest that interaction with the ECM in the perivascular niche, involving such proteins as laminins, is likely to be an extrinsic mechanism by which cells survive after radiation. Given these findings, exploring approaches to disrupt the interaction with the ECM to serve as a radiation sensitizer should be an immediate priority. Such an approach has recently been demonstrated with Notch signaling, a key component in GSC maintenance [16, 53], where Notch inhibition alone had a modest effect on GSC survival but when combined with radiation, had a major impact on GSC survival. Reports have suggested a cross-talk between Notch signaling, integrins, and laminins which indicates that the integrin-laminin relationship may be amenable to targeting for radiation sensitization [54–57]. Targeting integrins alone is also being actively explored in the context of malignant gliomas [58]. Our results highlight the role of laminins in the perivascular niche and suggest that their contribution is greater than that of a structural protein but rather are key in GSC maintenance.
The α chains may serve as a good starting point for functional analysis as they mediate most receptor binding events and the expression of the 5 α chains is responsible for generating laminin diversity throughout the body [22]. Laminins α1, α2, and α5 are structurally similar and contain a short arm consisting of globular domains while laminins α3 and α4 have a highly truncated arm [22]. Mutant mice of laminin alpha chains have further demonstrated the tissue specific expression and function of laminins. Within the nervous system, laminin α2 deficient mice display a radial glia detachment phenotype [28] and laminin α2 and α4 mice have myelination defects [23]. Expression and functional studies in the neoplastic brain will likely contribute key information with regards to the differing roles of the diverse laminin chains in tumorigenic processes.
In the context of brain tumors, GSCs reside within a perivascular niche [13] and interact with non-stem tumor cells and host tissue (including endothelial cells, pericytes, immune cells, and reactive astrocytes). Our results indicate that laminin is generated from the endothelial cells and non-GSCs (non-stem tumor cells) and suggest a model in which the GSCs have a receptor (i.e. integrin α6) and the ligand is being provided by the niche cells. The concept of stem cells requiring signals from the niche for their maintenance is well-established and is supported by several examples in brain tumors. For example, GSCs have elevated levels of interleukin (IL)-6 receptor and non-GSCs secrete IL-6, which via STAT3 signaling is required for GSC maintenance [59]. There are also several examples involving the vasculature. In a mouse glioma model, endothelial cells secrete endothelial nitric oxide synthase (eNOS) which activates Notch signaling in GSCs [60]. In human glioma models, endothelial cells influence GSC growth and self-renewal via Notch signaling by presenting appropriate ligands [16, 53]. Additionally, a secreted factor analysis of human brain vasculature identified the mammalian target of rapamycin (mTOR) pathway as being critical for GSC growth [61]. These examples highlight the importance of cell communication within the niche and future studies integrating the role of the niche cells in promoting GSC maintenance are likely to be informative.
To date, there is limited direct evidence for the function of laminins in GSCs and the perivascular niche. Previous studies examining the perivascular niche may have, in part, addressed the contribution of laminins as the exterior of the vasculature is enriched in laminins. These contributions encompass a variety of diverse biological processes including survival, migration/invasion, adhesion, and stem-cell maintenance. Our work highlights the role of laminin α2 in GSC survival, which has also been reported when integrin α6 is attenuated. This is likely to reflect an inherent role of the ECM signaling in the perivascular niche. Laminins also have a major role in glioma cell migration and invasion, which has been demonstrated by previous studies and while yet to be fully elucidated, is likely to involve laminin interactions on blood vessels [31]. An additional role laminin signaling may have is that of stem-cell maintenance. Additionally, laminins may have an instructive role in the recently characterized phenomenon of GSCs assuming EC phenotypes [62–65]. Future studies evaluating a role for perivascular ECM in this process will be critical in determining mechanism by which this plasticity occurs in brain tumors. To fully appreciate stem cell maintenance is a difficult task as many assays measure self-renewal as a surrogate in combination with survival and proliferation (i.e. sphere formation assay). Due to their location in the niche and their association with many key signaling pathways (EGFR, mitogen-activated protein kinase), it is likely that laminins promote stem cell maintenance and subsequent signaling focused studies will be required to determine the exact mechanisms [22, 66]]. Laminins may also play a role in adhesion to the niche [20]. This is a relatively unexplored area and many questions remain unresolved as to how the niche instructs the GSC phenotype. It is likely that laminins play a central role in this process and may involve adhesion receptors such as integrin α6, but live imaging studies may be required to fully appropriate interactions.
Supplementary Material
Table 1. Summary of association of laminin α2, integrin α6, and integrin β1 with GBM molecular subtypes.
Table summarizing number of samples evaluated using TCGA for each GBM molecular subtype (Classical, Mesenchymal, Neural, and Proneural) and p values calculated with the use of the Wilcoxon rank-sum test for laminin α2 between each subtype.
| Association of Laminin α2, Integrin α6, and Integrin β1 with GBM molecular subtype | |||
| Subtype | Number of Samples | ||
| Classical | 51 | ||
| Mesenchymal | 56 | ||
| Neural | 27 | ||
| Proneural | 54 | ||
| Statistics – p-values based on Wilcoxon rank-sum test | |||
| Laminin α2 | |||
| Mesenchymal | Neural | Proneural | |
| 0.436 | 1.4×10−5 | 4.5×10−14 | Classical |
| 0.001 | 1.8×10−12 | Mesenchymal | |
| 0.001 | Neural | ||
Acknowledgements
We thank the members of the Rich lab for experimental insight and critical comments on the manuscript. We thank C. Shemo, M. Morgan, and S. O’Bryant for flow cytometry assistance, as well as D. Satterfield, L. Ehinger, J. Funkhouser, and J. Faison for technical assistance. We thank the Cleveland Clinic Foundation Tissue Procurement Service and S. Staugatis, R. Weil, and M. McGraw. We also thank Dr. Holly Colognato (SUNY Stony Brook University) and Dr. Mattson (National Institute on Aging) for insightful comments on the manuscript. Work in the Rich lab is supported by the Pediatric Brain Tumor Foundation of the US, Brain Tumor Society, Goldhirsh Foundation, and James D. McDonnell Foundation. This work was also supported by National Institutes of Health grants CA112958 (JNR), CA116659 (JNR), CA154130 (JNR), CA151522 (ABH), CA137443 (AES), NS063971 (AES), CA128269 (AES), CA101954 (AES), CA116257 (AES), CA109748 (CLG), CA152883 (CLG), CA127620 (CLG), CA108786 (REM), NS20023 (REM), a National Research Service Award CA142159 (JDL), and a Pathway to Independence Award CA157948 (JDL). JNR was a Damon Runyon-Lilly Clinical Investigator supported by the Damon Runyon Cancer Research Foundation. ABH is supported by the National Brain Tumor Society. AES is supported by funding from the Cancer Genome Atlas (TCGA) Project, the Ben & Catherine Ivy Foundation, the Kimble Foundation, the Peter B. Cristal Endowment, and the Ohio Department of Development Technology (09-071). PEH was supported by the Pathological Society of Great Britain and Ireland. JDL and MV were supported by an American Brain Tumor Association Fellowship (JDL’s sponsored by the Joelle Syverson Fund). This publication was made possible by the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 to JDL from National Institutes of Health/National Center for Research Resources (NCRR).
References
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 3.Venere M, Fine HA, Dirks PB, Rich JN. Cancer stem cells in gliomas: identifying and understanding the apex cell in cancer's hierarchy. Glia. 59:1148–1154. doi: 10.1002/glia.21185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 5.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 6.Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 7.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 9.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 10.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444 doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 11.Cheng L, Wu Q, Guryanova OA, et al. Elevated invasive potential of glioblastoma stem cells. Biochem Biophys Res Commun. 406:643–648. doi: 10.1016/j.bbrc.2011.02.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lathia JD, Heddleston JM, Venere M, Rich JN. Deadly teamwork: neural cancer stem cells and the tumor microenvironment. Cell Stem Cell. 8:482–485. doi: 10.1016/j.stem.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 15.Penuelas S, Anido J, Prieto-Sanchez RM, et al. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–327. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Zhu T, Costello MA, Talsma CE, et al. Endothelial cells create a stem cell niche in glioblastoma by providing Notch ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 18.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lathia JD, Rao MS, Mattson MP, Ffrench-Constant C. The microenvironment of the embryonic neural stem cell: lessons from adult niches? Dev Dyn. 2007;236:3267–3282. doi: 10.1002/dvdy.21319. [DOI] [PubMed] [Google Scholar]
- 20.Kokovay E, Goderie S, Wang Y, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 23.Colognato H, ffrench-Constant C, Feltri ML. Human diseases reveal novel roles for neural laminins. Trends Neurosci. 2005;28:480–486. doi: 10.1016/j.tins.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Halfter W, Dong S, Yip YP, et al. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lathia JD, Patton B, Eckley DM, et al. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J Comp Neurol. 2007;505:630–643. doi: 10.1002/cne.21520. [DOI] [PubMed] [Google Scholar]
- 26.Hall PE, Lathia JD, Miller NG, et al. Integrins are markers of human neural stem cells. Stem Cells. 2006;24:2078–2084. doi: 10.1634/stemcells.2005-0595. [DOI] [PubMed] [Google Scholar]
- 27.Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 28.Loulier K, Lathia JD, Marthiens V, et al. beta1 integrin maintains integrity of the embryonic neocortical stem cell niche. PLoS Biol. 2009;7:e1000176. doi: 10.1371/journal.pbio.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graus-Porta D, Blaess S, Senften M, et al. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 30.Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawataki T, Yamane T, Naganuma H, et al. Laminin isoforms and their integrin receptors in glioma cell migration and invasiveness: Evidence for a role of alpha5-laminin(s) and alpha3beta1 integrin. Exp Cell Res. 2007;313:3819–3831. doi: 10.1016/j.yexcr.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 32.Ljubimova JY, Fugita M, Khazenzon NM, et al. Association between laminin-8 and glial tumor grade, recurrence, and patient survival. Cancer. 2004;101:604–612. doi: 10.1002/cncr.20397. [DOI] [PubMed] [Google Scholar]
- 33.Pollard SM, Yoshikawa K, Clarke ID, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Fael Al-Mayhani TM, Ball SL, Zhao JW, et al. An efficient method for derivation and propagation of glioblastoma cell lines that conserves the molecular profile of their original tumours. J Neurosci Methods. 2009;176:192–199. doi: 10.1016/j.jneumeth.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Irizarry RA, Bolstad BM, Collin F, et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29:4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eyler CE, Wu Q, Yan K, et al. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 146:53–66. doi: 10.1016/j.cell.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z, Bao S, Wu Q, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly JJ, Stechishin O, Chojnacki A, et al. Proliferation of human glioblastoma stem cells occurs independently of exogenous mitogens. Stem Cells. 2009;27:1722–1733. doi: 10.1002/stem.98. [DOI] [PubMed] [Google Scholar]
- 42.Huang P, Rani MR, Ahluwalia MS, et al. Endothelial expression of TNF receptor-1 generates a proapoptotic signal inhibited by integrin alpha6beta1 in glioblastoma. Cancer Res. 2012;72:1428–1437. doi: 10.1158/0008-5472.CAN-11-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyazaki T, Pan Y, Joshi K, et al. Telomestatin impairs glioma stem cell survival and growth through the disruption of telomeric G-quadruplex and inhibition of the proto-oncogene, c-Myb. Clin Cancer Res. 2012;18:1268–1280. doi: 10.1158/1078-0432.CCR-11-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Hall PE, Lathia JD, Caldwell MA, Ffrench-Constant C. Laminin enhances the growth of human neural stem cells in defined culture media. BMC Neurosci. 2008;9:71. doi: 10.1186/1471-2202-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leins A, Riva P, Lindstedt R, et al. Expression of tenascin-C in various human brain tumors and its relevance for survival in patients with astrocytoma. Cancer. 2003;98:2430–2439. doi: 10.1002/cncr.11796. [DOI] [PubMed] [Google Scholar]
- 48.Behrem S, Zarkovic K, Eskinja N, Jonjic N. Distribution pattern of tenascin-C in glioblastoma: correlation with angiogenesis and tumor cell proliferation. Pathol Oncol Res. 2005;11:229–235. doi: 10.1007/BF02893856. [DOI] [PubMed] [Google Scholar]
- 49.Ziu M, Schmidt NO, Cargioli TG, et al. Glioma-produced extracellular matrix influences brain tumor tropism of human neural stem cells. J Neurooncol. 2006;79:125–133. doi: 10.1007/s11060-006-9121-5. [DOI] [PubMed] [Google Scholar]
- 50.Hambardzumyan D, Becher OJ, Rosenblum MK, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witt H, Mack SC, Ryzhova M, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 20:143–157. doi: 10.1016/j.ccr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cordes N, Seidler J, Durzok R, et al. beta1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene. 2006;25:1378–1390. doi: 10.1038/sj.onc.1209164. [DOI] [PubMed] [Google Scholar]
- 53.Hovinga KE, Shimizu F, Wang R, et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 28:1019–1029. doi: 10.1002/stem.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campos LS, Decker L, Taylor V, Skarnes W. Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J Biol Chem. 2006;281:5300–5309. doi: 10.1074/jbc.M511886200. [DOI] [PubMed] [Google Scholar]
- 55.Estrach S, Cailleteau L, Franco CA, et al. Laminin-binding integrins induce dll4 expression and notch signaling in endothelial cells. Circ Res. 109:172–182. doi: 10.1161/CIRCRESAHA.111.240622. [DOI] [PubMed] [Google Scholar]
- 56.Karsan A. Notch and integrin affinity: a sticky situation. Sci Signal. 2008;1:pe2. doi: 10.1126/stke.12pe2. [DOI] [PubMed] [Google Scholar]
- 57.Li H, Chang YW, Mohan K, et al. Activated Notch1 maintains the phenotype of radial glial cells and promotes their adhesion to laminin by upregulating nidogen. Glia. 2008;56:646–658. doi: 10.1002/glia.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tabatabai G, Tonn JC, Stupp R, Weller M. The Role of Integrins in Glioma Biology and Anti-Glioma Therapies. Curr Pharm Des. doi: 10.2174/138161211797249189. [DOI] [PubMed] [Google Scholar]
- 59.Wang H, Lathia JD, Wu Q, et al. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells. 2009;27:2393–2404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charles N, Ozawa T, Squatrito M, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galan-Moya EM, Le Guelte A, Lima Fernandes E, et al. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO Rep. 12:470–476. doi: 10.1038/embor.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Hallani S, Boisselier B, Peglion F, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 133:973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 64.Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 65.Soda Y, Marumoto T, Friedmann-Morvinski D, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci U S A. 108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campos LS, Leone DP, Relvas JB, et al. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.