Abstract
Objective
To determine if Amyotrophic Lateral Sclerosis (ALS) risk varies according to body mass index (BMI) captured up to three decades earlier.
Methods
At baseline 537,968 women and 562,942 men in five ongoing cohorts reported height, current weight and weight at age 18/21. During 14-28 years of follow-up 1,153 participants developed ALS. Cohort-specific Cox proportional hazards models were used to estimate rates that were then pooled with random effects models.
Results
Lower BMI at baseline was associated with ALS; for each 5-unit increase in BMI, ALS rates were 21% lower (95%CI: 14% to 27%). Compared to individuals with healthy BMI, ALS rates were significantly lower among the overweight (RR=0.76 [95%CI: 0.62-0.93]) and obese (RR=0.73 [95%CI: 0.55-0.96]). Among never-smokers the association persisted: RR=0.75 (95%CI: 0.65-0.85) for each 5-unit increase. Excluding the first seven years of follow-up, the associations were materially unchanged suggesting that weight loss from undiagnosed disease does not fully explain the findings. Overall, 75% of men and women had a healthy BMI at age 18/21, 15% of men and 8% of women were overweight or obese; there was no association with ALS risk although power was limited.
Conclusion
These findings support an association between lower premorbid BMI and ALS.
Keywords: Amyotrophic Lateral Sclerosis, Body Mass Index, Risk, Epidemiology, Cohort, Nutrition
INTRODUCTION
It has been reported that amyotrophic lateral sclerosis (ALS) patients tend to be lean and physically active in life,(1) and exhibit hypermetabolism at diagnosis.(2-5) Likewise, in the Cu/Zn superoxide dismutase-1 animal model of ALS, metabolism is higher and weight is lower in mutant mice compared to wildtype.(4) However, the epidemiological evidence in humans of an association between pre-morbid body mass index (BMI) and future ALS risk is scarce and the few published studies have conflicting results, possibly due to methodological limitations. A small case-control study from a US clinical setting found that ALS patients were twice as likely to have “always been slim” as controls with other neurological conditions.(1) In a case-control study set in a tertiary clinic in the Netherlands ALS patients reported having a lower BMI during adulthood than controls and were less likely to be obese.(6) The time frame of the recalled pre-morbid or adulthood BMI in relation to disease onset was not given in either study nor was smoking (which is related to weight and to ALS risk) considered in either analyses. Conversely, in a population-based case-control study in Washington State those with a BMI>=26 kg/m2 five years prior to diagnosis had a 70% higher risk of ALS compared to those with BMI<21 kg/m2, whereas at the time of diagnosis the same ALS cases had significantly lower BMI than controls (>=26 kg/m2 vs <21kg/m2: OR=0.4; 95% CI:0.2-0.8).(7, 8) BMI at diagnosis is an inadequate proxy for adulthood BMI because some weight loss will have occurred by then. There is an absence of large-scale, prospective data on pre-morbid BMI and risk of ALS. We therefore examined the association in five well-established large cohorts where BMI was reported prior to disease onset.
METHODS
Study Population
The study population comprised participants in the following cohorts: the Nurses’ Health Study (NHS), the Health Professionals Follow-up Study (HPFS), the Cancer Prevention Study II Nutrition Cohort, the Multiethnic Cohort (MEC), and the National Institutes of Health–AARP Diet and Health Study (NIH-AARP).
The NHS cohort was established in 1976 when 121,700 female registered nurses from 11 US states, aged 30 to 55 years, responded to a mailed questionnaire about disease history and lifestyle.(9) In 1980, 103,298 women returned an expanded questionnaire including diet. The HPFS began in 1986 when 51,529 male health professionals (dentists, optometrists, pharmacists, podiatrists, and veterinarians), aged 40 to 75 years, answered a similar mailed questionnaire.(10) Follow-up questionnaires are mailed to the participants in both studies every 2 years to update information on potential risk factors for chronic diseases and to ascertain whether major medical events have occurred. The CPS-II Nutrition cohort comprises over 86,400 men and 97,700 women, aged 50 to 79 years, from 21 states with population-based cancer registries who completed a mailed questionnaire in 1992 to investigate the relation between diet and other lifestyles factors and the risk of incident cancer.(11) Similar questionnaires were sent in 1997 and every 2 years afterwards to update exposure information and newly diagnosed diseases. The MEC study consists of over 96,900 men and 118,800 women, aged 45 to 75 years at baseline, living in Hawaii and California (primarily Los Angeles) and mainly from the following 5 self-reported racial/ethnic groups: African -American, Japanese -American, Latino, Native Hawaiian, and white.(12) From 1993 to 1996, participants entered the cohort by completing a self-administered mailed questionnaire. Additional questionnaires were mailed to the participants at 5-year intervals. The NIH-AARP cohort included over 340,000 men and 227,000 women, aged 50 to 71 years, residing in 1 of 6 states or 2 metropolitan areas with high quality cancer registries in 1995-1996.(13) A total of 318,261 participants (187,499 men and 130,762 women) responded to the follow-up survey in 1996-1997. All studies included were reviewed and approved by the institutional review board of the institution at which each study was conducted.
Ascertainment of ALS
Follow-up of ALS in the CPS-II Nutrition, MEC, and NIH-AARP was through a search of the National Death Index. Vital status of the participants in these studies was determined by automated linkage with the National Death Index. The underlying and contributing causes of death were coded according to the International Classification of Diseases, Ninth Revision. All individuals with code 335.2 (motor neuron disease) listed as the underlying or contributing cause of death were considered to have had ALS. In a previous validation study,(14) it was found that ALS was the primary diagnosis listed on death certificates in the majority of instances where code 335.2 was listed as a cause or contributory cause of death.
In NHS and HPFS, incident ALS was also documented. In each biennial follow-up questionnaire, participants were asked to report a specific list of medically diagnosed conditions (initially not including ALS) and “any other major illness.” ALS was added to the list of specific conditions on the NHS questionnaires in 1992 onwards and on the HPFS questionnaires in 2000 onwards. We requested permission to contact the treating neurologist and for release of relevant medical records from participants who reported a diagnosis of ALS on the open question on major illnesses or on the specific question. Because of the rapidly progressive nature of the disease (median survival 1.5 to 3 years),(15-17) many participants with ALS died before we could send the release request for medical records, so the request was sent to the closest family member. After obtaining permission, we asked the treating neurologists to complete a questionnaire to confirm the diagnosis of ALS and to rate the certainty of the diagnosis (definite, probable, or possible) and send medical records. Starting in 2004 the questionnaire was modified to include the El Escorial criteria. The final confirmation for our study purposes was made by a neurologist with experience in ALS diagnosis based on the review of medical records. We relied on the diagnosis made by the treating neurologist if the information in the medical record was insufficient or if it could not be obtained. Only participants with definite and probable ALS are included as cases in the primary analyses. When we were unable to confirm (i.e., obtain a copy of the medical record or the neurologist's questionnaire) incident self-reported ALS, we classified the participant as having ‘possible ALS’ and excluded him or her from the primary analysis unless death occurred during follow-up and ALS was listed on the death certificate.
Exposure Ascertainment
In HPFS, MEC, NIH-AARP and CPSII-N height, current weight, and weight in early adulthood (ages 18 or 21 depending on the cohort) were self-reported at baseline. BMI was calculated as weight (kg) divided by height (m) squared. Similarly, in the NHS, height was reported at study entry in 1976, and combined with weight at age 18 and with current weight reported on the 1980 and 1994 questionnaires to calculate BMI. Correlations between self-reported height and weight and direct measurements have been high, r>0.9.(18, 19) BMI was categorized using standard World Health Organization categories for underweight (BMI <18.5 kg/m2), healthy weight (18.5 to 24.9 kg/m2; reference), overweight (25.0 to 29.9 kg/m2) and obese (≥ 30.0 kg/m2 ). Where sample size permitted these categories were further divided to determine, for example, the risk associated with a modestly increased BMI of 23 to <25 kg/m2 compared to a reference of 18.5 to <23 kg/m2.
Data Analysis
Each participant contributed person-time of follow-up from the return date of the baseline questionnaire to the date at onset of first ALS symptoms (in NHS and HPFS), death from ALS or any other cause, or the end of follow-up, which ever came first. The end of follow-up was June 2008 for the NHS, January 2008 for the HPFS, December 2006 for the CPS-II Nutrition, December 2007 for the MEC, and December 2008 for the NIH-AARP.
The analyses were conducted separately in each cohort. Because of the long follow-up in the NHS, the cases and person-time experienced during follow-up were split into the following 2 uncorrelated segments: 1980 to 1994 and 1994 to 2008. In accordance with the underlying theory of survival analysis, blocks of person-time in different periods are asymptotically uncorrelated, regardless of the extent to which they are derived from the same persons. Therefore, pooling the estimates from the 2 periods is equivalent to using a single period but takes advantage of the updated weight and confounder assessment in 1994.
Cox proportional hazards regression analysis was used to estimate the relative rates (RRs) and 95% confidence intervals (CIs) for BMI for each cohort. Men and women were analyzed separately in MEC, CPS-II Nutrition and NIH-AARP. To obtain better age adjustment, the Cox proportional hazards models were stratified by age in single years. The log RRs were pooled using a random-effects model and were weighted by the inverse of their variances. Multivariable Cox proportional hazards regression was used to adjust for additional potential confounders, including: smoking (never smoked, pack-years:<10, 10-<20, 20-<30, 30-<40, 40+), vitamin E from food and supplements (quintiles), education attained (less than high-school, high-school, more than high-school), and physical activity (low, moderate, high). There are few established risk factors for ALS. When considering potential confounders, we chose variables strongly associated with BMI for which there is also some evidence of their being risk factors for ALS. Because smoking is strongly associated with lower BMI, we repeated the analyses among never smokers. To minimize the possibility of including participants who already had symptoms of ALS, including weight loss, at the time of completing the baseline questionnaire or participants with underlying undiagnosed disease, additional analyses that excluded the first four years and the first seven years of follow-up were conducted. Analyses were performed using a commercially available statistical software package (SAS version 9.2; SAS Institute, Cary, North Carolina) and STATA (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, Texas).
RESULTS
Table 1 gives the baseline characteristics of the five cohorts combined. We documented 1,153 participants with ALS in over 562,942 men and 537,968 women. Following exclusions of individuals with missing or extreme BMI there remained 1,124 participants with ALS among 552,455 men and 520,059 women. At baseline 49% of women and 39% of men had a healthy BMI (18.5 to <25 kg/m2), while 49% of women and 66% of men were overweight or obese (>=25 kg/m2). Underweight (<18.5 kg/m2) participants were more likely to smoke or use supplemental vitamin E than participants with a healthy BMI (18.5-<25 kg/m2) who in turn were more likely to smoke or use supplemental vitamin E than overweight (25-<30 kg/m2) or obese (30+ kg/m2) participants. Those with healthy BMI were more physically active than the underweight, overweight or obese. Education level did not vary greatly with BMI except that obese participants were less likely to have obtained education beyond high-school. (Table 1)
Table 1.
Age-adjusted Characteristics of Study Participants at Baseline According Body Mass Index: CPS-II Nutrition (1992), HPFS (1986), MEC (1993-1997), NHS (1980) and NIH-AARP (1996).
| Body Mass Index at Baseline (kg/m2) | ||||||
|---|---|---|---|---|---|---|
| <18.5 | 18.5-<23 | 23-<25 | 25-<27.5 | 27.5-<30 | 30+ | |
| Men | ||||||
| No. of participants | 3,621 | 74,801 | 112,179 | 165,235 | 99,278 | 97,341 |
| No. of ALS cases | 7 | 131 | 138 | 202 | 105 | 86 |
| Age, mean (SD)* | 63.3 (7.3) | 61.8 (8.0) | 61.4 (7.6) | 61.4 (7.0) | 61.2 (6.6) | 60.6 (6.3) |
| BMI , mean (SD) | 17.2 (1.1) | 21.7 (1.0) | 24.0 (0.6) | 26.2 (0.7) | 28.6 (0.7) | 33.1 (3.1) |
| Current smokers, % | 24.0 | 17.1 | 12.4 | 10.7 | 9.8 | 9.1 |
| Education > high school, % | 71.2 | 76.8 | 77.9 | 75.1 | 71.7 | 68.7 |
| Physical activity, highest tertile, % | 26.3 | 34.2 | 33.3 | 29.3 | 25.7 | 20.3 |
| Vitamin E supplement use, % | 25.0 | 23.2 | 19.3 | 17.4 | 17.0 | 15.7 |
| Women | ||||||
| No. of participants | 11,093 | 159,256 | 96,627 | 98,507 | 59,037 | 95,539 |
| No. of ALS cases | 12 | 150 | 99 | 85 | 41 | 68 |
| Age, mean (SD)* | 59.4 (10.2) | 57.0 (9.9) | 58.6 (8.8) | 59.3 (8.2) | 59.7 (7.9) | 59.1 (7.7) |
| BMI, mean (SD) | 17.5 (0.9) | 21.2 (1.2) | 24.0 (0.6) | 26.2 (0.8) | 28.7 (0.7) | 34.4 (4.0) |
| Current smokers, % | 28.0 | 18.4 | 16.1 | 15.2 | 13.9 | 11.8 |
| Education > high school, % | 72.2 | 73.8 | 70.3 | 67.8 | 65.0 | 63.8 |
| Physical activity, highest tertile, % | 24.3 | 28.0 | 25.1 | 22.0 | 20.0 | 15.7 |
| Vitamin E supplement use, % | 29.6 | 23.5 | 21.7 | 21.6 | 23.0 | 21.5 |
Abbreviations: HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; CPS-IIN, Cancer Prevention Study II Nutrition; MEC, Multiethnic Cohort study; NIH, National Institutes of Health; ALS, amyotrophic lateral sclerosis; BMI, Body Mass Index.
Values are not age-adjusted.
We found that lower BMI at baseline was associated with higher rates of ALS. For a 5 unit increase in BMI the pooled multivariable relative rate (RR) of ALS was 0.79 (95% CI: 0.73, 0.86, p<0.0001; p for heterogeneity across cohorts=0.32). Compared to individuals with a healthy BMI, the risk of ALS was significantly lower among the overweight (RR=0.76 [95% CI: 0.62-0.93]) and obese (RR=0.73 [95% CI: 0.55-0.96]). Finer categorization of WHO BMI categories revealed a trend where RRs were further lowered with the degree of excess weight: compared to participants with BMI of 18.5 to <23 kg/m2 the pooled multivariable-adjusted RR of ALS was 0.80 for those with a BMI of 23 to <25 kg/m2; 0.71 for BMI of 25 to <27.5 kg/m2; 0.62 for BMI of 27.5 to <30 kg/m2, and 0.63 for BMI of ≥ 30.0 kg/m2.(Table 2) Results were similar when men and women were analyzed separately.(Table 2) Smoking appeared to be a risk factor for ALS and was strongly correlated with BMI in all five cohorts. However, the findings were materially unchanged when restricted to never smokers (Table 2): pooled multivariable-adjusted RR was 0.75 (95% CI: 0.65-0.85, p<0.0001; p for heterogeneity across cohorts=0.83) for a 5 unit increase in BMI. (Figure) When the first four years or seven years of follow-up were excluded the associations were slightly attenuated. For a 5 unit increase in BMI the four year lag pooled multivariable-adjusted RR was 0.80 (95% CI: 0.72-0.89, p<0.0001; p for heterogeneity across cohorts=0.20), and the seven year lag pooled multivariable RR was 0.82 (95% CI: 0.72-0.93, p=0.002; p for heterogeneity across cohorts=0.11).
Table 2.
Pooled Relative Risks (95% Confidence Intervals) of Amyotrophic Lateral Sclerosis by Body Mass Index
| <18.5 kg/m2 | 18.5-<23 kg/m2 | 23-<25 kg/m2 | 25-<27.5 kg/m2 | 27.5-<30 kg/m2 | >=30 kg/m2 | |
|---|---|---|---|---|---|---|
| All participants | ||||||
| Person years | 181,656 | 3,142,552 | 2,752,788 | 3,363,465 | 1,977,513 | 2,425,294 |
| Cases | 19 | 281 | 237 | 287 | 146 | 154 |
| RR (95% CI) | 1.61 (0.88-2.94) | Ref | 0.80 (0.67-0.95) | 0.71 (0.59-0.85) | 0.62 (0.49-0.78) | 0.63 (0.49-0.81) |
| p-value | 0.068 | 0.013 | <0.001 | <0.001 | <0.001 | |
| p for trend (p for heterogeneity) | <0.001 (0.32) | |||||
| Men | ||||||
| Person years | 36,062 | 875,223 | 1,339,882 | 1,920,755 | 1,116,440 | 1,051,137 |
| Cases | 7 | 131 | 138 | 202 | 105 | 86 |
| RR (95% CI) | 2.52 (0.34-18.5) | Ref | 0.68 (0.53-0.86) | 0.64 (0.50-0.83) | 0.59 (0.45-0.78) | 0.58 (0.36-0.94) |
| p-value | 0.36 | 0.002 | 0.001 | <0.001 | 0.027 | |
| p for trend (p for heterogeneity) | 0.004 (0.12) | |||||
| Women | ||||||
| Person years | 145,594 | 2,267,329 | 1,412,906 | 1,442,710 | 861,073 | 1,374,157 |
| Cases | 12 | 150 | 99 | 85 | 41 | 68 |
| RR (95% CI) | 1.25 (0.70-2.26) | Ref | 0.96 (0.75-1.25) | 0.79 (0.61-1.04) | 0.64 (0.41-0.99) | 0.68 (0.51-0.92) |
| p-value | 0.45 | 0.78 | 0.09 | 0.05 | 0.01 | |
| p for trend (p for heterogeneity) | <0.001 (0.83) | |||||
| Never smokers | ||||||
| Person years | 81,190 | 1,413,405 | 1,161,320 | 1,330,219 | 759,798 | 974,657 |
| Cases | 10 | 102 | 83 | 88 | 47 | 45 |
| RR (95% CI) | 2.74 (1.39-5.41) | Ref | 0.85 (0.63-1.14) | 0.71 (0.53-0.96) | 0.64 (0.48-1.00) | 0.59 (0.41-0.86) |
| p-value | 0.004 | 0.27 | 0.03 | 0.05 | 0.006 | |
| p for trend (p for heterogeneity) | <0.001 (0.83) | |||||
Adjusted for study, age, physical activity (low, medium, high), pack-years smoked (never, <10, 10-<20, 20-<30, 30-<40, 40+), education (some highschool, highschool, beyond highschool), total vitamin E intake (quintiles).
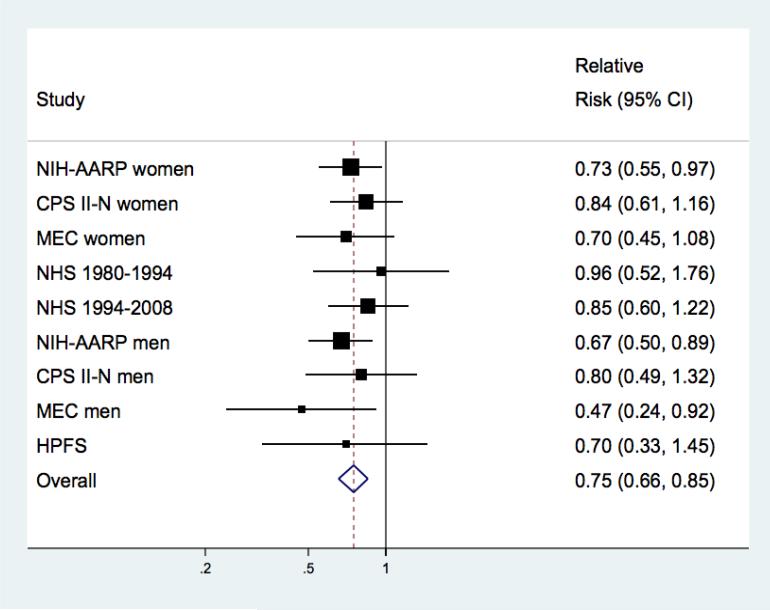
Figure. Pooled Relative Risks (95% Confidence Intervals) of Amyotrophic Lateral Sclerosis by Body Mass Index Among Never Smokers.
Study-specific and pooled multivariable relative risk and 95% confidence interval of amyotrophic lateral sclerosis for a 5 unit increase in BMI among never smokers (375 cases). The squares and horizontal lines correspond to the study-specific multivariable relative risk (RR) and 95% confidence interval (CI), respectively. The area of the squares reflects the study weight (inverse of the variance). The diamond represents the pooled multivariable RR and 95% CI. Pooled RR =0.75 (95% CI: 0.65-0.85; p<0.0001); p for heterogeneity =0.83. Among never smokers, with a BMI of 18.5-<23 as the reference category the RRs (95% CI) were 2.74 (1.39, 5.41) for BMI <18.5; 0.85 (0.63-1.14) for BMI 23-<25; 0.71 (0.53-0.96) for BMI 25-<27.5; 0.69 (0.48-1.00) for a BMI 27.5-<30; and 0.59 (0.41-0.86) for BMI of 30+
Overall, 75% of men and women had a healthy BMI at age 18/21, 13% of men and 6% of women were overweight, under 2% were obese; the remaining 10% of men and 17% of women were underweight. The correlation between BMI at baseline and BMI at age 18/21 was 0.40, p<0.001. There was no association between BMI at age 18/21 and ALS risk which may in part be due to low power. (data not shown)
DISCUSSION
This study, comprising data from five large cohort studies, prospectively examined the association between BMI measured before disease onset and future ALS risk. Men and women in the lower healthy BMI range (18.5-<23 kg/m2) were found to have higher rates of ALS than those with a BMI of 23 kg/m2 or above, and rates monotonically decreased as BMI increased. These findings persisted when analyses were restricted to never smokers who have on average higher BMIs than smokers and when the first seven years of follow-up were excluded.
A number of case-control studies have examined the association between premorbid BMI and ALS. Two studies reported that recalled adulthood BMI (the timeframe in relation to diagnosis was not indicated) was lower in ALS patients than their matched controls(1, 6) although neither adjusted for smoking (in one study the authors also found that ALS patients were more likely to report having “always been slim” than controls with other neurological disorders). A third study found that BMI at diagnosis, but not recalled BMI from five years prior to diagnosis, was lower among ALS patients than population-based controls.(7, 8) While the low incidence of ALS necessitated a case-control design for past studies there is potential for methodological bias. For example, even with in-person interview rather than self-reporting, case-control studies are susceptible to recall bias in the assessment of the exposure or confounders. In addition, selection bias is difficult to avoid; volunteer controls in a population-based study may be more health conscious, including weight conscious, than the general population of the same age and gender resulting in an obfuscation of lower premorbid BMI in ALS patients, if it existed. Smoking is among the strongest correlates of BMI(20) and unless adjusted for could result in confounding bias of a BMI and ALS association.(1, 6) In addition, weight is affected by disease, and perhaps by underlying disease prior to diagnosis, rendering weight around the time of diagnosis a poor correlate of pre-morbid BMI in a study of ALS etiology (reverse causation).
The strengths of the current study include the prospective design and the many participants with ALS. Taken together, these cohorts are more likely to be representative of the whole spectrum of patients with ALS, avoiding selection that is likely when patients are recruited in ALS tertiary care centers. In addition, recall bias is minimized because BMI was ascertained prospectively before participant knowledge of their future disease outcome. An important limitation of the present study is the use of ALS mortality as a proxy for ALS incidence which may result in underreporting of ALS occurrence. The short duration of the disease course suggests that mortality may be a good surrogate for incidence. Bias due to underreporting is unlikely to explain the findings unless the underreporting of ALS on death certificates is strongly related to premorbid BMI. In addition, the use of mortality could result in inclusion of prevalent ALS at baseline. By excluding ALS occurrence in the first four years or seven years of follow-up, the chance of our findings being wholly explained by reverse causation are reduced, but it remains possible that the hypermetabolic state reported in ALS starts many years before the onset of neurological symptoms. The findings of the current study support previous studies that suggested ALS patients are leaner.
BMI at diagnosis is one predictor of disease progression and survival. A recent analysis of clinical trial data found a U-shaped association with BMI at enrollment; patients with BMI of 30 to <35 kg/m2 had better survival than those with BMI of <30 kg/m2 or with BMI of 35+ kg/m2.(21) These findings confirmed a previous report of poorer survival among malnourished (BMI ≤ 18.5 kg/m2) ALS patients.(22) Energy balance is impaired in ALS.(23, 24) Patients have decreased food intake as a consequence of dysphagia and denervation of muscle leads to loss of lean body mass. At the same time, and paradoxically,(25) resting energy expenditure is higher than expected.(3-5) A possible explanation for the association between BMI and ALS, if causal, is that individuals at risk for ALS are more likely to be hypermetabolic throughout adulthood. One study of hypermetabolism in ALS reported that 50% of patients exhibited hypermetobolism at diagnosis and 80% showed no change in metabolic status over a 2 year follow-up suggesting that hypermetabolism occurs early in the disease course.(4) Further, prior to their diagnosis ALS patients have been reported to have used cholesterol-reducing drugs less frequently and have better lipid profiles,(6) and to have been hospitalized less for cardiovascular disease than controls.(26) Another possibility is that strenuous physical activity explains the association between low BMI and ALS risk. In theory, excessive physical activity results in physiological changes that correspond with proposed pathogenesis of ALS: increased oxidative stress and excitotoxicity of motor neurons.(27, 28) One case-control study suggested that self-reported vigorous activity was associated with a two-fold increase in the risk of ALS,(29) while two case-control studies using validated questionnaires for assessing physical activity levels in leisure and work time throughout life found no association with ALS.(28, 30) One of these studies did report that cases were 50% more likely to have participated in organized sports in high school compared to control subjects.(28) Similarly, it was reported by Scarmeas et al that ALS patients were twice as likely to have been varsity athletes as controls with other neurological conditions.(11)
In summary, in this large longitudinal investigation we found that ALS risk was about 30-40% lower among overweight or obese individuals than in those with healthy weight. This finding suggests that either the hypermetabolic state observed in ALS may start many years before the clinical disease onset, or that some factor associated with obesity may be protective against ALS.
ACKNOWLEDGEMENTS
FUNDING
This work was supported by grant R01 NS045893 from the NIH/National Institute of Neurological Diseases and Stroke. Nurses’ Health Study is funded by NIH program project P01 CA87969 and Health Professional Follow-up Study by NIH program project P01 CA055075. Role of the Sponsors: The funding agencies had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: Authors have not completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest at this time.
References
- 1.Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Premorbid weight, body mass, and varsity athletics in ALS. Neurology. 2002;59(5):773–5. doi: 10.1212/wnl.59.5.773. [DOI] [PubMed] [Google Scholar]
- 2.Kasarskis EJ, Berryman S, Vanderleest JG, Schneider AR, McClain CJ. Nutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of death. Am J Clin Nutr. 1996;63(1):130–7. doi: 10.1093/ajcn/63.1.130. [DOI] [PubMed] [Google Scholar]
- 3.Desport JC, Preux PM, Magy L, Boirie Y, Vallat JM, Beaufrere B, et al. Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. Am J Clin Nutr. 2001;74(3):328–34. doi: 10.1093/ajcn/74.3.328. [DOI] [PubMed] [Google Scholar]
- 4.Bouteloup C, Desport JC, Clavelou P, Guy N, Derumeaux-Burel H, Ferrier A, et al. Hypermetabolism in ALS patients: an early and persistent phenomenon. Journal of neurology. 2009;256(8):1236–42. doi: 10.1007/s00415-009-5100-z. Epub 2009/03/24. [DOI] [PubMed] [Google Scholar]
- 5.Funalot B, Desport JC, Sturtz F, Camu W, Couratier P. High metabolic level in patients with familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10(2):113–7. doi: 10.1080/17482960802295192. Epub 2008/09/17. [DOI] [PubMed] [Google Scholar]
- 6.Sutedja NA, van der Schouw YT, Fischer K, Sizoo EM, Huisman MH, Veldink JH, et al. Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2011;82(6):638–42. doi: 10.1136/jnnp.2010.236752. [DOI] [PubMed] [Google Scholar]
- 7.Nelson LM, McGuire V, Longstreth WT, Jr., Matkin C. Population-based case-control study of amyotrophic lateral sclerosis in western Washington State. I. Cigarette smoking and alcohol consumption. Am J Epidemiol. 2000;151(2):156–63. doi: 10.1093/oxfordjournals.aje.a010183. [DOI] [PubMed] [Google Scholar]
- 8.Nelson LM, Matkin C, Longstreth WT, Jr., McGuire V. Population-based case-control study of amyotrophic lateral sclerosis in western Washington State. II. Diet. Am J Epidemiol. 2000;151(2):164–73. doi: 10.1093/oxfordjournals.aje.a010184. [DOI] [PubMed] [Google Scholar]
- 9.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 10.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–8. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 11.Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490–501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 12.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–57. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 14.Weisskopf MG, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, Ascherio A. Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am J Epidemiol. 2004;160:26–33. doi: 10.1093/aje/kwh179. [DOI] [PubMed] [Google Scholar]
- 15.Magnus T, Beck M, Giess R, Puls I, Naumann M, Toyka KV. Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve. 2002;25(5):709–14. doi: 10.1002/mus.10090. [DOI] [PubMed] [Google Scholar]
- 16.Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology. 2002;59(2):280–2. doi: 10.1212/wnl.59.2.280. [DOI] [PubMed] [Google Scholar]
- 17.del Aguila MA, Longstreth WT, Jr., McGuire V, Koepsell TD, van Belle G. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60(5):813–9. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 18.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5(4):561–5. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 19.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, et al. Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obesity research. 2005;13(8):1466–75. doi: 10.1038/oby.2005.177. Epub 2005/09/01. [DOI] [PubMed] [Google Scholar]
- 21.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44(1):20–4. doi: 10.1002/mus.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desport JC, Preux PM, Truong TC, Vallat JM, Sautereau D, Couratier P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology. 1999;53(5):1059–63. doi: 10.1212/wnl.53.5.1059. [DOI] [PubMed] [Google Scholar]
- 23.Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(30):11159–64. doi: 10.1073/pnas.0402026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasarskis EJ. What does body mass index measure in amyotrophic lateral sclerosis and why should we care? Muscle Nerve. 2011;44(1):4–5. doi: 10.1002/mus.22113. Epub 2011/05/25. [DOI] [PubMed] [Google Scholar]
- 25.Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2010;10(1):75–82. doi: 10.1016/S1474-4422(10)70224-6. [DOI] [PubMed] [Google Scholar]
- 26.Turner MR, Wotton C, Talbot K, Goldacre MJ. Cardiovascular fitness as a risk factor for amyotrophic lateral sclerosis: indirect evidence from record linkage study. J Neurol Neurosurg Psychiatry. 2012;83(4):395–8. doi: 10.1136/jnnp-2011-301161. Epub 2011/11/11. [DOI] [PubMed] [Google Scholar]
- 27.Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Hypotheses to explain the association between vigorous physical activity and amyotrophic lateral sclerosis. Med Hypotheses. 1991;34(2):144–8. doi: 10.1016/0306-9877(91)90183-y. [DOI] [PubMed] [Google Scholar]
- 28.Longstreth WT, McGuire V, Koepsell TD, Wang Y, van Belle G. Risk of amyotrophic lateral sclerosis and history of physical activity: a population-based case-control study. Archives of neurology. 1998;55(2):201–6. doi: 10.1001/archneur.55.2.201. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto K, Kihira T, Kondo T, Kobashi G, Washio M, Sasaki S, et al. Lifestyle factors and risk of amyotrophic lateral sclerosis: a case-control study in Japan. Ann Epidemiol. 2009;19(6):359–64. doi: 10.1016/j.annepidem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Veldink JH, Kalmijn S, Groeneveld GJ, Titulaer MJ, Wokke JH, van den Berg LH. Physical activity and the association with sporadic ALS. Neurology. 2005;64(2):241–5. doi: 10.1212/01.WNL.0000149513.82332.5C. [DOI] [PubMed] [Google Scholar]