Synopsis
Numerous targeted therapies are being developed for patients with CLL. CAR-modified T cells targeting CD19 expressed by normal and malignant B cells is a unique therapy and recent results from four different trials highlight the dramatic potential of this therapy for patients with relapsed CLL. Since adoptive transfer of CAR-modified T cells is a novel approach to cancer therapy there are issues for the medical oncologist to consider when evaluating current and future clinical trials for patients with CLL. Herein, we review the impact of CAR design, T cell production, T cell dose, conditioning regimens, and tumor burden at the time of CAR-modified T cell infusion on the efficacy of this therapy.
Keywords: Chimeric Antigen Receptor, Chronic Lymphocytic Leukemia, CD19, Adoptive cell therapy, Cell engineering
Introduction
Chronic Lymphocytic Leukemia (CLL) is the target for numerous new investigational drugs and immunotherapies. Unique among these is the genetic modification of T cells to B cell antigens through the gene-transfer of a chimeric antigen receptor (CAR), which is composed of an antigen-binding component fused to T cell signaling domains. A patient’s own T cells are genetically modified and then adoptively transferred back to the patient to mediate killing of malignant, and normal, B cells. Over the past 10 years, work initiated at our center1, has transitioned this technology from pre-clinical models to clinical trials with evidence of promising results.2–7 However, there are important details that should be considered when evaluating and comparing the various CAR-modified T cells under study since this therapy is unlike any traditionally used by the medical oncologist. The goal of this article is to describe and evaluate these details that include CAR design, T cell production and dose, prior conditioning chemotherapy regimens, and tumor burden and discuss how they may affect the treatment response in patients with CLL.
Clinical Trial Results
Clinical outcomes of 16 patients with CLL treated with CAR-modified T cells targeted to the B cell specific CD19 antigen have recently been reported from four trials conducted at various academic medical centers.2–7 The NCI reported their results with 4 patients with relapsed CLL treated with fludarabine and cyclophosphamide followed by CD19-targeted CAR-modified T cells. These patients, previously treated with an average of four chemotherapy regimens, had variable anti-CD19 responses including a complete remission (CR) of greater than 15 months in duration. In addition, several patients developed anticipated B cell aplasia as a consequence of their treatment and exhibited systemic serum cytokine elevations consistent with robust CAR-modified T cell activation. Investigators at the University of Pennsylvania (UPenn)3,4 reported the results of 3 CLL patients treated with CD19-targeted CAR-modified T cells, of which 2 patients had relapsed disease and 1 patient was chemotherapy-naïve, that were treated with bendamustine or pentostatin plus cyclophosphamide as conditioning therapy prior to T cell infusion. Two of the patients had ongoing CRs while the third achieved a partial remission (PR). Similar to the clinical outcomes at the NCI, one of these patients experienced a prolonged (> 6 months) B cell aplasia. We recently reported the largest cohort of CLL patients treated with CD19-targeted T cells (Fig 1).2 Outcomes in these patients included objective responses with lymph node reductions and B cell aplasia.2 Furthermore, our trial included a unique secondary endpoint evaluating the requirement for conditioning therapy prior to gene-modified T cell infusion. Lastly, investigators at the Baylor College of Medicine reported the results of 6 patients with B cell malignancies, one of which had CLL.7 While no objective response was detected, the patient did have stable disease (SD) for 10 months after T cell infusion. Of note, this trial did not include prior conditioning chemotherapy.
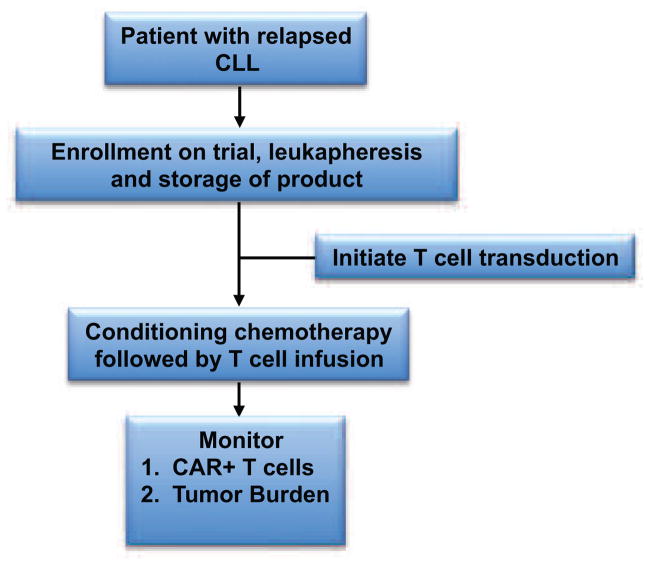
FIGURE 1. MSKCC Treatment Schema using CAR-modified T cells for patients with relapsed CLL.
Patients with relapsed CLL are eligible for enrollment, leukapheresis, and infusion with CAR-modified T cells after treatment with conditioning chemotherapy.
Overall, the toxicities reported among the different trials were quite similar including fevers, rigors, hypotension, and B cell aplasia.2–6 These toxicities began approximately 1 to 21 days after initial T cell infusion. Furthermore, the toxicities appeared to be coincident with peaks in cytokine production. 2–6 Collectively, in two of these patients their symptoms resolved and cytokine levels decreased after initiation of steroid therapy.3
All of the reported trials present clinical evidence to support in vivo CD19 targeted T cell efficacy. However, closer inspection of results and comparison of the trials focusing on elements of CAR design, T cell production, and patient selection allows for a better understanding regarding disparities in results from the individual trials, providing insight for more rational designs of future CARs and therapeutic clinical trials. Furthermore, this discussion will allow the medical oncologist to critically evaluate the multiple clinical trials involving gene-modified T cell therapy available for their patients with CLL (Table 1).
TABLE 1. Active clinical trials for adults with CLL.
Listed are currently accruing clinical trials using autologous CAR-modified T cells targeted to the CD19 antigen for patients with CLL.
| Clinical Trial Identifier | CAR | Gene Transfer | Disease status | T cell Escal. | Conditioning Therapy | Trial Site |
|---|---|---|---|---|---|---|
| NCT00586391 | 19z* vs 1928z | γ-retrovirus | Relapsed | Yes | CY | Dallas, TX |
| NCT00709033 | 19z** vs 1928z | γ-retrovirus | Relapsed | Yes | CY | Dallas, TX |
| NCT00924326 | 1928z | γ-retrovirus | Relapsed | No | CY+FLU IL2*** | Washington, DC |
| NCT00968760**** | 1928z | Electroporation SB-Transposase | Relapsed | Yes | BEAM+R ± IL2 | Houston, TX |
| NCT01653717 | 1928z | Electroporation SB-Transposase | 8 weeks from last chemo | Yes | CY+FLU | Houston, TX |
| NCT01416974 | 1928z | γ-retrovirus | MRD***** | Yes | CY | New York, NY |
| NCT00466531 | 1928z | γ-retrovirus | Relapsed | Yes | CY | New York, NY |
| NCT01029366 | 19BBz | lentivirus | Relapsed | No | Investigators Choice | Philadelphia, PA |
MRD=Minimal Residual Disease. CY=Cyclophosphamide. FLU=Fludarabine. SB=Sleeping Beauty.
Patients are infused with a mixture of T cells modified with either the 19z or 1928z CAR.
Patients are infused with a mixture of T cells modified with either the 19z or 1928z CAR. The 19z CARs are transduced into EBV+ T cells, while the 1928z CARs are transduced into normal peripheral, polyclonal T cells.
IL2 is not given as a lymphodepleting agent but as a T cell growth factor
This is the only trial listed where the T cells are administered as part of an autologous stem cell transplant
This trial is evaluating CAR-modified T cells as a consolidation regimen. Patients treated with an initial chemotherapy regimen that have residual disease after completing this regimen are infused with 1928z+ T cells.
CAR Design
CARs are generally classified as being of first generation, second generation, or third generation design. This classification relates to the signal transduction domains incorporated within the CAR (Fig 2). First generation CARs most commonly consist of a CD3ζ signaling element, which when combined with an anti-CD19 single chain variable fragment (scFv) successfully redirects T cells to mediate killing of B cells in vitro and in vivo in immunodeficient preclinical animal models.1,8 However, these first-generation CARs ultimately have been found to have limited in vivo efficacy with little evidence of T cell persistence in these models.9–11 The reason for this limited efficacy is related to T cell biology: T cells are optimally activated when they encounter antigen for the first time if they receive two signals, one mediated by CD3ζ (signal 1) and the other mediated by a co-stimulatory receptor, most commonly CD28 (signal 2).1 This two-signal paradigm for efficient T cell activation could be recapitulated through second-generation CARs that included co-stimulatory T cell cytoplasmic signal domains proximal to CD3ζ cytoplasmic signal domains (Fig 2).9,10 T cells modified to express second generation CARs demonstrated enhanced in vivo tumor killing and persistence. While CD28 is the most commonly utilized costimulatory signaling domain, others have modified second generation CARs to include the costimulatory signal domains of 41BB, OX40, DAP10, and CD27.10,12,13 Studies have demonstrated that additional signal domains enhance gene-modified T cell function by increasing cytokine secretion and enhancing T cell proliferation and persistence.12–14 Third generation anti-CD19 CARs, which have two co-stimulatory domains combined with CD3ζ, demonstrate impressive results in pre-clinical animal models, but have not been evaluated in CLL patients to date.15,16
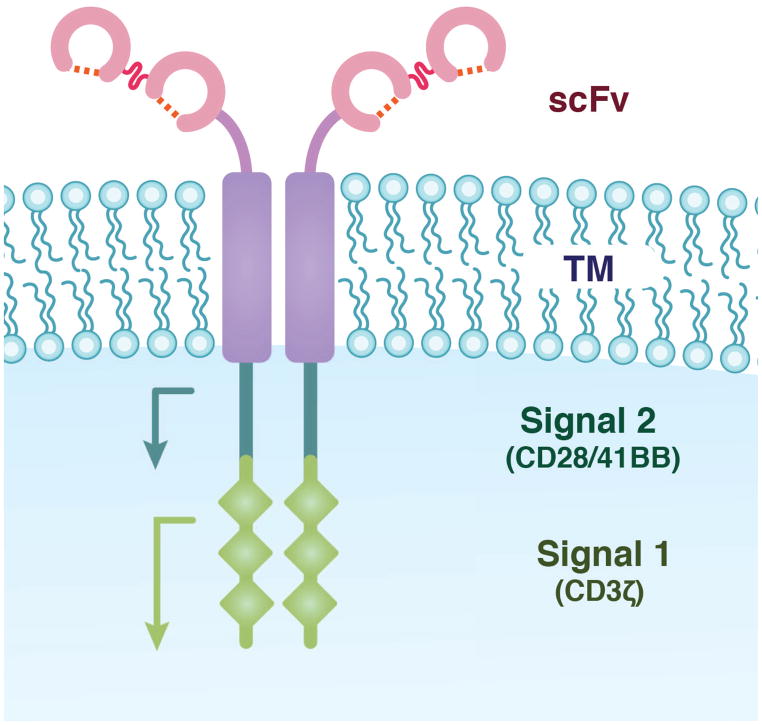
FIGURE 2. The Chimeric Antigen Receptor.
Most CARs are composed of the antigen-binding domains of a scFv, fused to the transmembrane (TM) region of a protein such as CD8, which is fused to signal transduction domains normally associated with a T cell receptor. The scFv binds an antigen and T cell activation is mediated in part by the two signal transduction domains. The three diamonds represent the three immunoreceptor tyrosine-based activation motifs present within CD3ζ.
Comparison of anti-CD19 CARs using different monoclonal antibody (Mab) derived scFv’s have not been performed, although one could speculate that if the binding affinities of the scFv’s were significantly different it could impact CAR-mediated T cell activation and consequent B cell killing. To this end, studies at MSKCCC utilized a different scFv, derived from the SJ25C1 hybridoma, when compared to studies at the NCI and UPenn wherein the anti-CD19 CAR utilized a scFv derived from the FMC63 hybridoma.
The four clinical trials involving CLL patients have all used second generation CARs, but the clinical trial results reported by Savoldo et al7 are unique for directly infusing a mixture of T cells genetically modified with a first generation CD3ζ CAR and a second generation CAR including the CD28 co-stimulatory domain. In a cohort of four patients (1 with CLL), investigators clearly demonstrated that T cells with second generation CARs enhanced persistence and/or expansion when compared to T cells modified with a first generation CAR.
Investigators at UPenn have the only trial for CLL patients using a CAR that has a co-stimulatory domain other than CD28, namely 41BB.3,4 At this time the only direct comparison of anti-CD19 second generation CARs with a CD28 or 41BB co-stimulatory domain (19-28z vs. 19-bbz) is in preclinical models and the results documenting protection against B cell malignancies have been contradictory, possibly due to the fact that the anti-CD19 scFvs were derived from different Mabs.10,12
T cell production
In most trials, CAR-modified T cells are generated ex vivo and include an initial activation step followed by a gene-transfer step (Fig 3). All trials activate T cells with agonistic Mab-mediated CD3 stimulation with or without additional CD28 co-stimulation.2–7 In three of the reported clinical trials gammaretroviral vectors were used for gene-transfer, while studies from UPenn utilized lentiviral vectors. However, given the small number of patients treated to date on these trials it is not yet possible to assess the superiority of one viral transfer system over the other. While in theory lentiviral gene transfer may increase safety given prior reports of leukemogenic integration sites associated with gammaretroviruses, in these cases the cells transduced were hematopoietic stem cells, not mature T cells.17,18 To date, there have been no reports of insertional oncogenesis with gammaretroviral vectors in the context of genetically modified mature lymphocytes. In fact, a recent report identified no long-term sequelae in 43 subjects infused with gammaretroviral transduced T cells in several clinical trials evaluating patients after an 11-year follow-up period.19
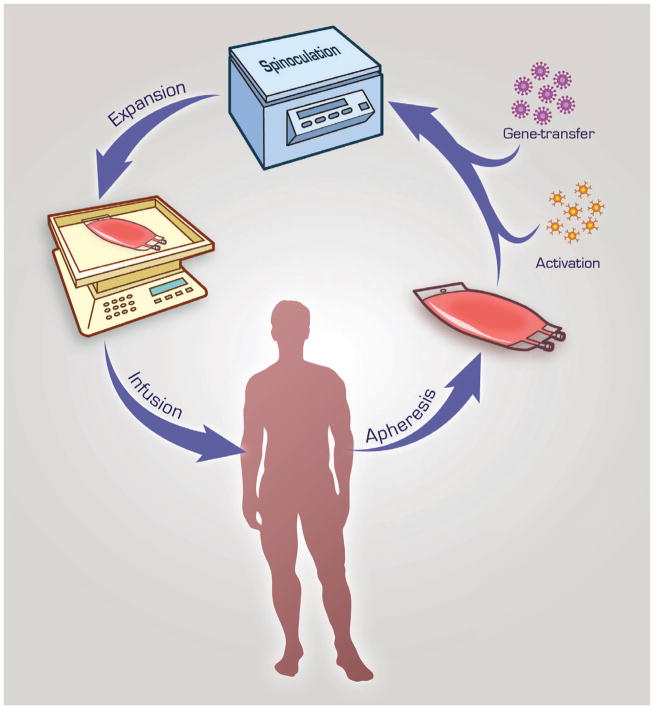
FIGURE 3. T cell isolation and gene-transfer.
Peripheral blood leukocytes are isolated from the patient and T cells are enriched and activated from this leukapheresis product with anti-CD3 and/or anti-CD28 ligation. Gene-transfer can be accomplished by retroviral transduction (depicted here with spinoculation), electroporation, RNA transfection, or via transposase activity. Afterwards, CAR-modified T cells are expanded, in this example with a Wave Bioreactor, and ultimately adoptively transferred back into the patient.
Another important consideration with respect to CAR-modified T cell technologies is how efficiently and rapidly gene-targeted T cells can be produced. This is highlighted by two trials for lymphoma patients that involved the genetic modification of T cells targeted to the CD20 antigen; an anti-CD20 CAR gene was introduced through electroporation and the cells were subsequently expanded after drug-selection.20,21 No objective responses were noted and a valid concern regarding these modest clinical outcomes was related to the long culture period required to produce the T cells potentially resulting in “exhausted” T cells with limited proliferative potential following infusion.22 In contrast, all CLL trials using retroviral vectors generated the requisite T cell dose within 1–3 weeks, and consisted of T cells that appear to have retained proliferative capacity.
An optimal anti-CD19 T cell product would be expected to provide immediate tumor control, by way of direct cytotoxicity, but also have the ability to generate long-term memory cells to mediate subsequent tumor immunosurveillance. Studies at our center and the NCI characterized the immunophenotype of the final T cell product and confirmed the expression of memory markers (CD62L 4 to 78%, CCR7 1–37%), suggesting that these T cells had retained proliferative potential and the capacity to become long-term memory cells.2,5,6
T cell dose, tumor burden, and conditioning treatment as predictors for optimal CAR-modified T cell function
Unlike standard therapeutic drugs, T cells have a completely different dynamic regulating their half-life and efficacy. For example, T cells ideally have the potential to proliferate and persist long after adoptive transfer making the half-life of these T cells incalculable and their effects indefinite. Scholler et al19 estimated the half-life of retrovirally gene-modified T cells infused into patients to be at least > 16 years. When CAR-modified T cell trials were being developed as a therapy for CLL and other indolent B cell malignancies, pre-clinical and clinical studies identified major determinants for T cell function to be T cell dose, tumor (or antigen) burden, and/or prior conditioning with chemotherapy or radiation therapy.10,23,24 Reflecting upon currently published clinical results we can now comment on how each of these variables appear to affect CAR-modified T cell function in patients with CLL.
T cell dose is an intriguing variable because it is possible that T cells will expand after transfer into optimally conditioned patients by homeostatic proliferation or in response to a pro-proliferative cytokine profile.22 Therefore, as the T cell dose increases, T cell expansion may plateau or decrease if limited by available cytokines or space in lymphoid tissues for expansion.25 To this end, results from these trials do not identify any correlation between T cell dose and clinical outcome.2–7 Specifically, an optimal anti-tumor response achieved at UPenn was in a patient treated with a T cell dose 40–80x lower than that infused into the other 2 patients reported in this cohort.3,4 Similarly, in our studies at MSKCC, we noted better objective responses in a lower dose cohort when compared to patients treated at a 3-fold higher CAR-modified T cell dose.2 Therefore, based on currently published reports there does not appear to be a correlation between T cell dose and clinical outcome within a large-range of clinically meaningful treatment doses (2 × 105 to 3.1 × 107 CAR+ T cells/kg).
We speculate that the T cell dose required for a positive clinical outcome may be affected by tumor burden. In fact, we have previously reported an inverse correlation between tumor burden and persistence of CAR-modified T cells in CLL patients treated on our protocol.2 A similar inverse rank-order is noted among the three CLL patients treated at UPenn. The best response in these studies, a CR with long-term B cell aplasia, was observed in the patient with the lowest estimated tumor burden, while a more modest PR response was noted in the patient with the greatest estimated tumor burden.3,4 These comparisons were assisted by the measurement of CLL tumor burden, calculated as the sum of the nodal tumor mass, blood tumor mass, and bone marrow tumor mass.3 A similar calculation for CLL tumor burden performed retrospectively on our treated patients was consistent with an inverse correlation between tumor burden and clinical outcome (data not shown). How the function of CAR-modified T cells is regulated by antigen and/or tumor burden is unknown but it is reasonable to speculate that infused T cells may be rendered non-functional through tolerance or exhaustion in the context of excessive tumor bulk and/or CD19 antigen expressed on normal B cells.
If tumor burden is an important regulator of clinical outcome then it follows that conditioning with chemotherapy or radiation therapy may enhance CAR-modified T cell function in part though debulking tumor mass prior to CAR-modified T cell infusion. Pre-clinical studies of CAR-modified T cells targeting B cell malignancies in immunocompetent mice suggest that optimal anti-CD19 T cell cytotoxic function and subsequent persistence is enhanced by lymphodepleting conditioning therapy prior to adoptive T cell transfer.24,26–28 Conditioning regimens used in these studies are variable and include γ-irradiation, cyclophosphamide chemotherapy, as well as anti-CD20 monoclonal antibody immunotherapy. While diverse, all these conditioning regimens have the ability to readily lymphodeplete mice. One recent pre-clinical study found that in the presence of overwhelming antigen, CAR+ T cells were sequestered in the lung and subsequently eliminated prior to encounter with tumor.24 This mechanism is likely most relevant when CAR-modified T cells target an abundant self-antigen such as CD19, which is expressed on normal B cells, but does not exclude other mechanisms attributed to conditioning regimens enhancing the function of adoptively transferred T cells such as homeostatic proliferation, cytokine sinks, and regulatory T cell depletion.22
Given the pre-clinical findings with respect to conditioning regimens, all reported clinical trials utilizing CD19-targeted T cells into CLL patients with the exception of the Savoldo et al7 trial have been designed to include conditioning chemotherapy prior to CAR-modified T cell infusion. The MSKCC clinical trial is to date the only one to compare cohorts of patients treated with and without prior conditioning chemotherapy.2 Our results of 8 CLL patients included 3 patients treated with CAR-modified T cells alone and 5 patients treated with cyclophosphamide and then CAR-modified T cells. Inclusion of cyclophosphamide conditioning chemotherapy before T cell infusion was associated with increased T cell persistence and improved clinical outcomes despite the fact that this cohort was infused with a lower dose of CAR-modified T cells than the non-conditioned cohort. It is important to note that patients treated with cyclophosphamide conditioning in the MSKCC studies had previously been treated with this agent as part of prior multi-chemotherapeutic regimens for their CLL. Therefore, the relapsed CLL tumor cells were likely resistant to cyclophosphamide. This assumption is further supported by an absence of tumor lysis, no decrease in absolute lymphocyte count, and no decrease in lymphadenopathy after infusion of the cyclophosphamide prior to infusion of the CAR-modified T cells. Therefore, the potential benefit of this conditioning therapy would be related to lymphodepletion of non-malignant chemo-sensitive normal B cells to reduce the CD19 antigen burden. In contrast, two of the patients reported by UPenn were treated with chemotherapeutic regimens not previously utilized in these patients and the other was treated with a regimen that they were currently responding to; all the regimens used are known to be highly active in CLL.3,4 Long-term effects, such as B cell aplasia 10 months after treatment are likely to be related to CAR-modified T cells, however, in these patients it is difficult to differentiate the observed tumor reduction mediated by highly active chemotherapy regimens from those mediated by the subsequently infused CAR-modified T cells. For example, one of the patients treated at UPenn was induced into a CR after treatment with bendamustine and CAR-modified T cells, but this patient had recovery of normal B cells and IgG serum levels.3,4 So it would be difficult to determine the role bendamustine played in inducing a CR in this particular patient. The NCI conditioned its 4 CLL patients with a combination of fludarabine and high-dose cyclophosphamide before infusion with CAR-modified T cells.5,6 Both drugs are highly active against CLL so the relative contributions of the chemotherapy and T cells in the overall tumor reduction remain difficult to assess.
The results from the clinical trials complement those from pre-clinical animal models demonstrating that prior conditioning therapy is critical to the subsequent anti-CD19 efficacy of CAR-modified T cells. Nevertheless, the trials reported to date have created new questions, which need to be addressed in future clinical studies. What is the optimal conditioning regimen? Should the goal of the conditioning treatment be merely lymphodepletion or should it also mediate substantial anti-tumor activity? Which of these variables enhance the efficacy of one conditioning regimen versus another?
Based on the available published clinical data of anti-CD19 CAR T cell therapy, the most active anti-tumor conditioning chemotherapy regimens are those which mediate tumor lysis since these regimens are associated with the best clinical outcomes. Therefore, we believe that most trials should include a tumor-responsive conditioning chemotherapy regimen, which in turn reduces tumor bulk, enhances tumor antigen presentation to foster endogenous antitumor immune responses, and enhances the persistence and function of adoptively transferred CAR-modified T cells. While the use of highly active chemotherapy regimens may blur the role of the CAR-modified T cells in the anti-tumor response, the latter may be assessed, in part, by predicted CART cell mediated long-term B cell aplasia, persistence of CAR-modified T cells, and loss of detectable clonal CLL tumor cell IgH rearrangement.
Future Directions
Promising clinical trial results have established the potential of anti-CD19 CAR-modified T cell therapy for patients with CLL and spurred the clinical investigation of this technology at multiple academic medical centers with currently 8 clinical trials using this technology enrolling patients with CLL (Table 1). The optimal co-stimulatory domain for targeting CLL may ultimately be addressed by a planned clinical trial at UPenn, Children’s Hospital of Pennsylvania, and MSKCC funded by a NIH Special Translational Research Acceleration Project (STRAP) award (Fig 4). In these studies, patients will be evaluated after infusion with two populations of T cells: one modified with a 19-28z CAR, derived from MSKCC, and the other modified with the UPenn 19-BBz CAR. Detection of both CAR-modified T cell populations by quantitative PCR may assess whether either T cell population expands better and/or persists longer in vivo. Additionally, lentiviral and gammaretroviral production systems will be compared head to head in these studies, with respect to gene transfer efficacy and CAR-modified T cell persistence.
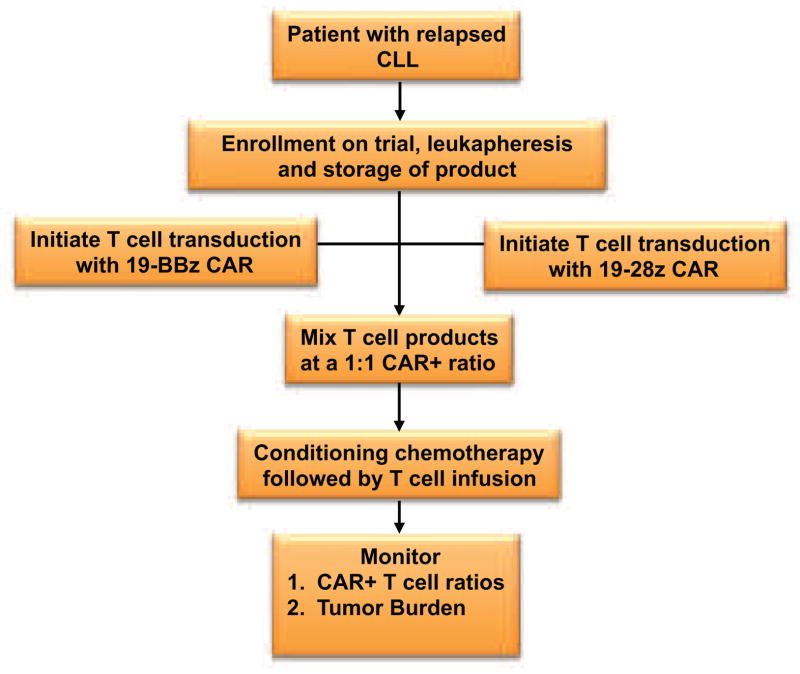
FIGURE 4. MSKCC and UPenn Treatment Schema comparing 19-28z and 19-BBz CAR-modified T cells in patients with relapsed CLL.
Patients with relapsed CLL at MSKCC or UPenn are eligible for enrollment, leukapheresis, and infusion with CAR-modified T cells after treatment with conditioning chemotherapy. The patients are infused with a mixture of CAR-modified T cells composed of an equal ratio of 19-28z+ T cells to 19-BBz+ T cells. Transduction of the 19-28z CAR occurs by gammaretroviral transduction, while transduction of the 19-BBz CAR occurs by lentiviral transduction. Production of both T cell groups occurs at the GMP facility located within the medical center treating the patient. Enhanced T cell persistence and/or proliferation will be determined by measuring the ratio of both CAR-modified T cell groups in treated patients.
Six of the currently open trials are performing T cell dose escalations to determine the maximum tolerated T cell dose. While clinical evidence suggests that T cell dose may not be a critical variable for optimal T cell function, these trials will allow the comparison of toxicities and benefits among multiple cohorts of patients treated under similar conditions. The results may finally suggest an acceptable dose of CAR-modified T cells, balancing toxicities and clinical outcomes.
Our review highlights the importance of tumor burden and effective tumor debulking conditioning regimens. At this time ongoing trials do not optimally evaluate tumor burden before and after treatment. However, use of the tumor burden calculation for CLL described in Kalos et al3 will allow for the reporting of tumor burden at the time of treatment and retrospective analyses of anti-tumor responses. Universal utilization of the CLL tumor burden calculation may allow prospectively a more rigorous evaluation of the suggested inverse correlation between tumor burden and CAR-modified T cell anti-tumor efficacy.
The clear pre-clinical and clinical evidence arguing for effective conditioning chemotherapy prior to CAR-modified T cell infusion is reflected by the fact that all currently open clinical trials include some form of conditioning therapy prior to T cell infusion (Table 1). Variability of these conditioning regimens is quite broad spanning single agent cyclophosphamide to a 5-drug regimen used in the context of an autologous stem cell transplant. While current comparison of clinical trial outcomes are unlikely to identify an optimal conditioning regimen it may help to further validate the role of prior conditioning to enhance or optimize subsequently transferred CAR-modified T cells. Ultimately, future clinical trials designed to compare conditioning regimens may need to be conducted to prospectively identify an ideal regimen.
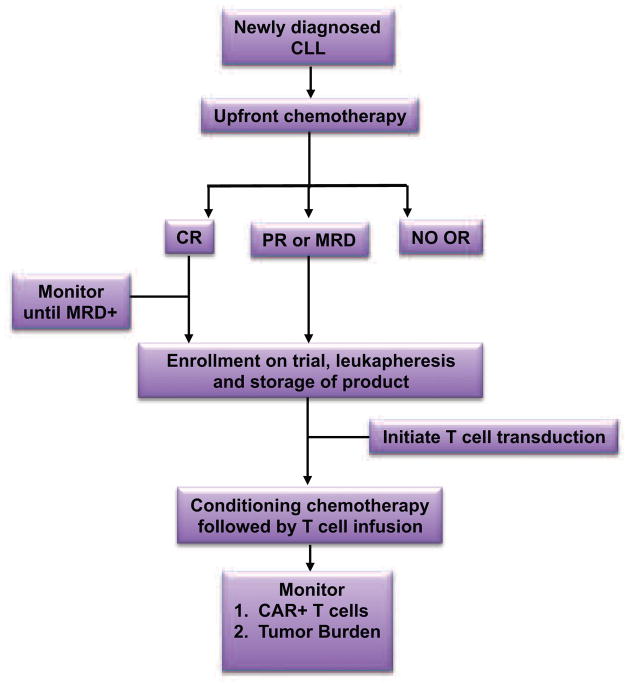
Initial first-in-man clinical trials using CAR-modified T cells treated only CLL patients with relapsed and/or chemo-refractory disease. Despite this poor prognosis patient population, there were clear instances of impressive clinical outcomes. Given the previously inferred inverse correlation between tumor burden and anti-CD19 CAR-modified T cell anti-tumor efficacy, we have recently opened a trial at MSKCC that utilizes anti-CD19 CAR-modified T cells as a consolidation regimen for CLL following completion of initial upfront chemotherapy. This trial is exclusively for CLL patients with detectable or minimal residual disease (PR or MRD) after completing standard frontline chemotherapy (Fig 5). The goal of this trial is to generate complete molecular remissions in patients with PR or MRD following upfront chemotherapy. The results from this trial could support the application of CAR-modified T cells at an earlier stage of disease progression if periods of remissions are increased. Results from this upfront trial could have a major impact on the treatment of patients with CLL by increasing the number of patients with complete molecular remissions, long-term disease control, and/or delaying the start of subsequent salvage therapies.
FIGURE 5. MSKCC Treatment Schema using CAR-modified T cells for patients with residual CLL.
This trial is open at MSKCC for patients with newly diagnosed CLL. Patients receive a complete course of standard combination chemotherapy after developing an indication for treatment. Afterwards, patients are stratified based on response to treatment. Patients with no response or stable disease (NO OR) are not eligible for the trial. Patients with a PR or MRD are eligible for enrollment, CAR-modified T cell production, and conditioning followed by infusion with T cells. Patients with a CR are monitored for relapse by flow cytometry or by a quantitative PCR for the IgH rearrangement associated with the CLL tumor cells. Detection of MRD makes the patient eligible for enrollment and treatment with CAR-modified T cells as above.
In conclusion, the early reports from these trials in patients with relapsed and/or refractory CLL clearly demonstrate the potential of CAR-modified T cell therapy. Significant work lies ahead and a cooperative effort between academic medical centers will be required to determine the optimal CAR design, prior conditioning regimen, and gene-transfer methodology in order to rationally design second generation clinical trials to treat CLL patients with optimized CAR-modified T cells. With a sustained collaborative effort by academic medical centers to this end, the medical oncologist may soon have an established novel and potentially curative approach for the treatment of CLL.
Key Points.
Numerous targeted therapies are being developed for patients with CLL.
CAR-modified T cells targeting CD19 expressed by normal and malignant B cells is a unique therapy and recent results from four different trials highlight the dramatic potential of this therapy for patients with relapsed CLL.
Since adoptive transfer of CAR-modified T cells is a novel approach to cancer therapy there are issues for the medical oncologist to consider when evaluating current and future clinical trials for patients with CLL.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marco L Davila, Email: davilam@mskcc.org, Leukemia Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY, 212-639-4056.
Renier Brentjens, Email: brentjer@mskcc.org, Leukemia Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY, 212-639-7053.
References
- 1.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 2.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2011 doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper LJ, Topp MS, Serrano LM, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 9.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 10.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 11.Brocker T, Karjalainen K. Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J Exp Med. 1995;181:1653. doi: 10.1084/jem.181.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song DG, Ye Q, Poussin M, et al. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119:696. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 14.Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 15.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong XS, Matsushita M, Plotkin J, et al. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18:413. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer A, Abina SH, Thrasher A, et al. LMO2 and gene therapy for severe combined immunodeficiency. The New England journal of medicine. 2004;350:2526. doi: 10.1056/NEJM200406103502422. [DOI] [PubMed] [Google Scholar]
- 18.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 19.Scholler J, Brady TL, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James SE, Orgun NN, Tedder TF, et al. Antibody-mediated B-cell depletion before adoptive immunotherapy with T cells expressing CD20-specific chimeric T-cell receptors facilitates eradication of leukemia in immunocompetent mice. Blood. 2009;114:5454. doi: 10.1182/blood-2009-08-232967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho WY, Blattman JN, Dossett ML, et al. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 26.Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012 doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochenderfer JN, Yu Z, Frasheri D, et al. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheadle EJ, Hawkins RE, Batha H, et al. Natural expression of the CD19 antigen impacts the long-term engraftment but not antitumor activity of CD19-specific engineered T cells. J Immunol. 2010;184:1885. doi: 10.4049/jimmunol.0901440. [DOI] [PubMed] [Google Scholar]