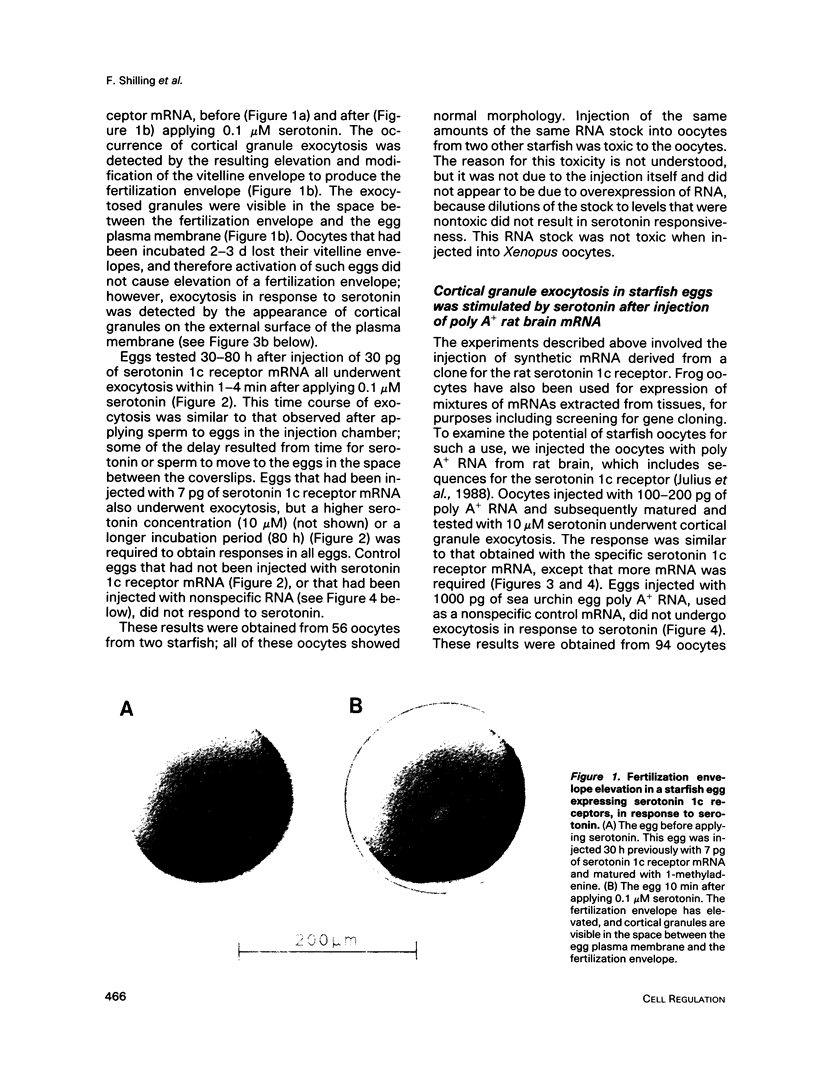
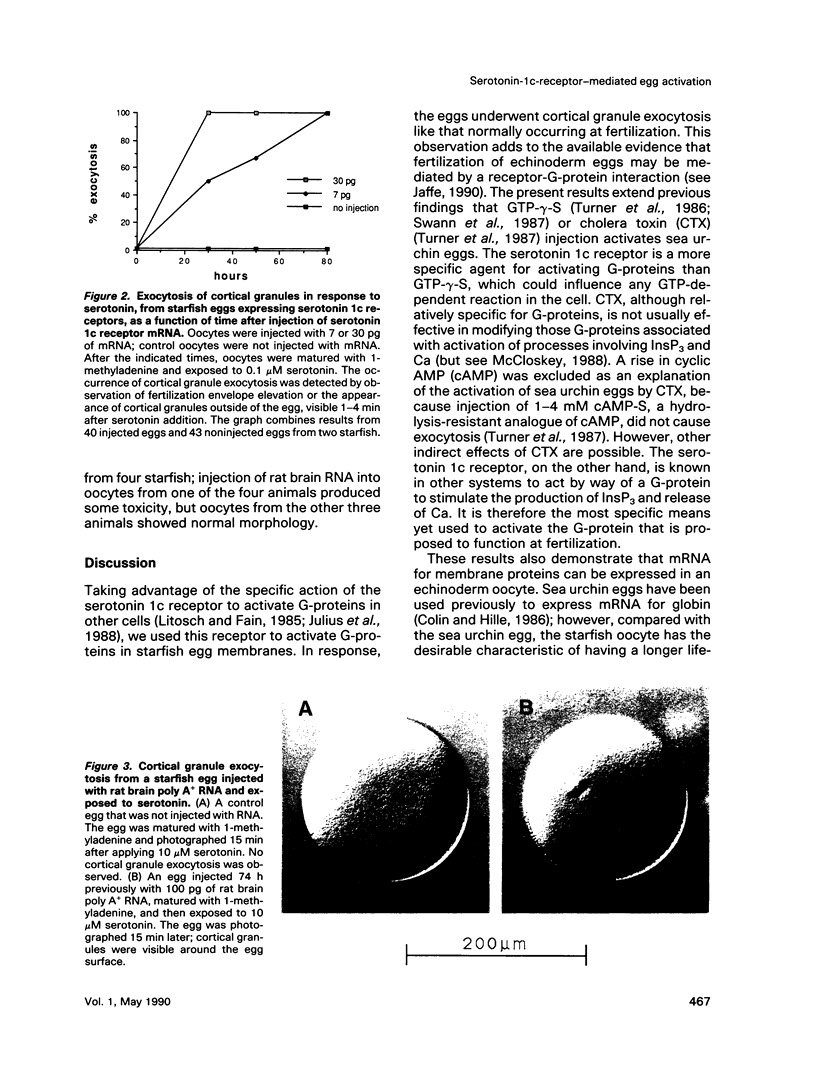
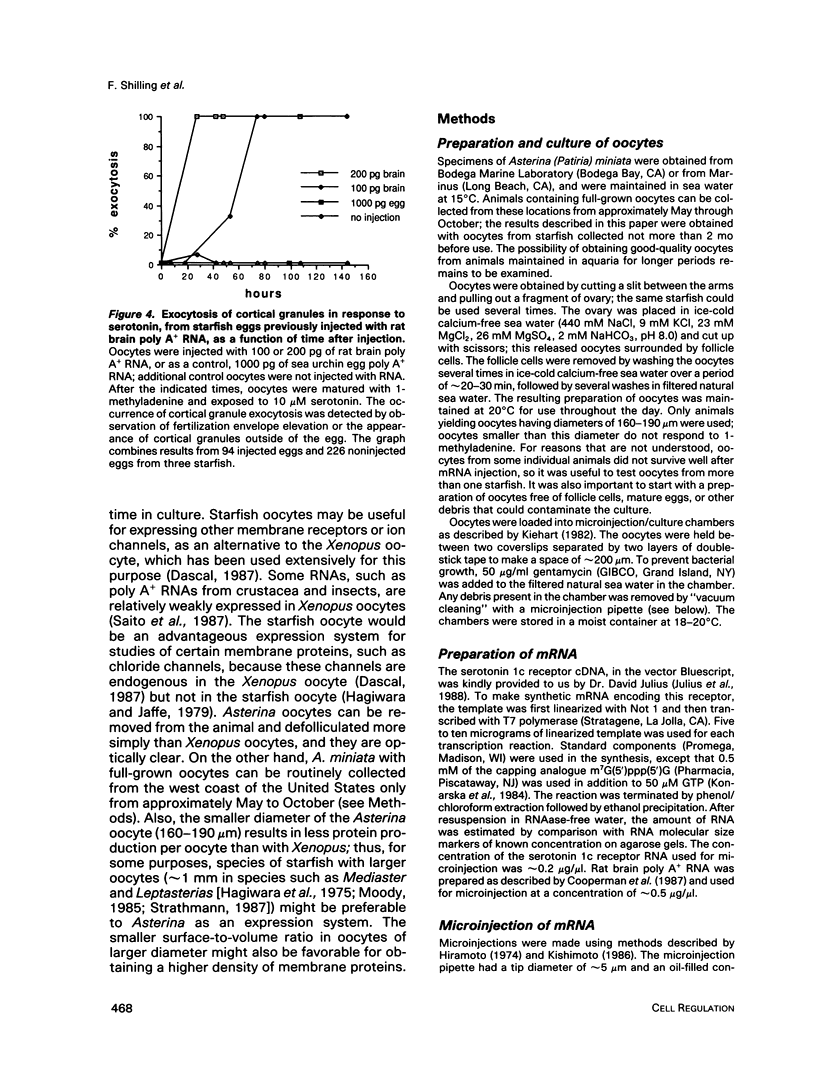
Abstract
Starfish oocytes were injected with mRNA for the serotonin 1c receptor or with rat brain poly A+ mRNA, incubated to allow expression of the membrane protein, then matured to eggs by addition of 1-methyladenine. Applying serotonin to these eggs caused cortical granule exocytosis like that occurring at fertilization. Because the serotonin 1c receptor specifically activates a G-protein, these results provide support for the hypothesis that sperm activate eggs by way of a receptor-G-protein interaction. The starfish oocyte may be a generally useful system for expression of exogenous mRNA for membrane proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Colin A. M., Hille M. B. Injected mRNA does not increase protein synthesis in unfertilized, fertilized, or ammonia-activated sea urchin eggs. Dev Biol. 1986 May;115(1):184–192. doi: 10.1016/0012-1606(86)90239-3. [DOI] [PubMed] [Google Scholar]
- Cooperman S. S., Grubman S. A., Barchi R. L., Goodman R. H., Mandel G. Modulation of sodium-channel mRNA levels in rat skeletal muscle. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8721–8725. doi: 10.1073/pnas.84.23.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto Y. A method of microinjection. Exp Cell Res. 1974 Aug;87(2):403–406. doi: 10.1016/0014-4827(74)90503-5. [DOI] [PubMed] [Google Scholar]
- Jones S. V., Barker J. L., Bonner T. I., Buckley N. J., Brann M. R. Electrophysiological characterization of cloned m1 muscarinic receptors expressed in A9 L cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4056–4060. doi: 10.1073/pnas.85.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Axel R., Jessell T. M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988 Jul 29;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Kiehart D. P. Microinjection of echinoderm eggs: apparatus and procedures. Methods Cell Biol. 1982;25(Pt B):13–31. doi: 10.1016/s0091-679x(08)61418-1. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Microinjection and cytoplasmic transfer in starfish oocytes. Methods Cell Biol. 1986;27:379–394. doi: 10.1016/s0091-679x(08)60359-3. [DOI] [PubMed] [Google Scholar]
- Kline D., Simoncini L., Mandel G., Maue R. A., Kado R. T., Jaffe L. A. Fertilization events induced by neurotransmitters after injection of mRNA in Xenopus eggs. Science. 1988 Jul 22;241(4864):464–467. doi: 10.1126/science.3134693. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984 Oct;38(3):731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Litosch I., Fain J. N. 5-Methyltryptamine stimulates phospholipase C-mediated breakdown of exogenous phosphoinositides by blowfly salivary gland membranes. J Biol Chem. 1985 Dec 25;260(30):16052–16055. [PubMed] [Google Scholar]
- McCloskey M. A. Cholera toxin potentiates IgE-coupled inositol phospholipid hydrolysis and mediator secretion by RBL-2H3 cells. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7260–7264. doi: 10.1073/pnas.85.19.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W. J. The development of calcium and potassium currents during oogenesis in the starfish, Leptasterias hexactis. Dev Biol. 1985 Dec;112(2):405–413. doi: 10.1016/0012-1606(85)90413-0. [DOI] [PubMed] [Google Scholar]
- Saito M., Ohsako S., Deguchi T., Kawai N. Glutamate receptors expressed in Xenopus oocyte by messenger RNA from invertebrate muscle. Brain Res. 1987 Dec;427(1):83–87. doi: 10.1016/0169-328x(87)90048-9. [DOI] [PubMed] [Google Scholar]
- Shapiro R. A., Scherer N. M., Habecker B. A., Subers E. M., Nathanson N. M. Isolation, sequence, and functional expression of the mouse M1 muscarinic acetylcholine receptor gene. J Biol Chem. 1988 Dec 5;263(34):18397–18403. [PubMed] [Google Scholar]
- Turner P. R., Jaffe L. A., Fein A. Regulation of cortical vesicle exocytosis in sea urchin eggs by inositol 1,4,5-trisphosphate and GTP-binding protein. J Cell Biol. 1986 Jan;102(1):70–76. doi: 10.1083/jcb.102.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R., Jaffe L. A., Primakoff P. A cholera toxin-sensitive G-protein stimulates exocytosis in sea urchin eggs. Dev Biol. 1987 Apr;120(2):577–583. doi: 10.1016/0012-1606(87)90260-0. [DOI] [PubMed] [Google Scholar]