Abstract
Although women have a lower risk of stroke during middle age, the menopausal transition is a time when many women develop cardiovascular risk factors. In addition, during the 10 years after menopause, the risk of stroke roughly doubles in women. Endogenous estrogen levels decline by 60% during the menopausal transition, leading to a relative androgen excess, which could contribute to the increased cardiovascular risk factors in women. Earlier onset of menopause may influence the risk of stroke, but the data are not clear. Because of the stroke risk associated with hormone therapy, this is only indicated for treatment of vasomotor symptoms, but some formulations may be safe than others. More research is needed to understand which women are at greatest stroke risk during midlife and to determine the safest formulation, dose, and duration of hormone therapy that will treat vasomotor symptoms without increasing the risk for stroke.
Introduction
Stroke is the 4th leading cause of death and a major cause of disability.1 The risk of stroke increases with age. Although overall age-adjusted stroke risk is higher in men than women, more strokes occur in women because of their longer life expectancy combined with very high stroke rates in the oldest age groups. Women, therefore, account for 60% of all stroke events.2 Studies have consistently shown that women have less favorable functional outcomes after stroke than men including greater disability at the time of hospital discharge and more physical impairments and limitations in activities during the post-stroke recovery period.3–5,6, 7 Discussing issues related to stroke in women is important because of the anticipated epidemic of stroke in women as the population ages.2 Recognition of stroke risk factors and treatment for the modifiable risks is essential and should not be delayed until older age. In fact, acceleration of mid-life risk factors for women seems to be associated with the menopausal transition,8, 9 so this is the ideal time frame for focusing on prevention in women. The reason for the higher risk profile after menopause is not entirely understood but is likely to be influenced by endogenous sex steroid hormones, especially estradiol. Our hypothesis is that stroke risk in women is related to menopause, in part due to hormonal changes and the acceleration of risk factors. To investigate this hypothesis, we have combined the observational data related to stroke in mid-life and the influence of menopause and hormone therapy (the “what”), with physiological evidence related to hormones and atherosclerosis (the “why”), which could help practitioners understand the reasons to focus on early and appropriate treatment to prevent stroke in women. We used this approach to highlight the evidence to guide clinical practice, but also to focus on gaps in knowledge and where future research is needed.
Stroke Incidence and Prevalence in Women During the Midlife
Incidence
Ischemic stroke is relatively uncommon among premenopausal women, but risk increases with advancing age. Several population-based epidemiologic studies have reported estimates of stroke incidence in midlife women including the age range when the majority of women experience menopause (Table 1).10–15 Among white women ages 45 to 54 estimates of stroke incidence, including ischemic, ICH and SAH, range from 0.58–1.02 per 1,000 per year. As an example, Petrea et al using 56 years of follow-up data from the Framingham Heart Study (FHS) reported a stroke incidence 0.82 per 1,000 person years among white women 45 to 54 years of age.14 Stroke rates roughly double among women 55 to 64 years of age. 10–15
Table 1.
Stroke Rates Among Men and Women Aged 45–54 Years
| Year | Study | Study Design | Time Frame |
Incident Events Only |
Stroke Endpoint | Stroke Rate | |
|---|---|---|---|---|---|---|---|
| Women | Men | ||||||
| 2009 | Framingham Heart Study14 |
Cohort | 1948– 2005 |
Yes | IS, ICH, SAH | 0.82 per 1,000 person yrs |
1.16 per 1,000 person yrs |
| 2007 | Riks-Stroke, Sweden13 | Population- based Surveillance |
2000– 2002 |
Yes | IS, ICH, unspecified stroke |
1.02 per 1,000/yr | 1.48 per 1,000 pop/yr |
| 2008 | Brain Attack Surveillance in Corpus Christi Project*12 |
Population- based Surveillance |
2000– 2006 |
No | IS, ICH | 0.97 per 1,000/yr (whites) 1.94 per 1,000/yr (Mexican Americans) |
NR |
| 2005 | Oxford Vascular Study17 |
Population- based Surveillance |
2002– 2005 |
No | IS, ICH, SAH, TIA |
1.25 per 1,000/yr | 1.37 per 1,000 /yr |
| 2004 | Greater Cincinatti/Northern Kentucky Stroke Study11 |
Population- based Surveillance |
1993– 1994 |
Yes | IS, ICH, SAH, unsepcified stroke, TIA |
0.59 per 1,000/yr (whites) 2.47 per 1,000/yr (blacks) |
0.95 per 1,000/yr (whites) 2.26 per 1,000/yr (blacks) |
| 1998 | Northern Manhatten Stroke Study15 |
Population- based Surveillance |
1993– 1996 |
Yes | IS, ICH, SAH | 0.76 per 1,000/yr (whites, blacks, Hispanics) |
1.75 per 1,000 pop/yr |
| 1996 | Rochester, MN10 | Population- based Surveillance |
1985– 1989 |
Yes | IS, ICH, SAH, unspecified stroke |
0.64 per 1,000/yr | 0.61 per 1,000 pop/yr |
Risk is among those 45–59 years
IS = Ischemic Stroke; ICH = intracerebral hemorrhage; SAH = subarachnoid hemorrhage; TIA = transient ischemic attack; pop=population
Age-specific stroke incidence data for minority women are limited. Among Mexican American women ages 45 to 59, stroke incidence has been estimated to be 1.94 per 1,000 per year.12 Among African American women ages 45 to 54, stroke incidence has been estimated to be 2.47 per 1,000 per year.11 These data point to higher stroke incidence for minority women compared with white women during the midlife.
Over the life course, age-adjusted stroke risk is higher in men compared with women.14 During the midlife specifically, most but not all studies suggest that men have greater stroke risk compared with women. For example, stroke incidence rates among those 45 to 54 from the FHS were 1.16 and 0.82 per 1,000 person years in men and women respectively.14 Other population-based US studies, as well as a population-based study from Sweden, also report greater stroke rates in men ages 45 to 54 compared with women of the same age (see Table 1).11, 13, 15
Interestingly, following the midlife, sex differences in stroke risk may decrease or reverse with advancing age;2 however, findings across studies have not been consistent with regard to patterns of sex differences in stroke risk with age.2, 13, 14, 16–18 Data from FHS demonstrate that compared with white men, white women 45 to 84 years have lower stroke risk than men, but this association is reversed in older ages such that women greater than 85 years have elevated risk compared with men.14 Similarly, a population-based study in Sweden found stroke incidence to be lower for women than men at ages 55 to 64 years but at 75 to 85 years of age this association reversed and women had higher incidence than men.13 Other studies, however, report excess stroke risk in men compared with women that persists after midlife or diminishes but does not reverse with age.16–18
Prevalence
Data on stroke prevalence in midlife women, as well as sex differences in stroke prevalence, is limited. Towfighi et al reported a prevalence of 2.5% among women ages 45 to 54 years of age data from the National Health and Nutrition Examination Survey (NHANES) for the time period 1999 to 2004. In this study, midlife women ages 45 to 54 years had a higher odds of having experienced a stroke compared with men of the same age (OR = 2.39, 95% CI 1.32 to 4.33), while no sex differences were noted in the younger (35–44) and older (55–64) age groups.19 Higher stroke prevalence in women compared with men 45 to 54 years of age was also shown in a more recent wave (2005–2006) of NHANES data.20 In contrast, data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study suggests that fewer women than men in the 45 to 54 year age range have had a stroke and that this sex difference decreases with age and is reversed among those greater than 85.2
If stroke prevalence is greater in midlife women compared with men we would expect to see supporting epidemiologic data with regard to sex differences in incidence, mortality or both. For example, if stroke incidence in women in this age range is greater than that for men this could result in increased prevalence; however, existing data do not support this hypothesis, with most studies reporting greater incidence in men in the midlife as described above. Alternatively, a post-stroke survival benefit in midlife women compared with men could result in an increased prevalence in women in this age range. Considering US national vital statistics data, women 45 to 74 years of age have a lower risk of stroke mortality (i.e., stroke deaths divided by person-time), on the magnitude of 25 to 35%, compared with men of the same age but this is driven in part by the lower incidence in women in this age range.2, 21 Case-fatality and post-stroke mortality rates in midlife women compared with men would more directly answer this question. In a recent analysis of data from the Nationwide Inpatient Sample, Towfighi et al reported that for the most recent time period (2005–2006), women aged 45 to 54 years were less likely to die in-hospital from stroke compared with their male counterparts even after adjustment for demographics, clinical factors, treatment with tPA, and hospital factors (OR = 0.94; 95% CI: 0.90–0.98).22 Sex differences in the 45 to 54 years age range were also reported for the 2001–2002 time period; however, absolute differences in in-hospital mortality were quite small (for example, 4.88% in women and 5.15% in men in 2005–2006).22 Recent findings from The Registry of the Canadian Stroke Network showed no sex differences in stroke case fatality among those 45 to 64 years.23
Menopause: Age at Onset and Risk Factors for Early Menopause
Age at Onset
The menopause is defined as the absence of menstrual periods for 12 consecutive months. The average age of menopause is 51 years, with a range from 40 to 60 years.24 The menopausal transition is associated with significant hormonal changes, most importantly, a decline of estradiol levels by about 60%.25 Post-menopause, the levels continue to decline and then plateau after 1 to 3 years. Altogether, estradiol levels decrease from 7 to 10-fold between pre- and post-menopause.26 During this same time period, circulating testosterone levels decrease more gradually as compared to the rapid decline in estradiol, such that the menopausal transition is associated with a relative androgen increase.27 Accurate measurement of estradiol and testosterone is dependent on the concurrent measurement of sex hormone-binding globulin (SHBG), which binds both estradiol and testosterone. This is because less than 2% of the biologically active estradiol and testosterone is represented by free levels in the circulation. SHBG levels also decline over the menopausal transition,25 and low levels are used to assess androgen excess by calculating the free androgen index (FAI), which equals total testosterone divided by SHBG in molar concentrations. This same method can be used to calculate the free estradiol index or FEI.28 SHBG levels increase in the setting of exogenous estrogens and thyroid replacement.29
Early decline in estradiol levels from early age at menopause could be detrimental to bone and blood vessel health. Cigarette smoking, malnutrition, and lower socioeconomic status have been associated with earlier menopause, but heredity appears to be the most important determinant of age at menopause. In fact, a study of sister pairs showed that about 85% of the phenotype variation of age at menopause was genetically determined.30
Primary ovarian insufficiency (the term preferred over premature ovarian failure) is defined as amenorrhea for at least 4 months, sex steroid deficiency, and two measurements of follicle-stimulating hormone (FSH) concentrations of > 40 IU/L at least 1 month apart in a woman aged less than 40 years.31 Because of the normal distribution of age at menopause, 40 years or less is used because it represents greater than 2 standard deviations below the mean age of menopause.32 The most recognized cause of primary ovarian insufficiency is Turner’s syndrome (45,X). The loss of all or part of the X chromosome leads to oocyte dysfunction because these cells require two active X chromosomes to allow differentiation. Therefore, oocytes are depleted by 10 years of age.33 Other known causes include cytotoxic drugs (cyclophosphamide, ifosfamide, and chlormethine) and auto-immune disorders (autoimmune lymphocytic oophoritis, polyglandular autoimmune syndrome).32 There is an ongoing effort to better understand this disorder because of the implications on bone health, as well as risk for cardiovascular disease and stroke.
Menopause and Stroke Risk Factors
Menopause is associated with an increase in multiple stroke risk factors. Cohort studies of healthy women moving through the menopausal transition have shown an increase in abdominal obesity, an increase in triglycerides, total cholesterol and LDL cholesterol, a decrease in HDL cholesterol, increased fasting glucose and other measures of insulin resistance, increased BMI, and increased blood pressure.34 Importantly, low SHBG (and FAI) has been associated with increased cardiovascular risk factors during the menopausal transition.8, 35 In addition, women have an increase in evidence of subclinical vascular disease early post-menopause, measured with carotid intimal medial thickness and adventitial diameter.9, 36 The accumulation of these risk factors are likely due to the androgen excess and estrogen decline,8 and may explain the doubling of stroke risk in the 10 years after menopause. Therefore it is imperative to focus on recognition and treatment of these risk factors in midlife women since menopause is often evident based on symptoms (amenorrhea, vasomotor symptoms), but also based on those aged 51 to 55 years without prior surgical hysterectomy. It is uncertain whether measurement of these hormones or screening for subclinical disease in midlife would impact cardiovascular disease and stroke prevention strategies, although this is an active area of research.37 A recent analysis of the Study of Women Across the Nation (SWAN) investigated the risk factors associated with menopause vs. chronological aging.38 These results showed that total cholesterol, LDL cholesterol, and apolipoprotein B substantially increased within 1 year of the last menstrual period, whereas the change in other risk factors were more suggestive of chronological aging (Table 2).38 A separate analysis of risk factor and carotid atherosclerosis changes across the menopausal transition in SWAN is also shown in Table 2.34
Table 2.
Association between menopause, final menstrual period, and cardiovascular risk factors
| Author, year | Risk factors | Findings | Comment |
|---|---|---|---|
| Matthews, 200134 | 1) CVD risk factors 2) Carotid atherosclerosis (CIMT and plaque) |
1) Increased SBP, pulse pressure, LDL-C, Triglycerides, fasting glucose, and BMI from first to fifth year PM 2) Increasing IMT assoc. with SBP, pulse pressure increases from pre-menopause to 1st yr PM, and increasing IMT between 1st and 5th yr PM associated with glucose change. |
An analysis of the Health Women Study showed no significant change in risk factors from premenopause to 1st year PM, but changes from 1st to 5th year PM were significant |
| Matthews, 200938 | TC, LDL-C, apo-B, HDL-C, Triglycerides, insulin, glucose, Lp(a), fibrinogen, Factor VIIc, tPA-ag, PAI-1, CRP, BMI, SBP, DBP | TC, LDL-C, and Apo-B were the only risk factors substantially increased around the FMP | This study analyzed the slope of change in risk factors during the year around the FMP. Other CVD risk factors were associated with chronological aging rather than the menopausal transition |
CVD = Cardiovascular disease; CIMT = Carotid intimal medial thickness; SBP = Systolic blood pressure; LDL-C = Low density lipoprotein cholesterol; BMI = Body mass index; PM = Post-menopause; TC = Total cholesterol; Lp(a) = Lipoprotein (a); tPA-ag = tissue plasminogen activator antigen; CRP = C reactive protein; DBP = Diastolic blood pressure; Apo-B = Apolipoprotein-B; FMP = Final menstrual period.
Epidemiology of Menopause and Stroke
Observational studies have demonstrated that younger, premenopausal women are protected from ischemic stroke compared with their male counterparts of a similar age, and that this protection may be lost with advancing age. Because of these findings, as well as supportive evidence from animal models, exposure to endogenous estrogen has been hypothesized to be protective for stroke in premenopausal women. To date, no study has investigated the relationship between endogenous hormones and stroke in premenopausal women or in women as they transition to menopause to directly test this hypothesis. The studies that have investigated the association between endogenous hormones and stroke, cardiovascular disease or death have been limited to post-menopausal women. The Study of Osteoporotic Fractures investigated FEI and stroke in women over age 65.28 In age adjusted analyses, a linear relationship between free estradiol and ischemic stroke risk was observed, such that those with the highest FEI had more than 2-fold increased risk of ischemic stroke (comparing highest to lowest quartiles). An early analysis of the Women’s Health Study (women 55 years of age or older) showed that, among hormone therapy non-users, those with cardiovascular events had a lower level of SHBG (p=0.03) and higher FAI (p=0.008) compared with those without cardiovascular events.39 Women in the lowest quartile of SHBG were 2.25-fold (95% CI: 1.03–4.91) more likely to have a cardiovascular event in the adjusted analysis. However, there was no difference in SHBG or FAI in women using hormone therapy, whether or not an event occurred.39 Lastly, the InChianti Study in Italy reported that women with higher total estradiol had a higher likelihood of age-adjusted mortality (HR = 1.03, 95% CI: 1.01–1.06), although the cause-specific mortality, such as from stroke, could not be determined.40 Given the logistical difficulty in measuring endogenous hormones over the life course, the focus from an epidemiologic perspective has been on the association between proxies for endogenous hormone exposures, such as age at menopause and number of reproductive years, and stroke risk. Prior OCP use related to the risk of stroke during menopause is unknown, although current use is associated with stroke in young women.41, 42
Stroke by Age at Menopause
There have been a handful of cohort studies conducted in various countries considering the association between age at menopause and stroke mortality, with findings to date suggestive of a null association. de Kleijn et al, using 20 years of follow-up data from a population-based cohort study in the Netherlands, found that age at menopause analyzed in quartiles of the distribution (≤44, 45–48, 49–51, >51) was not associated with stroke mortality adjusting for age, HRT, hypertension, BMI and socioeconomic status.43 Jacobsen et al similarly reported no association between age at natural menopause modeled in three-year age categories and stroke mortality in a large Norwegian cohort study.44 Data from two prospective cohort studies of US adults also support no association between age at natural menopause and stroke mortality.45, 46 Finally, in a Japanese cohort study, age at menopause was not associated with stroke mortality, with the exception that women with menopause at 47–48 years had elevated risk of stroke death compared with women with menopause after 50 years of age.47
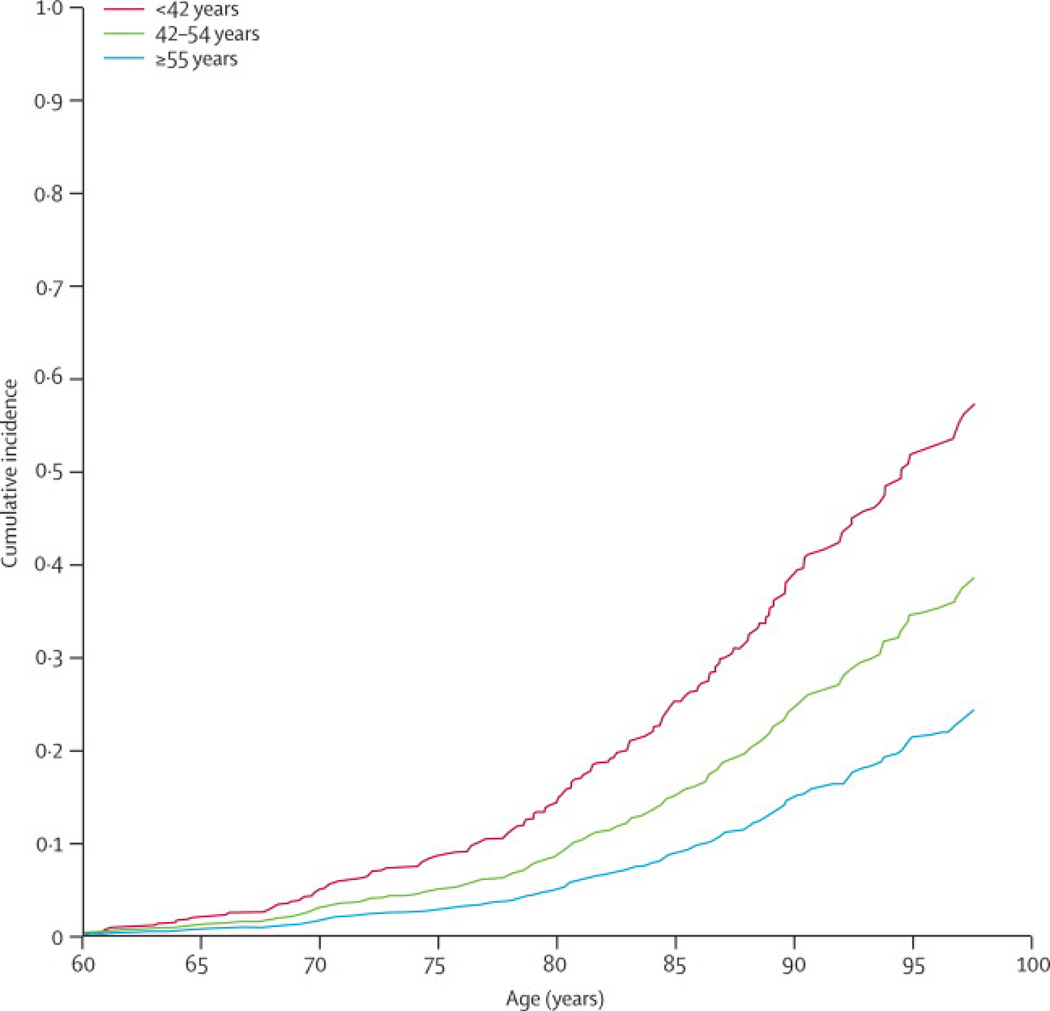
Studies considering the association between age at menopause and incident stroke risk are few in number and somewhat inconsistent in findings. Hu et al found that age at natural menopause modeled categorically in 5-year age categories or in a continuous manner (i.e., 1-year decrease in age at menopause) was not associated with risk of total stroke, ischemic stroke, or hemorrhagic stroke among never users of hormone therapy in the Nurse’s Health Study.48 Choi et al in a cohort study of post-menopausal Korean women who never used hormone therapy found no association between age at natural menopause (<40, 40–44, 45–49, 50–54, ≥55) and risk of total stroke, ischemic stroke or hemorrhagic stroke, although the number of events in some categories was small.49 In contrast, Lisabeth et al, using data from the Framingham Heart Study found that women with natural menopause before age 42 had twice the ischemic stroke risk compared (RR = 2.03, 95% CI: 1.16–3.56) with women with natural menopause at age 42 and older adjusting for age, stroke risk factors and post-menopausal estrogen use (Figure 1).50 Results from a Japanese cohort also suggested that women who underwent menopause before age 40 were more than twice as likely to have an ischemic stroke as those with menopause between 50 and 54 years of age adjusting for age and risk factors; however, this study included women with both natural and surgical menopause and findings were driven by those with surgical menopause.51 In a case-control study conducted in Spain age at menopause (>53 versus ≤53) was not associated with the odds of non-cardioembolic stroke accounting for age and stroke risk factors.52
Figure 1.
Kaplan Meier event curve for stroke risk associated with age at menopause in the Framingham cohort.50
Interestingly, in this same study lifetime estrogen exposure less than 34 years, defined as age at menarche to age at menopause, was associated with an increased odds of non-cardioembolic stroke (OR = 1.51, 95% CI: 1.13–2.03), raising the hypothesis that longer exposure to endogenous hormones may be protective for stroke and measures related to age at menopause may not necessarily reflect this exposure. However, a Japanese study considering the association between reproductive years and stroke mortality did not find such an association.47 Alternative measures of lifetime exposure to endogenous estrogens which include additional information on pregnancies and breastfeeding have also been proposed, although their association with stroke has not been studied.43 Further, anti-Mullerian hormone levels have been shown to be highly predictive of age at menopause,53 and therefore may be a reasonable surrogate for studying hormonal exposures and stroke in younger women, particularly those with established high risks, such as existing cardiovascular disease, diabetes, cigarette smoking, and hypertension.
Hormone Therapy and Stroke
Existing Clinical Trials
Randomized clinical trial data indicate that use of estrogen plus progestin, as well as estrogen alone, taken orally increases stroke risk in healthy post-menopausal women.54, 55 The Women's Health Initiative (WHI), a randomized trial of 16,608 post-menopausal women found that estrogen plus progestin increased ischemic stroke risk by 44%, with no effect on hemorrhagic stroke.55 In the WHI trial, among 10,739 post-menopausal women with a hysterectomy, it was found that conjugate equine estrogen (CEE) alone increased risk of ischemic stroke by 55% and that there was no significant effect on hemorrhagic stroke.54 Meta-analyses of existing trials have confirmed these findings suggesting a roughly 30% elevated total stroke risk with HRT use compared with no use.56–58 Recently, LaCroix et al reported long-term health outcomes for the WHI Estrogen Alone Trial participants. Participants were followed for an average of 10.7 years with a median CEE use of 5.9 years. They found that stroke risk was no longer elevated during the post-intervention period (HR = 0.89; 95% CI: 0.64–1.24).59
In post-menopausal women with known coronary heart disease, the Heart and Estrogen/Progestin Replacement Study (HERS) found that estrogen plus progestin did not reduce stroke risk of nonfatal or fatal stroke.60 The Women's Estrogen for Stroke Trial (WEST) found that estrogen alone in postmenopausal women with recent stroke or TIA had no effect on recurrent stroke (fatal and nonfatal combined), although there was an early onset of stroke events in the estradiol arm in the first 6 months after randomization.61
Ongoing Clinical Trials
Currently, there are two ongoing trials that may help to shed light on the association of HRT and stroke. The Kronos Early Estrogen Prevention Study (KEEPS) is a randomized placebo controlled clinical trial comparing 0.45 mg of oral estrogen and a transdermal estrogen skin patch, as well as a progesterone, with placebo among healthy, recently post-menopausal women (i.e., within 3 years of menopause).37 The primary outcome of this study is rate of change in carotid intimal medial thickness. The Early Versus Late Intervention Trial with Estradiol (ELITE) is a second ongoing randomized placebo controlled clinical trial with the goal of examining the effects of estrogen (1 mg 17β-estradiol daily) taken orally on the progression of early subclinical atherosclerosis and cognitive decline in healthy post-menopausal women (http://clinicaltrials.gov/ct2/show/NCT00114517?term=estradiol&rank=12). The design of this study includes randomizing women according to their years since menopause, < 6 years or ≥ 10 years, to receive either estrogen or a placebo. Although not designed to specifically consider stroke, these trials should provide evidence for or against the role of HRT, including issues surrounding timing and route of administration, in the progression of atherosclerosis and whether a larger trial powered for hard cardiovascular outcomes, including heart disease and stroke, may be warranted.
Route of Administration
It is hypothesized that transdermal estrogen may be safer with respect to stroke risk than oral estrogen given the lack of exposure to first-pass liver metabolism and the lack of increase in clotting factors and inflammatory markers.62 Limited observational data is available exploring the different routes of administration of estrogen and stroke risk. In a nested case-control study of post-menopausal women in the United Kingdom’s General Practice Research Database, there was a suggestion of greater risk of transient ischemic attack (TIA) associated with current use of oral estrogen (OR = 1.47 compared with nonuse; 95% CI: 1.09–1.97) than with current use of transdermal patches (OR = 0.86 compared with nonuse; 95% CI, 0.43–1.73), although the authors report that there were only a small number of users of transdermal patches (actual number not reported).63 Results were not reported for ischemic or hemorrhagic stroke because of small numbers. In a more extensive and updated nested case-control study of the same database, Renoux et al reported an adjusted rate ratio of stroke of 0.95 (95% CI: 0.75–1.20) comparing current users of transdermal HRT with non users.64 Interestingly, this association was modified by dose such that stroke risk was not increased for users of low dose (≤50 µg) estrogen patches (RR = 0.81; 95% CI 0.62–1.05) but was increased for users of high dose (>50 µg) patches (RR = 1.89; 95% CI 1.15–3.11) compared with non users. For comparison, current users of oral HRT had a higher risk of stroke than non users (RR = 1.28; 95% CI 1.15–1.42) which was not modified by dose of the oral HRT. The authors also provided a direct comparison of stroke risk for users of transdermal versus oral therapies. The results suggest that stroke risk is roughly 25% less in those who use transdermal HRT compared with oral (RR = 0.74; 95% CI 0.580.95).64 Of note, these observational studies were focused on women who were on average ten to twenty years post menopause (average age 62 and 70 years respectively) in comparison to younger, recently menopausal women which are the target of the ongoing KEEPS and ELITE.
Dose of Hormone Therapy
“Estrogenic dose” may depend on the type (estradiol versus congregated equine estrogen (CEE)) and dose of estrogen used. As existing clinical trials were based on single HRT regimens, no trial data directly comparing stroke risk for varying types and doses of estrogen are available; however, there is limited observational data on this subject. Most recently, in the nested case-control study by Renoux et al described above, the association of oral and transdermal HRT with stroke were reported for low and high dose estrogen formulations. Considering the transdermal route, as highlighted above the authors found that stroke risk was not increased with current use of low dose patches but was increased with current use of high dose (>50 µg) patches compared with non use (RR = 1.89; 95% CI: 1.15–3.11). In contrast, stroke risk was elevated to a similar extent among current users of low (≤0.625 mg equine estrogen or ≤2 mg of estradiol) and high dose (>0.625 mg equine estrogen or >2 mg of estradiol) oral therapies (low RR = 1.25; 95% CI 1.12–1.40; high RR = 1.48; 95% CI: 1.16–1.90). 64 In the earlier analysis of the same dataset, Arana et al also reported a dose response association between HRT and odds of TIA.63 Current users of medium dose HRT defined as oral CEE of 0.625–1.24 mg or 50 µg of transdermal estrogen had a 50% greater odds of TIA (RR = 1.48,;95% CI: 1.12–1.96), while current users of high does HRT defined as oral CEE of ≥ 1.25 mg or 100 µg of transdermal estrogen had a two-fold increased odds of TIA (OR = 1.96; 95% CI: 1.34–2.87) compared with non users after accounting for confounding factors. No association with stroke was observed among users of low dose HRT.63 While not new, these results are in general agreement with results from the Nurse’s Health Study. In this study, Grodstein et al also demonstrated a dose response relationship between oral CEE and risk of stroke. Compared with never users, post-menopausal women taking ≥ 0.625 mg of CEE were 35% more likely to develop a stroke (RR = 1.35; 95% CI 1.08–1.68) and women taking ≥ 1.25 mg were 63% more likely to develop a stroke (RR = 1.63; 95% CI 1.18–2.26) after accounting for age and risk factors. Women taking 0.30 mg did not have an elevated risk of stroke (RR = 0.54; 95% CI: 0.28–1.06).65
Mono versus Dual Therapy
Clinical trial data indicate that the use of both estrogen plus progestin and estrogen alone (taken orally) increase stroke risk in post-menopausal women.54, 55 Newer observational data by Renoux et al from their nested case-control study also support no difference in the association of HRT, taken orally, and stroke comparing users of mono and dual therapies.64 The odds of stroke were 1.35 (95% CI: 1.16–1.58) and 1.24 (95% CI 1.08–1.41) for users of mono and dual therapy respectively. Among users of transdermal therapy, stroke risk was not elevated for users of mono (RR = 1.02; 95% CI: 0.78–1.34) or dual therapies (RR = 0.76; 95% CI: 0.47–1.22).64 Although not significant, similar results for TIA were observed by Arana et al combining data on route of administration.63
The Timing Hypothesis for Hormone Therapy and Stroke
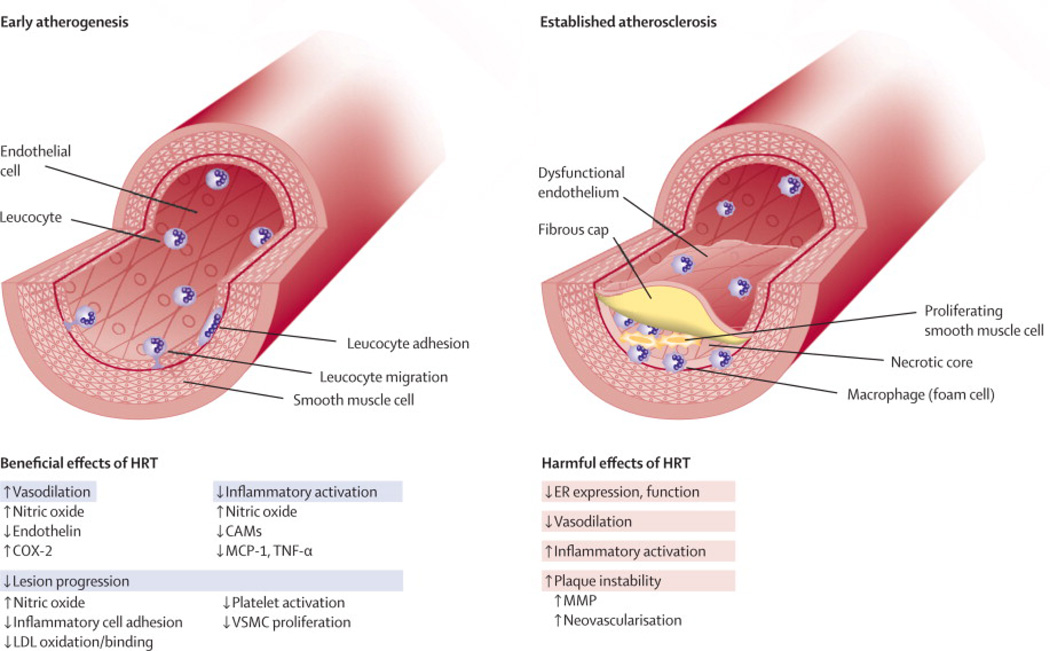
One of the most important lessons learned from the clinical trials of hormone therapy in post-menopausal women is that once women are 10 years or more past menopause, the main effect appears to be harm rather than benefit from exogenous estrogens or progesterone. The lack of benefit was shown in a study of APO E deficient mice, in which treatment with estrogen for mice with mature plaques did not lead to inhibition of progression of the plaque.66 However, for vessels with less advanced atherosclerosis, estrogen treatment was associated with lesser fatty streaks, the initial sign of atherosclerosis.66 Endothelial dysfunction can be improved or reversed with physiological levels of estrogen replacement during the early stage of atherosclerosis. Whereas, with more advanced atherosclerotic lesions, the cellular biology differs such that the response to late initiation of exogenous hormone therapy is more likely to lead to inflammatory and hemostatic abnormalities that promote progression or instability of the lesion.67 A summary of the beneficial and harmful effects of hormone therapy are shown in Figure 2.67
Figure 2.
Differential effects of estrogens depending on the stage of atherosclerosis.67
Timing was shown to be important in the WHI secondary analysis of women categorized by the number of years since menopause and the risk of having a coronary artery disease event.68 Women who were 10 years or less since menopause received a nonsignificant benefit from hormone therapy (HR = 0.76; 95% CI: 0.50–1.16), whereas there was no significant benefit in those 10–19 years post-menopause (HR = 1.10; 95% CI: 0.84–1.45), and an increased risk in women 20 years or more since menopause (HR = 1.28; 95% CI: 1.03–1.58; p value for trend, 0.02). Interestingly, this trend was not seen in women who had stroke events, as there was a risk of stroke with treatment regardless of age at enrollment or years since menopause.68 A similar pattern of increased risk with hormone therapy use regardless of age or timing of initiation was shown in the Nurses’ Health Study.69 In a medical records study of over 50,000 women in the United Kingdom General Practice Research Database, for women less than 55 years old at the time of exposure to estrogen, the adjusted HR for stroke was 1.52 (95% CI: 1.11–2.08), whereas, the effect of estrogen exposure for MI was essentially neutral (HR 0.91; 95% CI: 0.69–1.20). 70 Although age and timing of hormone therapy initiation may play a role in the risk/benefit ratio for heart disease, women appear to be at the same risk for stroke regardless of age at initiation.
The reasons for the differences in risk by age for stroke and heart disease are poorly understood. One potential explanation is that the pro-thrombotic properties of hormone therapy also increase the risk for venous thromboembolism,70, 71 which could then lead to ischemic stroke via paradoxical embolism through a patent foramen ovale or other right to left cardiac shunt. In fact, a recent longitudinal study showed that increased thrombin generation at baseline is associated with the risk for ischemic stroke, but not CHD, and this effect was primarily seen in women, but not men.72 The etiology of stroke is rarely reported to the level of detail in large cohorts, clinical trials, or database studies in order to allow speculation about causation with hormone therapy exposure. In addition, the etiology of stroke is heterogeneous and is undetermined (i.e. no identifiable cause) in about 35% of patients.73
Current Clinical Guidelines for HT
The American Heart Association guidelines specifically state that hormone therapy should not be prescribed for prevention of heart disease or stroke.74–76 The European Menopause and Andropause Society released a position statement for women with known coronary heart disease, which recommends that women who are experiencing menopausal symptoms should be individually evaluated for their baseline risk for developing breast cancer, venous thromboembolism, and coronary heart disease recurrence. After weighing the risks of these events against quality of life, “the lowest effective estrogen dose should be used for the shortest possible time.”77 Also, for women at increased risk of CHD, transdermal HT should be the first choice over oral formulations.77
Summary and Conclusions
Although women have a lower incidence of stroke than men during midlife, their risk doubles in the decade after menopause, emphasizing the need to screen for and manage risk factors that are also increasing during this time period. There may also be an increased risk for women with very early onset of menopause, but it is unknown whether these women should receive replacement therapy. Although the risk of stroke is low and early age of menopause is uncommon, further research is needed for optimum prevention of cardiovascular events. Hormone therapy is still the most effective treatment for menopausal symptoms, but there is no timing for exposure in midlife that seems to protect women from stroke, whereas for heart disease, the risk appears lowest in the early post-menopausal time frame. More research is needed to determine the safest and most effective formulation, dose, and duration of hormone therapy that will treat vasomotor symptoms without increasing the risk for stroke. The ongoing KEEPS and ELITE will shed light on the association between HRT, including information on route of administration and timing of initiation, and progression of atherosclerosis – information that should inform the design of future clinical trials. In the meantime, it is important for women to maximize a healthy lifestyle throughout midlife to reduce the overall risk for stroke and cardiovascular disease.78
Search Strategy: Incidence or prevalence AND stroke; Menopause AND stroke; Primary Ovarian Insufficiency; Premature ovarian failure; sex steroids or Sex hormone-binding globulin or hormone therapy or estrogens AND stroke
Database: PubMed, and references cited in identified articles from 1966 to present.
Inclusion criteria: Studies with multivariable adjusted analysis to determine risk of stroke based on age at menopause. Observational or post-hoc studies of treatment with hormone therapy focused on age or timing or initiation, published since 2000 and up to August 2011. Other original studies, as well as reviews relevant to the topic of this manuscript were also included.
Exclusion criteria: Case reports, case series, or studies with less than 30 subjects, or with too small a sample size to perform adjusted analyses.
Acknowledgments
Dr. Bushnell receives funding support from NINDS K02 NS058760.
Dr. Lisabeth receives funding support from NINDS R01 NS38916, R01 NS062675, R01 NS070941, and NHLBI R01 HL098065.
Role of the funding source:
The funding sources provided effort towards research time for Drs Bushnell and Lisabeth.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest statement:
Author contribution:
Dr. Bushnell and Dr. Lisabeth each performed the literature searching, drafted an equal distribution of sections in this manuscript, and reviewed/edited the final draft.
References
- 1.Roger V, Go A, Lloyd-Jones D, et al. Heart Disease and Stroke Statistics 2011 Update: A report from the American Heart Association. Circulation. 2010 doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roquer J, Rodriguez A, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34:1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 4.Kapral MK, Fang J, Hill MD, et al. Sex differences in stroke care and outcomes. Results from the registry of the Canadian Stroke Network. Stroke. 2005;36:809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 5.Gargano J, Reeves M. Sex differences in stroke recovery and stroke-specific quality of life. Stroke. 2007;38:2541–2548. doi: 10.1161/STROKEAHA.107.485482. [DOI] [PubMed] [Google Scholar]
- 6.Gargano JW, Wehner S, Reeves MJ. Sex differences in acute stroke care in a statewide Stroke registry. stroke. 2008;39:24–29. doi: 10.1161/STROKEAHA.107.493262. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Sánchez P, Fuentes B, Fernández-Domínguez J, et al. Young Women Have Poorer Outcomes than Men after Stroke. Cerebrovasc Dis. 2011;31:455–463. doi: 10.1159/000323851. [DOI] [PubMed] [Google Scholar]
- 8.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K, Lassila HC, Meilahn EN, Bunker C, Matthews KA, Kuller LH. Carotid atherosclerosis in premenopausal and postmenopausal women and its association with risk factors measured after menopause. Stroke. 1998;29:1116–1121. doi: 10.1161/01.str.29.6.1116. [DOI] [PubMed] [Google Scholar]
- 10.Brown RD, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–380. [PubMed] [Google Scholar]
- 11.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 12.Lisabeth LD, Smith MA, Sanchez BN, Brown DL. Ethnic disparities in stroke and hypertension among women: the BASIC project. Am J Hypertens. 2008;21:778–783. doi: 10.1038/ajh.2008.161. [DOI] [PubMed] [Google Scholar]
- 13.Lofmark U, Hammarstrom A. Evidence for age-dependent education-related differences in men and women with first-ever stroke. Results from a community-based incidence study in northern Sweden. Neuroepidemiology. 2007;28:135–141. doi: 10.1159/000102141. [DOI] [PubMed] [Google Scholar]
- 14.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40:1032–1037. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 16.Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74:317–321. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 18.Vega T, Zurriaga O, Ramos JM, et al. Stroke in Spain: epidemiologic incidence and patterns; a health sentinel network study. J Stroke Cerebrovasc Dis. 2009;18:11–16. doi: 10.1016/j.jstrokecerebrovasdis.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Towfighi A, Saver J, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69:1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- 20.Towfighi A, Markovic D, Ovbiagele B. Persistent sex disparity in midlife stroke prevalence in the United States. Cerebrovasc Dis. 2011;31:322–328. doi: 10.1159/000321503. [DOI] [PubMed] [Google Scholar]
- 21.Ayala C, Croft J, Greenlund K, et al. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995–1998. Stroke. 2002;33:1197–1201. doi: 10.1161/01.str.0000015028.52771.d1. [DOI] [PubMed] [Google Scholar]
- 22.Towfighi A, Tai W, Markovic D, Ovbiagele B. Sex-Specific Temporal Trends in In-Hospital Mortality After Stroke Among Middle-Age Individuals in the United States. Stroke. 2011 Jul 28; doi: 10.1161/STROKEAHA.110.612648. [Epub ahead of print]: [DOI] [PubMed] [Google Scholar]
- 23.Kapral M, Degani N, Hall R, et al. Gender differences in stroke care and outcomes in Ontario. Womens Health Issues. 2011;21:171–176. doi: 10.1016/j.whi.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 24.te Velde E, Pearson P. The variability of female reproductive aging. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 25.Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21:103–113. doi: 10.1016/0378-5122(94)00869-9. [DOI] [PubMed] [Google Scholar]
- 26.McKinlay SM. The normal menopause transition: An overview. Maturitas. 1996;23:137–145. doi: 10.1016/0378-5122(95)00985-x. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Ding J, Bush T, et al. Relative androgen excess and increased cardiovascular risk after menopause: A hypothesized relation. Am J Epidem. 2001;154:489–494. doi: 10.1093/aje/154.6.489. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Yaffe K, Lui LY, et al. Prospective study of endogenous circulating estradiol and risk of stroke in older women. Arch Neurol. 2010;67:195–201. doi: 10.1001/archneurol.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selby C. Sex hormone binding globuling: origin, function, and clinical significance. Ann Clin Biochem. 1990;27:532–541. doi: 10.1177/000456329002700603. [DOI] [PubMed] [Google Scholar]
- 30.de Bruin J, Bovenhuis H, van Noord P, et al. The role of genetic factors in age at natural menopause. Hum Reprod. 2001;16:2014–2018. doi: 10.1093/humrep/16.9.2014. [DOI] [PubMed] [Google Scholar]
- 31.Coulam C, Adamson S, Annegers J. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- 32.De Vos M, Devroey P, Fauser P. Primary ovarian insufficiency. Lancet. 2010;376:911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 33.Sybert V, McCauley E. Turner's Syndrome. N Engl J Med. 2004;351:1227–1238. doi: 10.1056/NEJMra030360. [DOI] [PubMed] [Google Scholar]
- 34.Matthews KA, Kuller LH, Sutton-Tyrrell K, Chang Y-F. Changes in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke. 2001;32:1104–1111. doi: 10.1161/01.str.32.5.1104. [DOI] [PubMed] [Google Scholar]
- 35.Mudali S, Dobs AS, Ding J, Cauley JA, Szklo M, Golden SH. Endogenous postmenopausal hormones and serum lipids: The Atherosclerosis Risk in Communities Study. J Clin Endocrinol & Metab. 2005;90:1202–1209. doi: 10.1210/jc.2004-0744. [DOI] [PubMed] [Google Scholar]
- 36.Wildman R, colvin A, Powell L, et al. Associations of endogenous sex hormones with the vasculature in menopausal women: The Study of Women's Health Across the Nation (SWAN) Menopause. 2008;15:414–421. doi: 10.1097/gme.0b013e318154b6f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harman S, Brinton E, Cedars M, et al. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 38.Matthews K, Crawford S, Chae C, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rexrode KM, Manson JE, Lee I-M, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–1693. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 40.Maggio M, Ceda G, Lauretani F, et al. Relationship between higher estradiol levels and 9-year mortality in older women: The Invecchiare in Chianti Study. J Am Geriatr Soc. 2009;57:1810–1815. doi: 10.1111/j.1532-5415.2009.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives. A meta-analysis. JAMA. 2000;284:72–78. doi: 10.1001/jama.284.1.72. [DOI] [PubMed] [Google Scholar]
- 42.Chan W-S, Ray J, Wai EK, et al. Risk of stroke in women exposed to low-dose oral contraceptives. A critical evaluation of the evidence. Arch Intern Med. 2004;164:741–747. doi: 10.1001/archinte.164.7.741. [DOI] [PubMed] [Google Scholar]
- 43.de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339–345. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- 44.Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and stroke mortality: cohort study with 3561 stroke deaths during 37-year follow-up. Stroke. 2004;35:1548–1551. doi: 10.1161/01.STR.0000131746.49082.5c. [DOI] [PubMed] [Google Scholar]
- 45.Cooper GS, Sandler DP. Age at natural menopause and mortality. Annals of epidemiology. 1998;8:229–235. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- 46.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162:1089–1097. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 47.Cui R, Iso H, Toyoshima H, et al. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. Journal of epidemiology / Japan Epidemiological Association. 2006;16:177–184. doi: 10.2188/jea.16.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 49.Choi SH, Lee SM, Kim Y, Choi NK, Cho YJ, Park BJ. Natural menopause and risk of stroke in elderly women. Journal of Korean medical science. 2005;20:1053–1058. doi: 10.3346/jkms.2005.20.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40:1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010;17:506–510. doi: 10.1097/gme.0b013e3181c7dd41. [DOI] [PubMed] [Google Scholar]
- 52.de Lecinana MA, Egido JA, Fernandez C, et al. Risk of ischemic stroke and lifetime estrogen exposure. Neurology. 2007;68:33–38. doi: 10.1212/01.wnl.0000250238.69938.f5. [DOI] [PubMed] [Google Scholar]
- 53.Broer S, Eijkemans M, Scheffer G, et al. Anti-Mullerian hormone predicts menopause: A long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2001;96:2532–2539. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 54.Hendrix SL, Wassertheil-Smoller S, Johnson KC, et al. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 55.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of Estrogen plus progestin on stroke in postmenopausal women. The Women's Health Initiative: A randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 56.Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke: a meta-analysis. Bmj. 2005;330:342. doi: 10.1136/bmj.38331.655347.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29:2031–2041. doi: 10.1093/eurheartj/ehn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magliano DJ, Rogers SL, Abramson MJ, Tonkin AM. Hormone therapy and cardiovascular disease: a systematic review and meta-analysis. Bjog. 2006;113:5–14. doi: 10.1111/j.1471-0528.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 59.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon JA, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen-progestin Replacement Study (HERS) Circulation. 2001;103:638–642. doi: 10.1161/01.cir.103.5.638. [DOI] [PubMed] [Google Scholar]
- 61.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. NEJM. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 62.Hemelaar M, van der Mooren M, Rad M, Kluft C, Kenemans P. Effects of non-oral postmenopausal hormone therapy on markers of cardiovascular risk: a systematic review. Fertility and Sterility. 2008;90:642–672. doi: 10.1016/j.fertnstert.2007.07.1298. [DOI] [PubMed] [Google Scholar]
- 63.Arana A, Varas C, Gonzalez-Perez A, Gutierrez L, Bjerrum L, Garcia Rodriguez LA. Hormone therapy and cerebrovascular events: a population-based nested case-control study. Menopause. 2006;13:730–736. doi: 10.1097/01.gme.0000233494.28335.71. [DOI] [PubMed] [Google Scholar]
- 64.Renoux C, Dell'aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519. [DOI] [PubMed] [Google Scholar]
- 65.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:922–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 66.Rosenfeld M, Kauser K, Martin-McNulty B, Polinsky P, Schwartz S, Rubanyi G. Estrogen inhibits the initiation of fatty streaks throughout the vasculature but does not inhibit intra-plaque hemorrhage and the progression of established lesions in apolipoprotein E deficient mice. Atherosclerosis. 2002;164:251–259. doi: 10.1016/s0021-9150(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 67.Mendelsohn M, Karas R. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 68.Rossouw J, Prentice R, Manson J, et al. postmenopausal hormone therapy and risk of cardiovascular disase by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 69.Grodstein F, Manson J, Stampfer M, Rexrode K. Postmenopausal hormone therapy and stroke. Role of time since menopause and age at initiation of hormone therapy. Arch Intern Med. 2008;168:861–866. doi: 10.1001/archinte.168.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiner MB K, Xie D, Tannen R. Hormone therapy and coronary heart disease in young women. Menopause. 2008;15:86–93. doi: 10.1097/gme.0b013e3180413e45. [DOI] [PubMed] [Google Scholar]
- 71.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy post-menopausal women. Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 72.Carcaillon L, Alhenc-Gelas M, Bejot Y, et al. Increased thrombin generation is associated with acute ischemic stroke but not with coronary heart disease in the elderly. The Three-City Cohort Study. Arterio Thromb Vasc Biol. 2011;31:1445–1451. doi: 10.1161/ATVBAHA.111.223453. [DOI] [PubMed] [Google Scholar]
- 73.Kolominsky-Rabas P, Weber M, Gefeller O, Neundoerfer B, Heuschmann P. Epidemiology of ischemic stroke subtypes according to TOAST criteria. Incidence, recurrence, and long-term survival in ischemic stroke subtypes: A population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 74.Mosca L, Benjamin E, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women 2011 update: A guideline from the American Heart Association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldstein L, Bushnell C, Adams R, et al. Guidelines for the primary prevention of stroke. A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010 [Google Scholar]
- 76.Furie K, Kasner S, Adams R, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 77.Schenck-Gustafsson K, Brincat M, Erel C, et al. EMAS position statement: Managing menopause in the context of coronary heart disease. Maturitas. 2011;68:94–97. doi: 10.1016/j.maturitas.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Lloyd-Jones D, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]