Abstract
Phosphoinositide 3’-kinase (PI3K) is a key node in the B cell receptor (BCR) pathway, which plays a crucial role in the trafficking, survival, and proliferation of chronic lymphocytic leukemia (CLL) cells. We review the biology of the PI3K with a focus on its relationship to the CLL microenvironment. We then discuss the biologic rationale underlying the development of PI3K inhibitors in CLL. Delta-isoform specific PI3K inhibitors such as GS1101 (formerly CAL-101) are highly selective for CLL cells and have progressed furthest in their clinical development. Though less specific, pan-PI3K inhibitors and dual PI3K/mTOR inhibitors have the potential to overcome possible resistance mechanisms to isoform-specific inhibition. In early phase clinical trials, PI3K inhibitors appear to be highly active in relapsed refractory CLL, including in high-risk disease such as del(17p). Like other BCR pathway antagonists, they typically induce early transient lymphocytosis with associated nodal response. We examine potential biomarkers for clinical response to PI3K inhibitors such as ZAP-70, IGHV status, and CCL3. We also explore where PI3K inhibition may fit in the evolving landscape of CLL therapy.
Keywords: Lymphoid Leukemia, Signaling Therapies
INTRODUCTION
Although chronic lymphocytic leukemia (CLL) usually responds well to initial chemotherapy, the disease inevitably relapses, and remains incurable by conventional therapy1. It has been hypothesized that after treatment, sanctuary sites such as the lymph nodes and bone marrow may harbor residual CLL cells that can later lead to relapse 2. Indeed, the protective role of the CLL microenvironment may be a key to understanding why stroma-exposed CLL cells are protected from undergoing apoptosis in response to treatment. The B cell receptor (BCR) pathway has been particularly identified as a key mediator of prosurvival signals in CLL cells3. Several novel kinase inhibitors are now in development to target various components of the BCR pathway4–7. A class effect of these BCR inhibitors is a ‘lymphocyte redistribution’ phenomenon, whereby a majority of patients initially develop a transient lymphocytosis while simultaneously achieving nodal reduction. This observation has led to the hypothesis that these agents may achieve their efficacy, at least in part, by mobilizing CLL cells out of sanctuary sites and into the peripheral blood, where they more readily die or can be killed by combination therapy.
Of the new agents targeting the BCR pathway, phosphoinositide-3 kinase (PI3K) inhibitors are amongst the most promising. Here, we review the scientific rationale underlying PI3K inhibition in CLL, as well as data from recent and ongoing clinical trials of PI3K inhibitors in CLL. We also discuss potential biologic predictive markers for PI3K clinical response, as well as where PI3K inhibitors may fit in to the evolving landscape of CLL therapy.
BIOLOGY OF PI3K IN CLL
Signaling cascades from several major pathways converge on PI3K, which serves as a key node regulating B cell function and survival. Although there are 3 classes of PI3K isoforms, only class I isoforms are thought to be directly related to oncogenesis8. Within class I, PI3K isoforms can be further subdivided into class IA (PI3K-alpha, -beta, and –delta) and class IB (PI3K-gamma)9. Although we have shown that activating mutations in PI3K are very rare in CLL, we did identify amplification of PIK3CA in about 3.5% of CLL10, 11. Furthermore, even in the absence of genetic activation, CLL cells generally express high levels of active PI3K (in particular the delta-isoform12), and great interest has therefore focused on elucidating the role PI3K plays in the pathogenesis of the disease.
The 3 best characterized pathways that activate PI3K include the BCR, receptor tyrosine kinases (RTKs), and cytokine/chemokine receptors (Figure 1). Of these, the BCR pathway is thought to play a dominant role in CLL. The BCR usually becomes activated in the presence of antigen (though tonic signaling has also been described13). Activated BCR recruits other kinases such as spleen tyrosine kinase (SYK) and LYN kinase, which phosphorylate immunoreceptor tyrosine-based activation motifs (ITAMs) on the cytoplasmic Ig domains of the receptor14. Stimulated RTKs, cytokine, and chemokine receptors also cause autophosphorylation of the tyrosine residue on the ITAMs and subsequent PI3K activation in immune cells15, though the importance of these pathways in CLL is variable.
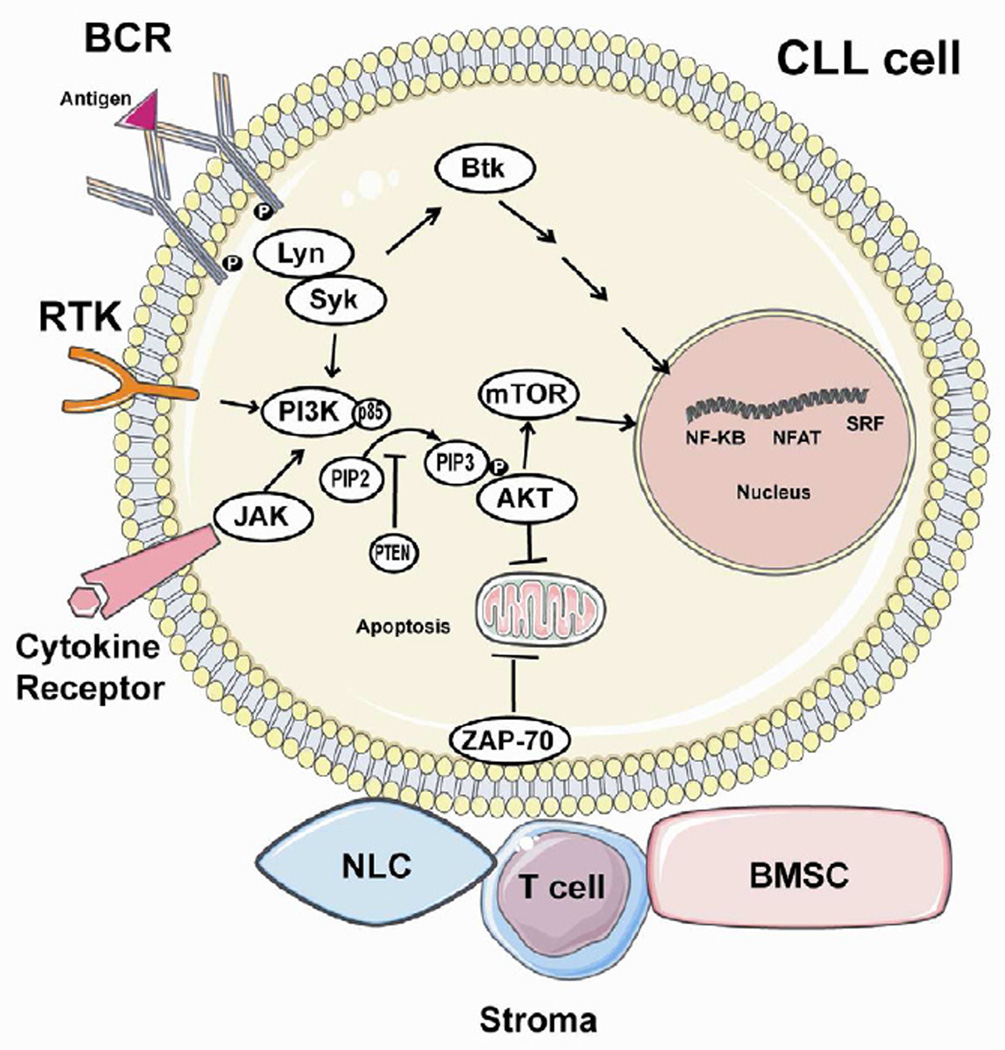
Figure 1. PI3K signaling and molecular interactions in the CLL cell.
Three important pathways converge on PI3K signaling, including the B cell receptor (BCR), thought to be the dominant pathway in CLL, as well as receptor tyrosine kinases (RTKs) and cytokine/chemokine receptors. The BCR usually becomes activated in the presence of antigen (though tonic signaling has also been described). Activated BCR recruits other kinases such as spleen tyrosine kinase (SYK) and LYN kinase, which phosphorylate immunoreceptor tyrosine-based activation motifs (ITAMs) on the cytoplasmic Ig domains of the receptor. RTKs can be stimulated by a variety of ligands, and also lead to autophosphorylation of the tyrosine residue on the ITAM. Stimulated cytokine and chemokine receptors can activate janus-activated kinase (JAK) tyrosine kinases, which phosphorylate tyrosine residues in proteins such as gp130 and IRS family members. These tyrosine-phosphorylated proteins interact with SH2 domains of the p85 subunit of PI3K, thereby activating the p110 kinase activity which is normally inhibited in the p85-p110 complex. This activation results in the conversion of PIP2 to PIP3, which has several downstream effects, many of which are mediated by AKT. The net result of PI3K activation is to promote CLL cell survival and proliferation, largely through activation of nuclear transcription factors such as NF-KB, NFAT, and SRF. These effects can be modulated by nearby stromal cells including T cells, nurse-like cells (NLC), and bone marrow stromal cells (BMSC).
Stimulation of each of these three pathways sets off a chain of downstream molecular interactions, the net result of which is to create Src homology 2 (SH2)-binding domains capable of binding the p85 regulatory subunit of PI3K16. Once this binding occurs, p85 can no longer inhibit the p110 catalytic domain of PI3K, thereby leading to PI3K activation. One of the primary functions of activated PI3K in B cells is to convert phosphatidylinositol(3,4)P2 into phosphatidylinositol (3,4,5)P3, leading to AKT phosphorylation, which then can go on to activate a wide variety of downstream kinases17. In addition to AKT, activated PI3K also promotes calcium mobilization and activation of other downstream kinases such as PKC-β, mammalian target of rapamycin (mTOR), and MAP kinase (ERK). These events promote increased proliferation of B cells, largely mediated by the upregulation of transcription factors such as nuclear factor KB (NF-KB) and nuclear factor of activated T cells (NFAT)18.
Although substantial evidence indicates that PI3K activation inhibits both the extrinsic and intrinsic pathways of apoptosis, the precise mechanism of these interactions remains incompletely understood. Activated AKT likely interferes with FasL expression, thereby decreasing levels of this primary mediator of extrinsic apoptosis19. Activated AKT has also been hypothesized to affect the intrinsic mitochondrial pathway of apoptosis by increasing the amount of the pro-apoptotic protein BAD that is sequestered by the anti-apoptotic protein BCL-XL, thereby pushing the cell farther from the threshold of apoptosis (i.e. decreasing ‘priming’ for apoptosis)20. Other interactions between AKT and the mitochondrial pathway of apoptosis are likely, and this remains an active area of investigation.
Beyond its direct effects on promoting B cell proliferation and inhibiting apoptosis, activated PI3K also has a profound influence on B cell trafficking by promoting CLL cell chemotaxis towards CXCL12/13, migration beneath stromal cells, and upregulation of CLL cell chemokine secretion21. Once CLL cells enter the stromal microenvironment, they become bathed in a variety of protective mediators such as CD40L, fibronectin, and BAFF, all of which likely send prosurvival signals through PI3K. We have shown that the net effect of these stromal interactions is to decrease CLL cell mitochondrial apoptotic priming, which may lead to resistance to a wide variety of therapies22
INHIBITION OF PI3K IN CLL
Given the key role that PI3K plays in CLL pathophysiology, the potential efficacy of small molecule PI3K inhibitors has been widely recognized. Several different PI3K inhibitors are in various stages of development, and can be divided into two main categories: delta-isoform specific and pan-PI3K inhibitors. A third category of dual inhibitors targeting both PI3K and mammalian target of rapamycin (mTOR) are also being investigated.
PI3K-δ Inhibition
Preclinical
Since the delta-isoform of the p110 catalytic subunit of PI3K is the predominant form expressed in leukocytes23, a delta-isoform specific PI3K inhibitor was the first logical target to pursue and is currently the furthest along in clinical development. GS1101 (formerly CAL-101) is a small molecule that specifically and potently inhibits the delta-isoform of PI3K6. The drug has been shown to induce apoptosis in primary CLL cells ex vivo in a time- and dose-dependent manner12. In vitro, GS1101 partially reverses the chemoresistance observed in stroma-exposed CLL cells and also reduces CLL cell chemotaxis into stroma22, 24. Importantly, GS1101 alone can induce a modest degree of apoptosis even in the presence of stroma21, 22.
Given its ability to release CLL cells from protective stroma niches, in vitro studies have been performed to determine which drugs might best complement the activity of GS1101 by blocking other pathways that contribute to CLL cell survival. The drug does appear to have at least additive effects in killing when combined with commonly used CLL chemotherapies such as fludarabine and bendamustine21.
Recent preclinical work has focused on developing rational combinations of GS1101 by utilizing agents with complimentary but targeted mechanisms of action. One such approach combined pharmacologic inhibition of PI3K-δ with lenalidomide, an immunomodulatory agent known to induce tumor flare at low doses and to have modest clinical activity in patients with relapsed refractory CLL25. Pharmacologic inhibition (or siRNA knockdown) of PI3K-δ was found to abrogate CLL cell activation and to reduce costimulatory molecule expression, as well as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) gene expression, which are thought to contribute to CLL survival in stroma26, 27. These findings suggest that combining GS1101 with lenalidomide might limit tumor flare and potentially augment the clinical activity of lenalidomide in patients with CLL.
We studied another approach of combining GS1101 with the BH3-mimetic drug ABT-737 to increase apoptotic killing of stroma-exposed CLL cells22. In vitro, stroma-exposed CLL cells were highly resistant to ABT-737 and only underwent modest levels of apoptotic killing with GS1101 alone. The combination of GS1101 and ABT-737 led to rapid and significant CLL cell killing. We showed that GS1101 also led to release of CLL cells from stroma, thereby increasing the level of CLL cell apoptotic priming, which may have accounted for their increased susceptibility to killing by BH3-mimetics. Ongoing investigation will be needed to confirm whether these observations hold true in the clinic.
Clinical
GS1101 (formerly CAL-101)
GS1101 was first evaluated in a large phase I study of approximately 190 patients with relapsed refractory hematologic malignancies28. The CLL subjects on this study (n=55) were heavilty pre-treated (median 5 prior therapies) and the majority (82%) had bulky lymphadenopathy. The most common symptomatic adverse event was grade 3 or higher pneumonia in 24% of patients. Grade 3 or higher hematological laboratory abnormalities included neutropenia (24%), thrombocytopenia (11%), and anemia (8%), which in most cases were not considered GS1101-related. Nodal partial response was seen in greater than 80% of patients, but the overall response rate was a modest 24% due to the fact that over half of the patients had an early, transient elevation in lymphocyte count thought to be related to mobilization of lymphocytes out of stroma. This redistribution lymphocytosis was associated with nodal response and did not represent disease progression, but it did preclude many patients from meeting iwCLL response criteria29. Patients did not appear to have any ill effects from persistent lymphocytosis. High risk patients such as those with del(17p) had similar clinical benefit, at least initially, as those with standard risk disease. The median progression free survival (PFS) reported thus far is 16 months, and 21 patients remain on study after 1 year with continued benefit, suggesting that responses to GS1101 can be durable. Given the important role that chemokines such as CCL3, CCL4, and CXCL13 play in the CLL microenvironment, it was notable that patients on GS1101 in this study experienced a rapid decline in plasma concentrations of these chemokines21. In addition, constitutive phosphorylation at AKT T308 was observed at baseline, and completely abrogated by GS1101. Both of these observations suggest pharmacodynamic inhibition of activated PI3K signaling.
GS1101 has substantial single agent activity, but the effect of the drug in mobilizing CLL cells from stroma also makes it a natural partner for combination studies, several of which are now underway. Initial data from a phase I study of GS1101 in combination with rituximab or bendamustine in patients with relapsed refractory CLL was recently presented30. These combinations have been well tolerated and have shown an impressive overall response rate of greater than 75% (by iwCLL criteria) in the initial 27 patients on study. Lymphocyte redistribution has been less apparent, particularly with bendamustine, likely due to the fact that mobilized CLL cells are killed rapidly. Registration studies for GS1101 are now underway, and other novel combinations with chemotherapy, antibodies, or other small molecule inhibitors will also be explored in both the frontline and relapsed settings.
Pan-PI3K Inhibition
Like PI3Kδ, the other class IA PI3K isoforms p110-alpha and p110-beta, as well as the class IB isoform p110-gamma are also expressed in leukocytes, including CLL cells; however, due to their widespread expression in other cell types, pan-PI3K inhibitors were expected to have too much toxicity to move forward into the clinic. Early animal data did suggest important potential toxicities including hyperglycemia due to inhibition of PI3K-alpha in the beta islet cells of the pancreas31. However, the introduction of pan-PI3K inhibitors into clinical trials in solid tumors demonstrated that these compounds were relatively well-tolerated 32, and raised the possibility of exploring their use in CLL.
Our group recently showed through an integrative genomic analysis that not only is PI3K-alpha expressed in CLL cells, but some CLL patients may also have amplification of the PI3KCA gene that leads to enrichment of the alpha subunit in their CLL cells11. Alpha subunit enrichment in this subset of CLL patients could theoretically render their disease resistant to a delta-isoform specific inhibitor. Furthermore, upregulation of alternative PI3K isoforms while on treatment with a PI3Kδ-specific inhibitor would be one possible way for patients to acquire drug resistance over time. These considerations raise the possibility that a pan-PI3K inhibitor may even have superior activity compared to a delta-isoform inhibitor.
Moreover, p110-gamma is known to be the predominant PI3K isoform expressed in T cells. For example, p110-gamma isoform knockout mice have an isolated T cell defect that, while not embryonically lethal, does confer significant immune dysfunction33. Given the critical role played by T cells in providing pro-survival signals to CLL cells in the microenvironment, p110-gamma isoform inhibition may significantly disrupt the CLL microenvironment, thereby facilitating CLL cell death.
Clinical
SAR245408 (S08)
SAR245408 (S08) is a class I pan-PI3K inhibitor that was previously found to be welltolerated in patients with solid tumors32. This initial study of 68 solid tumor patients found a DLT of grade 3 rash (4%) when dosing was scheduled on days 1–21 in 28-day cycles. When the schedule was switched to continuous daily dosing, no further DLTs were observed. SAR245408 (S08) was found to inhibit both the PI3K and ERK signaling pathways, suggesting that the drug was pharmacodynamically active. An arm focused on relapsed CLL and lymphoma was added to this study, and 25 total patients were enrolled. At the first report, of 7 CLL/SLL patients, 5 were evaluable. In these 5 patients with refractory CLL, SAR245408 (S08) was well-tolerated34. Although none of the 5 patients met formal iwCLL criteria for response, 3 patients (60%) benefited from a nodal partial response with transient lymphocytosis and remained on treatment at 12–18 month follow-up. Though these early results in CLL have been promising, the availability of SAR245408 (S08) for clinical trials is currently limited due to a change in the drug formulation.
Dual PI3K/mTOR Inhibition
Preclinical
CLL cells may also develop resistance to a delta-isoform specific PI3K inhibitor through activation of the RAS/MEK/ERK pathway, which eventually leads to mTOR activation35. The disappointing activity of mTOR inhibitors as monotherapy in CLL5 has raised the question of whether PI3K preferentially activates alternative downstream messengers such as NF-KB or PKCβ, thereby leading to resistance to mTOR inhibition. A drug able to inhibit both PI3K and mTOR would have the potential to overcome this type of resistance mechanism. Furthermore, since PI3K inhibitors directly induce apoptosis in CLL cells, and mTOR inhibitors primarily cause induction of growth arrest, these complementary mechanisms of action further justify the potential utility of such a combined blockade approach. Therefore, a dual pan-PI3K/mTOR inhibitor has the potential to be highly active in CLL.
Clinical
SAR245409 (S09)
SAR245509 (S09) is a small molecule dual PI3K/mTOR inhibitor currently being evaluated in clinical trials, which although primarily a pan-PI3K inhibitor, does have some activity against mTOR. In a PTEN-deficient mantle cell lymphoma cell line, the drug inhibited PI3K and ERK, and led to a marked decrease in proliferation markers such as Ki-6736. SAR245509 (S09) was relatively well-tolerated in a phase I dose-escalation study of patients with advanced solid tumors37. A phase I dose expansion cohort of 16 patients with non-Hodgkin lymphoma found the most common related adverse events to be nausea (25%), elevated liver enyzmes (18.8%) and diarrhea (12.5%). Two patients with mantle cell lymphoma remained on study with clinical benefit for over 1 year36. The drug is therefore now being explored at the recommended phase 2 dose of 50 mg BID in a large, multicenter phase II study in patients with CLL, follicular, and mantle cell lymphomas. A phase I study of SAR245509 (S09) in combination with bendamustine and/or rituximab in the same patient population is also underway. Additional combination studies of SAR245509 (S09) are also in development.
PREDICTING RESPONSE TO PI3K INHIBITION
Nodal response rates have been high in the early trials of GS1101, yet a substantial minority of patients either does not respond or progresses relatively shortly after starting on therapy. Therefore, in parallel with ongoing clinical trials of PI3K inhibitors are efforts to develop predictive biomarkers for response to these drugs. Both conventional CLL prognostic markers (such as ZAP-70 and immunoglobulin heavy chain [IGHV] status) as well as novel biomarkers are currently being evaluated.
ZAP-70 is a cytoplasmic tyrosine kinase, normally associated with the T cell receptor, which is aberrantly upregulated in the malignant B cells of a subset of patients with CLL38. ZAP-70 positivity (defined as >20% expression) is associated with activation of the BCR pathway, unmutated IGHV status, and clinically more aggressive CLL. ZAP-70 may also increase CLL cell responsiveness to the chemokines CCL19, CCL21, and CXCL12, thereby leading to increased CLL cell motility39. Given these observations that increased ZAP- 70 expression is associated with signaling through the BCR pathway, it will be interesting to see whether PI3K inhibitors will be more effective in patients with ZAP-70 positive CLL.
Unmutated IGHV status predicts a shorter time to first treatment and overall worse prognosis compared to mutated IGHV status40. Pre-clinical data have suggested that mutation status may serve as a biomarker of response to PI3K inhibition. In response to IgM–mediated BCR stimulation in vitro, functional gene groups including signal transduction, transcription, cell-cycle regulation, and cytoskeletal organization were all up-regulated in unmutated but not mutated CLL cases41. Furthermore, IGHV unmutated CLL cells cultured ex vivo are more prone to spontaneous apoptosis and more dependent on stromal prosurvival signals from cell-to-cell contact and soluble factors compared to mutated CLL cells42. We have also shown that IGHV unmutated CLL cells have increased levels of mitochondrial apoptotic priming as compared to their mutated counterparts22. All of these observations suggest that patients with unmutated IGHV would benefit preferentially from PI3K inhibitors compared to mutated patients, and detailed reports from the clinical trials of PI3K inhibitors should demonstrate whether this is true.
Novel biomarkers of response to PI3K inhibition are also in development. Of these, the leading candidate is CCL3, a chemokine produced by both normal and malignant lymphocytes that acts through the chemokine receptors CCR1 and CCR5 as a chemoattractant for other lymphocytes and adaptive immune cells43. When the BCR pathway is activated, CCL3 is secreted in high levels by CLL cells44, particularly in lymph node-derived CLL45, suggesting that stroma enhances CCL3 expression. Based on these observations, agents that target the BCR pathway and disrupt stromal support signals should theoretically lead to decreased CCL3 levels. Indeed, PI3K-δ inhibition21 has been shown to decrease CLL cell secretion of CCL3 in a nurselike cell model in vitro. Moreover, patients treated with GS1101 were found to normalize their CCL3 levels in the peripheral blood after 28 days21. These preclinical and clinical findings make CCL3 a promising predictive biomarker for PI3K inhibitors; however, it should be noted that since nearly all patients normalized CCL3 levels, it is not clear that CCL3 level alone will be predictive of clinical response. In addition to measurement of CCL3, future trials will also incorporate genetic biomarker evaluations, such as the novel mutations SF3B1, NOTCH1, MYD88 and others recently described in CLL10, 46, 47.
Utilizing both conventional and novel biomarkers will hopefully help to optimize the use of PI3K inhibitors by targeting the use of these agents to those patients who will benefit most. Utilizing these agents in high-risk CLL patients who typically have short-lived responses to chemotherapy, especially those who are ZAP-70 positive and IGHV unmutated, may potentially represent an important therapeutic advance in CLL.
CONCLUSION
An improved understanding of the key molecular pathways fueling the survival and growth of CLL cells is an important advance that heralds the development of selective, well-tolerated, and effective therapies. PI3K, within the BCR pathway, is a key node being targeted. The unique pattern of nodal response with redistribution lymphocytosis observed with BCR pathway inhibitors including PI3K inhibitors may serve both as a pharmacodynamic marker and also facilitate potent combination therapies. Given that the iwCLL 2008 response criteria were devised prior to knowledge of the lymphocyte redistribution effect of BCR pathway antagonists, the currently reported formal response rates for these agents underestimate their clinical benefit. For this reason, leading investigators have proposed modifying the traditional response criteria to recognize the benefits patients derive from nodal response in the presence of persistent lymphocytosis48. Given the significant toxicities associated with modern chemoimmunotherapy regimens, the approval of BCR pathway-targeted agents such as PI3K inhibitors would represent a new therapeutic paradigm. If PI3K inhibitors could be used either as monotherapy or in combination with other novel agents to achieve long term disease remission without the toxicity and inconvenience of chemotherapy, this would provide significant benefit for CLL patients, particularly for those unable to tolerate chemoimmunotherapy, such as older patients or those with comorbidities.
Despite the excitement about these new agents, several important questions need to be addressed in future studies. For example, which approach to PI3K inhibition will strike the best balance between efficacy and toxicity--delta-isoform selective or pan-PI3K inhibitors? Why are certain patients resistant to PI3K inhibitors at baseline, and which resistance mechanisms will evolve in patients who initially respond to treatment? How durable will the responses observed in early phase trials be? Will PI3K inhibitors be effective enough as monotherapy or in combination with antibodies to obviate the need for chemotherapy in CLL, or will they need to be combined with chemotherapy to achieve a durable effect? Is there a role for PI3K inhibitors as maintenance in the post chemoimmunotherapy or post allogeneic stem cell transplantation setting?
These challenging but important questions will need to be answered with carefully constructed clinical trials. Equally essential to the successful future development of PI3K inhibitors will be the correlative studies embedded within these trials, which will help us learn how best to combine novel agents, and what mechanisms of resistance may arise. PI3K inhibitors are one of several exciting new approaches to the treatment of CLL. Moving forward, the expected approval of such targeted therapies will be a major advance for patients with CLL.
Key Points.
PI3K is a key node in B cell receptor (BCR) signaling.
The biology of PI3K signaling provides a strong rationale for targeting this kinase in CLL.
Delta-isoform inhibitors such as GS1101 (formerly CAL-101), pan-PI3K inhibitors such as SAR245408 (S08), and dual pan-PI3K/mTOR inhibitors such as SAR245409 (S09) are all in development.
Early phase clinical trials have found these agents to be highly active and well-tolerated.
ZAP-70, IGHV, and CCL3 are all potential biomarkers for response to PI3K inhibitors.
The place of PI3K inhibitors in the landscape of CLL therapy is evolving.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. The New England journal of medicine. 2005;352(8):804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114(16):3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103(12):4389–4395. doi: 10.1182/blood-2003-12-4312. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13):2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zent CS, LaPlant BR, Johnston PB, et al. The treatment of recurrent/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) with everolimus results in clinical responses and mobilization of CLL cells into the circulation. Cancer. 2010;116(9):2201–2207. doi: 10.1002/cncr.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34(Pt 5):647–662. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- 9.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30(4):194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Lawrence MS, Wan Y, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365(26):2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JR, Hanna M, Tesar B, et al. Integrative Genomic Analysis Implicates Gain of PIK3CA at 3q26 and MYC at 8q24 in Chronic Lymphocytic Leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(14):3791–3802. doi: 10.1158/1078-0432.CCR-11-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nature reviews. Immunology. 2006;6(4):283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 14.Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- 15.Koyasu S. The role of PI3K in immune cells. Nature immunology. 2003;4(4):313–319. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- 16.Chantry D, Vojtek A, Kashishian A, et al. p110delta, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. The Journal of biological chemistry. 1997;272(31):19236–19241. doi: 10.1074/jbc.272.31.19236. [DOI] [PubMed] [Google Scholar]
- 17.So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem J. 2012;442(3):465–481. doi: 10.1042/BJ20112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinohara H, Kurosaki T. Comprehending the complex connection between PKCbeta, TAK1, and IKK in BCR signaling. Immunological reviews. 2009;232(1):300–318. doi: 10.1111/j.1600-065X.2009.00836.x. [DOI] [PubMed] [Google Scholar]
- 19.Uriarte SM, Joshi-Barve S, Song Z, et al. Akt inhibition upregulates FasL, downregulates c-FLIPs and induces caspase-8-dependent cell death in Jurkat T lymphocytes. Cell death and differentiation. 2005;12(3):233–242. doi: 10.1038/sj.cdd.4401549. [DOI] [PubMed] [Google Scholar]
- 20.She QB, Solit DB, Ye Q, O'Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer cell. 2005;8(4):287–297. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3'-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davids MS, Deng J, Wiestner A, et al. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012 doi: 10.1182/blood-2012-02-414060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanhaesebroeck B, Welham MJ, Kotani K, et al. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4330–4335. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niedermeier M, Hennessy BT, Knight ZA, et al. Isoform-selective phosphoinositide 3'-kinase inhibitors inhibit CXCR4 signaling and overcome stromal cell-mediated drug resistance in chronic lymphocytic leukemia: a novel therapeutic approach. Blood. 2009;113(22):5549–5557. doi: 10.1182/blood-2008-06-165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman SE, Lapalombella R, Gordon AL, et al. The role of phosphatidylinositol 3-kinase-delta in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood. 2011;117(16):4323–4327. doi: 10.1182/blood-2010-11-315705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YK, Shanafelt TD, Bone ND, Strege AK, Jelinek DF, Kay NE. VEGF receptors on chronic lymphocytic leukemia (CLL) B cells interact with STAT 1 and 3: implication for apoptosis resistance. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2005;19(4):513–523. doi: 10.1038/sj.leu.2403667. [DOI] [PubMed] [Google Scholar]
- 27.Konig A, Menzel T, Lynen S, et al. Basic fibroblast growth factor (bFGF) upregulates the expression of bcl-2 in B cell chronic lymphocytic leukemia cell lines resulting in delaying apoptosis. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 1997;11(2):258–265. doi: 10.1038/sj.leu.2400556. [DOI] [PubMed] [Google Scholar]
- 28.Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM, et al. CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110d Demonstrates Clinical Activity and Pharmacodynamic Effects in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. ASH. 2010 [Google Scholar]
- 29.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharman J, de Vos S, Leonard J, Furman R, et al. A Phase 1 Study of the Selective PI3K Inhibitor CAL-101 (GS-1101) in Combination with Rituximab and/or Bendamustine in Patients with Relapsed or Refractory CLL. ASH. 2011 [Google Scholar]
- 31.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer cell. 2003;4(4):257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 32.Edelman G, Bedell G, Shapiro SS, Pandya EL, Cwak C, et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients with advanced malignancies. ASCO. 2010 [Google Scholar]
- 33.Martin AL, Schwartz MD, Jameson SC, Shimizu Y. Selective regulation of CD8 effector T cell migration by the p110 gamma isoform of phosphatidylinositol 3-kinase. Journal of immunology. 2008;180(4):2081–2088. doi: 10.4049/jimmunol.180.4.2081. [DOI] [PubMed] [Google Scholar]
- 34.Brown JR, Davids MS, Rodon J, Abrisqueta P, DeCillis AP, et al. Phase I Trial of SAR245408 (S08), a Pan-PI3K Inhibitor, in Patients with CLL and Lymphoma. ASH. 2011 [Google Scholar]
- 35.Drakos E, Rassidakis GZ, Medeiros LJ. Mammalian target of rapamycin (mTOR) pathway signalling in lymphomas. Expert Rev Mol Med. 2008;10:e4. doi: 10.1017/S1462399408000586. [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulos K, Abrisqueta P, Chambers G, et al. A Phase I Dose Escalation Expansion Cohort Study of the Safety, Pharmacokinetics and Pharmacodynamics of SAR245409 (S09), an Orally Administered PI3K/mTOR Inhibitor, in Patients with Lymphoma. American Society of Hematology Annual Meeting; San Diego, CA. 2011. [Google Scholar]
- 37.Lorusso P, Markman J, Tabernero R, Shazer L, Heath E, et al. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765, a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced solid tumors. ASCO. 2009 [Google Scholar]
- 38.Wiestner A, Rosenwald A, Barry TS, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101(12):4944–4951. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 39.Richardson SJ, Matthews C, Catherwood MA, et al. ZAP-70 expression is associated with enhanced ability to respond to migratory and survival signals in B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2006;107(9):3584–3592. doi: 10.1182/blood-2005-04-1718. [DOI] [PubMed] [Google Scholar]
- 40.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–1854. [PubMed] [Google Scholar]
- 41.Guarini A, Chiaretti S, Tavolaro S, et al. BCR ligation induced by IgM stimulation results in gene expression and functional changes only in IgV H unmutated chronic lymphocytic leukemia (CLL) cells. Blood. 2008;112(3):782–792. doi: 10.1182/blood-2007-12-127688. [DOI] [PubMed] [Google Scholar]
- 42.Coscia M, Pantaleoni F, Riganti C, et al. IGHV unmutated CLL B cells are more prone to spontaneous apoptosis and subject to environmental prosurvival signals than mutated CLL B cells. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2011;25(5):828–837. doi: 10.1038/leu.2011.12. [DOI] [PubMed] [Google Scholar]
- 43.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177(6):1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burger JA, Quiroga MP, Hartmann E, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113(13):3050–3058. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 475(7354):101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borchmann P, Eichhorst B, Hellmann M, et al. [Hematology 2008] Dtsch Med Wochenschr. 2008;133(25–26):1400–1404. doi: 10.1055/s-2008-1081088. [DOI] [PubMed] [Google Scholar]
- 48.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(23):2820–2822. doi: 10.1200/JCO.2012.43.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]