SUMMARY
Activation of the transcription factor NF-κB is essential to innate immune function and requires strict regulation. The micronutrient zinc modulates proper host defense and zinc deficiency is associated with elevated inflammation and worse outcomes in response to bacterial infection and sepsis. Previous studies suggest that zinc may regulate NF-κB activity during innate immune activation but a mechanistic basis to support this is lacking. Herein we report that the zinc transporter, SLC39A8 (ZIP8), is a transcriptional target of NF-κB and functions to negatively regulate pro-inflammatory responses through zinc-mediated down-modulation of IKK activity in vitro. Accordingly, fetal fibroblasts obtained from Slc39a8 hypomorphic mice exhibited dysregulated zinc uptake and increased NF-κB activation. Consistent with this, mice provided zinc-deficient dietary intakes developed excessive inflammation to polymicrobial sepsis in conjunction with insufficient control of IKK. Our findings identify a novel negative feedback loop that directly regulates innate immune function through coordination of zinc metabolism.
INTRODUCTION
The innate immune system constitutes the front line of host defense by triggering inflammation, a primordial response designed to protect the host against pathogen invasion (Takeuchi and Akira, 2010). Upon recognition of pathogen-associated molecular patterns, the Toll-like receptor (TLR) pathway becomes activated in immune cells that include monocytes, macrophages, dendritic cells, and nonprofessional cells such as lung epithelia (Kawai and Akira, 2010). Inflammatory mediators are then rapidly released to further alert the remainder of the immune system. TLR signaling initiates recruitment of adaptor molecules such as TRIF, TIRAP and MyD88 (Takeuchi and Akira, 2010). In turn, the danger signal is transmitted coordinately through a series of molecular events that involve the IRAK family, TRAF6 and TAK1, leading to activation of IκB kinase (IKK) and mitogen-activated protein kinases (MAPKs) (Hayden and Ghosh, 2008; Johnson and Lapadat, 2002). Activation of the IKK complex, which includes IKKα, IKKβ and NEMO, results in IκB phosphorylation and degradation, thereby allowing phosphorylated NF-κB dimers to translocate into the nucleus and bind κB sites located within target gene promoters to activate transcription (Hayden and Ghosh, 2008). Simultaneously, activation of MAPKs up-regulates ERKs, JNKs, and p38, leading to the activation of the transcriptional factor AP-1 (Johnson and Lapadat, 2002). IKKβ can also activate ERKs through crosstalk (Tpl2-MEK1/2) between the NF-κB and MAPKs/AP-1 pathways (Banerjee et al., 2006; Waterfield et al., 2004)
Coordination of the initial host response to infection through regulation of the NF-κB and MAPK pathways must be tightly regulated in order to maintain proper immune balance, thereby maximizing host defense while simultaneously minimizing collateral damage (Liew et al., 2005). In order to achieve precise balance, multiple counter-regulatory elements have evolved within these pathways that include but are not limited to IκBα (Chiao et al., 1994), MyD88s (Burns et al., 2003), IRAKM (Kobayashi et al., 2002), A20 (Boone et al., 2004) and NLRC5 (Cui et al., 2010). The expression and function of many of these negative regulators including IκBα, A20, MyD88s and IRAKM are themselves activated by TLR ligands and thus constitute classic negative regulatory feedback loops that ensure attenuation of the TLR response in a threshold-dependent manner (Ruland, 2011).
Sepsis is the leading cause of death in critically ill patients in the United States (Angus et al., 2001) and its incidence has increased over the past two decades (Martin et al., 2003). A major cause of sepsis-related morbidity and mortality is overwhelming inflammation, referred to as the “cytokine storm”, driven by the excessive production of inflammatory mediators within hours of pathogen invasion (Hotchkiss and Karl, 2003; Warren, 1997). The magnitude of the innate immune response to infection directs the cumulative host response with respect to tissue injury and survival (Abraham and Singer, 2007). Importantly, sepsis patients that encounter an exaggerated initial inflammatory response are more susceptible to tissue injury and mortality, but it remains unclear why or how this occurs (Parrillo, 1993).
Zinc, an essential trace element, facilitates the coordination of innate and adaptive immunity (Rink and Haase, 2007). Zinc deficiency causes immune dysfunction resulting in increased morbidity and mortality following infection (Caulfield et al., 2004), whereas zinc supplementation prevents the incidence of infectious diseases and improves immune function (Brooks et al., 2005; Prasad et al., 2007). Zinc metabolism is primarily coordinated by zinc transporters. In mammals, these transmembrane-spanning proteins are encoded by two solute-linked carrier (SLC) gene families: that include fourteen SLC39 (a.k.a. ZIP) family members and that include ten SLC30 (a.k.a. ZnT) family members. SLC39 transporters increase cytosolic zinc content by promoting extracellular uptake or release from subcellular organelles, whereas SLC30 transporters function as counter-regulators that decrease intracellular zinc levels (Lichten and Cousins, 2009).
Despite recent advances (Aydemir et al., 2009; Kitamura et al., 2006; Nishida et al., 2009; Yu et al., 2011), much remains unknown regarding the influence of zinc transporters on zinc metabolism relative to innate immunity (Lichten and Cousins, 2009). Zinc metabolism changes rapidly in response to systemic infection in humans, leading to rapid mobilization of intravascular zinc into vital organs with a consequent decrease in circulating plasma zinc levels (hypozincemia) (Cousins and Leinart, 1988; Gaetke et al., 1997; Sobocinski et al., 1978). Zinc redistribution is primarily driven by induction of key zinc transporters, as exemplified by the up-regulation of Slc39a14 (ZIP14) mediated by IL-6 (Liuzzi et al., 2005). Importantly, lower than expected circulating zinc levels correlate with higher mortality in septic humans (Wong et al., 2007) although the consequences of altered zinc metabolism in this setting are not yet known.
Using a mouse model of polymicrobial sepsis, we first reported that zinc deficiency increases systemic inflammation and mortality, whereas zinc supplementation suppresses inflammation and improves survival, suggesting that zinc metabolism plays an important regulatory role during the early stage of sepsis (Bao et al., 2010; Knoell et al., 2009). Our group and others have identified SLC39A8 (ZIP8) as the most significantly up-regulated transporter in response to cytokines, bacteria and sepsis, suggesting its unique role in innate immune function (Begum et al., 2002; Besecker et al., 2008; Besecker et al., 2011). Herein, we report the discovery that ZIP8 is a negative feedback regulator of NF-κB and innate immune activation in response to infection through coordination of zinc metabolism. These novel findings help to bridge an existing gap in our fundamental understanding of how zinc affects innate immunity and host defense.
RESULTS
Pro-inflammatory stimuli induce SLC39A8 expression in an NF-κB dependent manner, resulting in zinc influx
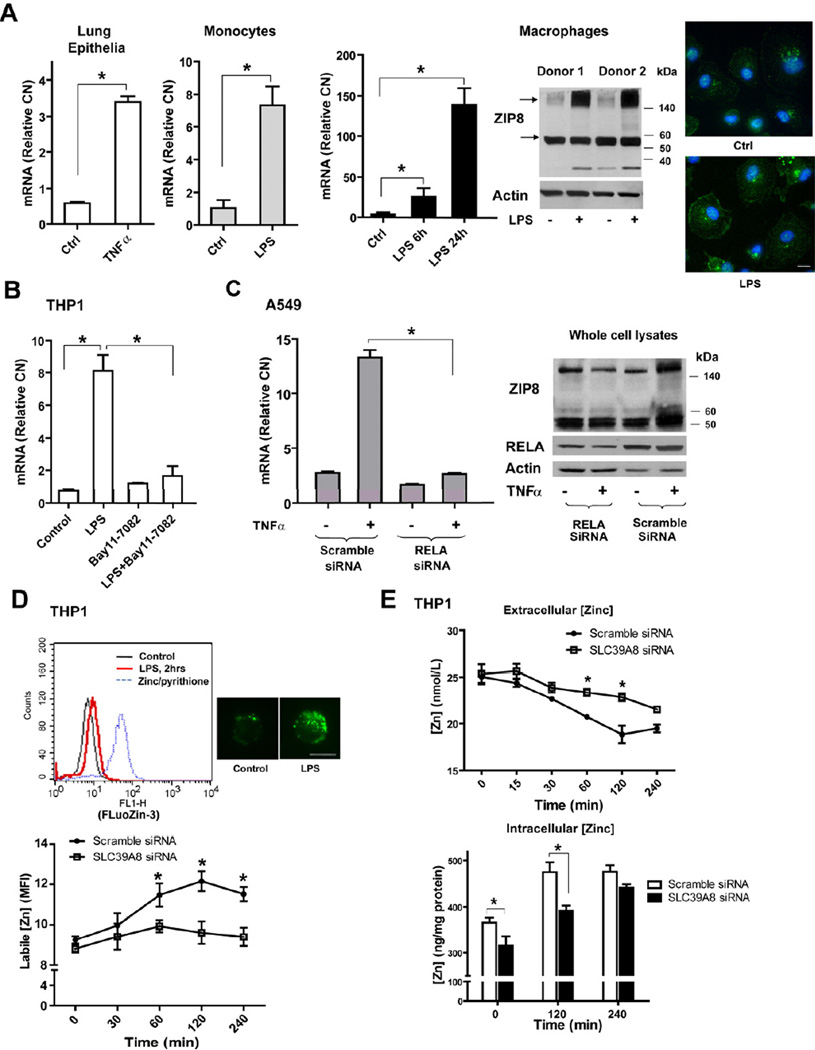
SLC39A8 expression is up-regulated in primary human lung epithelia, monocytes and macrophages in response to TNFα or LPS. SLC39A8 (ZIP8) protein localizes mainly to the plasma membrane, in addition to lysosomal or mitochondrial membranes (Aydemir et al., 2009; Besecker et al., 2008). ZIP8 is heavily glycosylated following stimulation, leading to increased expression of a high molecular weight, membrane-associated protein (Figure 1A and Figure S1A–B), suggesting that additional post-transcriptional (or translational) regulatory events are at play in controlling protein function (that will require further study). Mouse Slc39a8 possesses 89% amino acid sequence and 96% ZIP domain sequence identity with human SLC39A8, implying a high degree of conservation (Girijashanker et al., 2008). As expected, LPS also induced Slc39a8 expression in mouse RAW 267.4 cells (Figure S1C). We next examined whether SLC39A8 expression is NF-κB dependent. Suppression of NF-κB by pharmacologic inhibition or RELA-specific siRNA significantly inhibited SLC39A8 expression induced by LPS or TNFα (Figure 1B–C).
Figure 1. SLC39A8 expression is induced by pro-inflammatory stimuli in a NF-κB-dependent manner and regulates zinc uptake.
(A) Human primary lung epithelia were treated with TNFα (10 ng/mL) for 16 hrs (n=3); human primary monocytes were treated with LPS 1.µg/mL) for 4 hrs (n=4); CD14+ monocyte-derived primary macrophages were treated with LPS (1 µg/mL) for 6 and 24 hrs. SLC39A8 mRNA levels were determined by real-time PCR analysis (Relative CN: relative copy number). Western blot was performed on membrane fractions with ZIP8 antiserum. Immunofluorescence staining shows ZIP8 localization (green) in macrophages, counterstained with DAPI (Scale bar: 10 µm). (B) THP1 cells were treated with LPS (1 µg/mL) for 4 hrs with Bay11–7082 (20 µM), added 30 min prior to LPS. (C) A549 cells were treated with TNFα (10 ng/mL) in combination with RELA siRNA (40 pmol), which was transfected 24 hrs before TNFα treatment. (D) Control siRNA or SLC39A8 siRNA -treated THP1 cells were stimulated with LPS (1 µg/mL), followed by FluoZin-3 staining. Intracellular labile zinc was quantified by flow cytometry (MFI, Mean fluorescence intensity). Representative composite flow cytometric histograms are shown and ZnSO4 (10 µM) /pyrithione (10 µM) served as a positive control. Two representative FluoZin-3 staining images are shown (Scale bar: 10 µm). Western analysis of ZIP8 silencing is shown in Figure 3A. (E) Total zinc levels in culture medium and cell pellets were detected by AAS or ICP-OES, respectively.
Since ZIP8 is a zinc importer, we then determined whether changes in ZIP8 expression result in changes in intracellular zinc content. Using the cell-permeable zinc indicator FluoZin-3, we revealed that intracellular labile zinc levels rapidly increased in response to LPS, but was significantly impaired in ZIP8-silenced cells (Figure 1D), indicating that ZIP8 plays an important role in regulating zinc uptake shortly following TLR activation. The representative pictures depict the unique pattern of intracellular labile zinc. We further observed a decrease in total zinc content in the culture medium with a corresponding increase within cells, establishing that elevation of intracellular labile zinc content occurs following transport across the plasma membrane via ZIP8 (Figure 1E). As expected, we also observed a significant increase in intracellular labile zinc levels when ZIP8 was over-expressed (Figure S1D).
SLC39A8 transcription is directly regulated by NF-κB
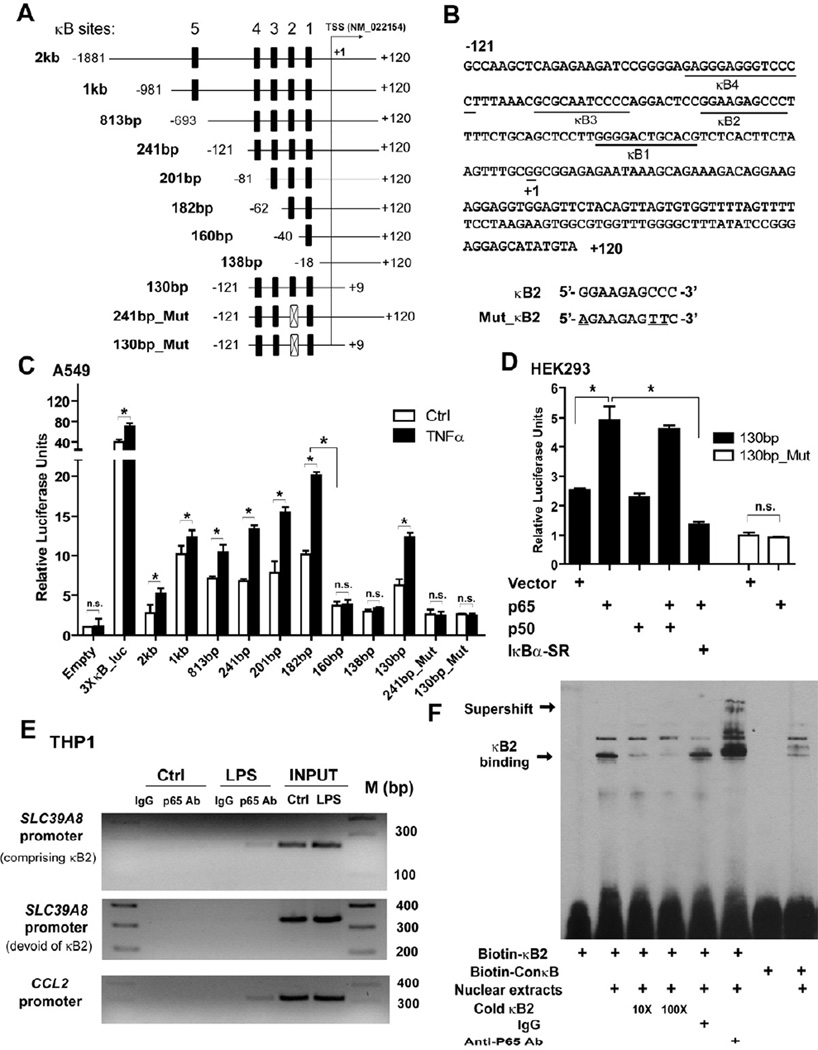
Inspection of the human SLC39A8 gene sequence revealed four potential transcript variants, indicating that its transcription may be initiated at multiple transcriptional start sites (TSS). Based on this, 5’RACE (rapid amplification of cDNA ends) was conducted and revealed two TSSs but only one was induced by TNFα (Figure S2A–C). The inducible TSS was in close proximity to the known TSS of the reference sequence (NM_022154.5) Based upon their close proximity, the known TSS served as the basis of subsequent studies (Aiba et al., 2008).
NF-κB activates gene transcription following physical interaction with the κB binding motif located within the regulatory region of target genes. Analysis of the human SLC39A8 gene (Match™) revealed five potential putative κB binding sites in the 5’-flanking region (Figure 2A). The sequences of κB 1–4 are shown in Figure 2B. To determine whether the κB sites are transcriptionally active, a 2 kilobase (kb) promoter fragment (−1881 to +120) of human genomic DNA was amplified and cloned into a pGL3 luciferase reporter. A series of plasmids with progressively truncated promoter regions were subsequently developed and used to locate a 100-bp region proximal to the TSS, containing κB 1–4, which was required for promoter activity (Figure 2A, C). Serial deletion of κB regions 1 through 4 identified κB2 as the critical binding site. Loss or site-mutagenesis of κB2 resulted in a decrease of both constitutive and inducible activity (Figure 2C and Figure S2D). Ectopic expression of p65 also induced κB2-mediated expression, whereas the IκBα-super repressor (SR) inhibited induction. Confirmation of the κB2 site was achieved by site directed-mutagenesis (Figure 2C–D). Chromatin immunoprecipitation (ChIP) analysis demonstrated in vivo binding of NF-κB p65 to the SLC39A8 promoter region and EMSA analysis revealed physical binding of κB2 oligonucleotides with activated NF-κB (p65) in vitro (Figure 2E–F and Figure S2E–F).
Figure 2. Transcriptional activation of the human SLC39A8 promoter by NF-κB.
(A) Schematic diagram depicts a 2 kb SLC39A8 promoter region and its serial deletion constructs that were cloned into a pGL3-Basic vector. (B) Sequence of the key promoter region and four potential putative NF-κB-binding sites. (C) Constructs were transfected into A549 cells, followed by TNFα (10 ng/mL) treatment for 24 hrs. Serial deletion identified κB2 as the critical binding site. The NF-κB reporter 3×κB-luc served as the positive control. Site-directed mutagenesis of the κB2 site resulted in the loss of promoter activity. (D) Ectopic expression of p65, but not p50, induced promoter activity of the 130-bp construct, which was further inhibited by IκBα-SR. Moreover, a mutated construct (130-bp_Mut) was not responsive to p65. (E) ChIP-based PCR analysis of p65-bound chromatin DNA, which was immunoprecipitated from THP1 cells treated with vehicle or LPS (1 µg/mL) for 1 hr. A 234-bp band containing κB2 was amplified from the SLC39A8 promoter region. The adjacent region devoid of κB2 was amplified as a negative control. CCL2 served as a positive control. (F) EMSA revealed the binding of biotin-labeled κB2 with nuclear extracts from TNFα-treated A549 cells. Binding was inhibited by an excess of unlabeled κB2 oligonucleotides (10× and 100×). Co-incubation with an anti-p65 antibody produced a super-shift. The biotin-labeled NF-κB consensus sequence (ConκB) served as a positive control.
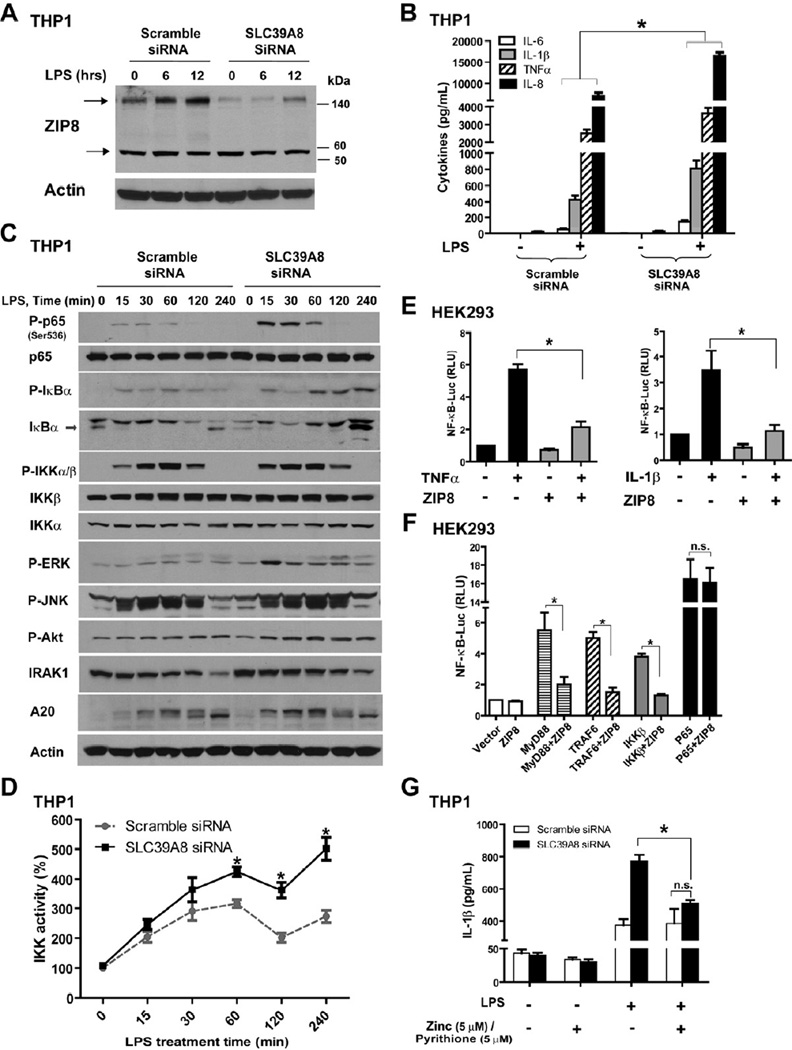
ZIP8 is a potent negative regulator of NF-κB activation
Transcriptional induction of ZIP8 by NF-κB revealed that ZIP8 may assist in coordination of host defense. We first determined the extent of immune activation following ZIP8 knockdown in human monocytes and lung epithelia. Under all conditions studied, suppression of ZIP8 expression resulted in increased production of pro-inflammatory mediators (cytokines) (Figure 3A–B and Figure S3A–B, S3E). Consistent with this, endogenous NF-κB binding activity was higher when ZIP8 expression was silenced in THP1 cells (Figure S3D). We then screened signaling effectors of the NF-κB and MAPK pathways (Figure 3C and Figure S3C). Suppression of ZIP8 expression increased phosphorylation of p65 and IκBα, two known substrates of IKKβ, suggesting that ZIP8 affects the activity of the IKK kinase complex. An increase in p-ERK, p-Akt and A20 levels was also observed. The minor change observed in phosphorylation of IKKα/β indicated that ZIP8 suppression did not significantly impact the activity of IKK upstream signaling intermediates. Consistent with this, IRAK1 was degraded with similar kinetics in both control and ZIP8-silenced cells. No appreciable differences in JNK phosphorylation were observed. We then immunoprecipitated the IKK complex and directly measured its activity. Suppression of ZIP8 significantly increased IKK activity in response to LPS, thereby providing evidence that ZIP8-mediated effects occur at or proximal to IKK (Figure 3D). Consistent with its role as a negative regulator, we observed that over-expression of ZIP8 inhibits NF-κB activation induced by TNFα or IL-1β (Figure 3E and Figure S3F). Knowing that over-expression of MyD88, TRAF6, IKKβ or p65 induce NF-κB (Cui et al., 2010), we co-transfected ZIP8 with each signaling molecule and found that overexpression of ZIP8 with either MyD88, TRAF6 or IKKβ, but not p65, inhibited NF-κB activation, indicating that the location of the ZIP8 effect is at or upstream of IKKβ (Figure 3F and Figure S3G). Taken together, these findings strongly indicate that ZIP8 negatively regulates NF-κB through down-modulation of IKK. Additionally, we observed that augmentation of NF-κB activation by ZIP8 knockdown was reduced by the addition of zinc in combination with pyrithione, an ionophore that facilitates zinc entry into the cytosol (Figure 3G). This observation led us to then determine whether the negative regulatory impact of ZIP8 on NF-κB is dependent on zinc.
Figure 3. ZIP8 modulates NF-κB activity and the inflammatory response.
(A and B) THP1 cells were transfected with either scrambled control or SLC39A8 siRNA for three days, followed by LPS (1 µg/mL × 6–12 hrs) treatment. ZIP8 silencing was evaluated by Western analysis. Cytokine levels in culture medium were determined by ELISA at 6 hrs. (C) Western analysis of signaling molecules in THP1 extracts following ZIP8 knockdown in combination with LPS treatment (P: phosphorylated). (D) IKK kinase activity was measured following immunoprecipitation of the IKK complex using an anti-IKKβ antibody. (E) ZIP8 over-expression inhibits NF-κB activation induced by TNFα or IL-1β. HEK293 cells were transfected with 3×κB-luc and ZIP8 cDNA construct, followed by treatment with TNFα (10 ng/mL) or IL-1β (20 ng/mL) for 24 hrs. (F) HEK293 cells were transfected with MyD88, TRAF6, IKKβ, or p65 expression plasmids together with the ZIP8 cDNA and 3×κB-luc constructs. Luciferase activity was measured 24 hrs after transfection. (G) Zinc addition normalized the extent of NF-κB activation in the cells subjected to ZIP8 knockdown. SiRNA knockdown was performed, followed by LPS (1 µg/mL) treatment for 8 hrs. Zinc (5 µM) and pyrithione (5 µM) were added 30 min prior to LPS.
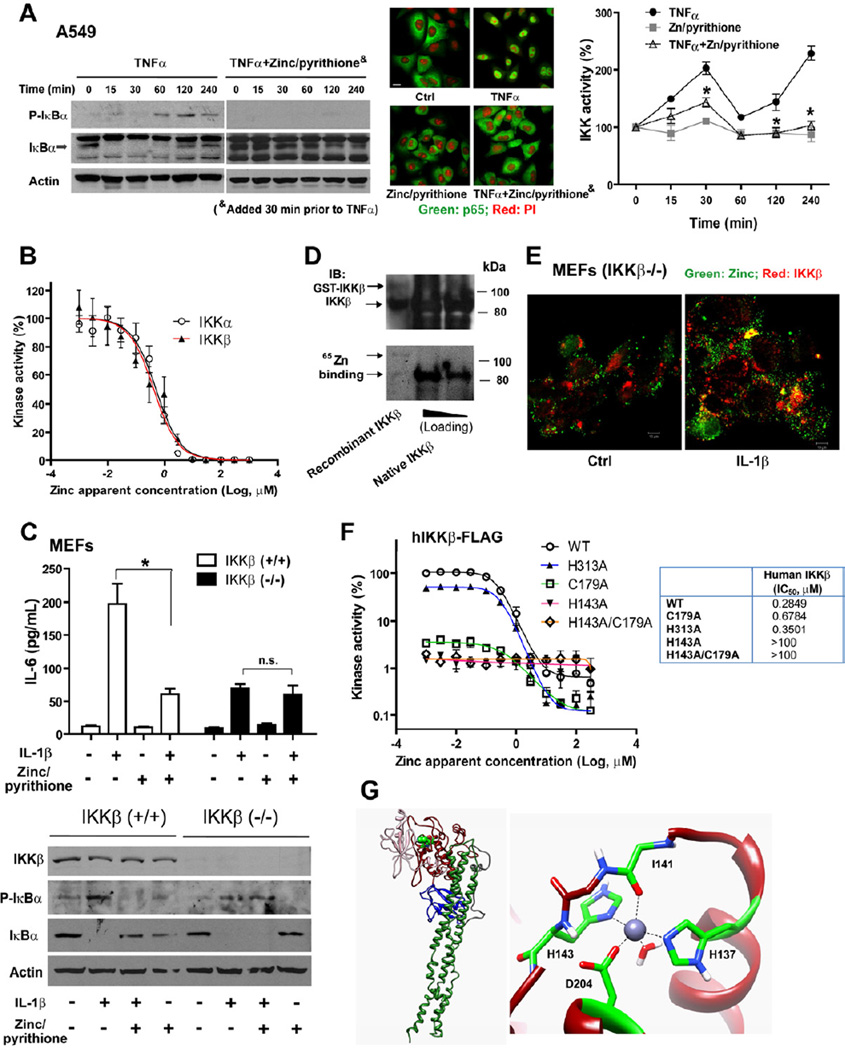
Zinc suppresses NF-κB activation through direct interaction with IKK
Zinc is known to inhibit NF-κB activation but the underlying mechanism remains unclear (Haase and Rink, 2009; Jeon et al., 2000). We observed that zinc significantly inhibited the expression of NF-κB-dependent transcripts or luciferase reporter (Figure S4A–B). The phosphorylation of IκBα was markedly inhibited and degradation of IκBα was nearly abolished, resulting in a significant reduction in p65 nuclear translocation. A significant time-dependent inhibition of IKK activity was also observed (Figure 4A). Meanwhile, zinc was not found to inhibit the activity of the E3 ligase SCFβ-TrCP complex that mediates IκBα ubiquitination and degradation (data not shown). We also observed that zinc inhibited NF-κB activity driven by overexpression of MyD88, TRAF6 and IKKβ, but not p65, mapping the location of the effect at IKK, identical to our previous findings involving ZIP8 co-transfection (Figure S4B). Given the remarkable similarity between ZIP8- and zinc-mediated effects that identified IKK complex as the target, we next determined whether zinc directly binds to and inhibits IKK. First we observed that recombinant IKKα or IKKβ was directly inhibited by zinc in a dose-responsive manner (IC50 for IKKα: 0.52 µM (95% CI: 0.42 to 0.64 µM); IC50 for IKKβ: 0.43 µM (95% CI: 0.32 to 0.58 µM) ) (Figure 4B). To understand whether zinc selectively inhibits IKKα and/or IKKβ, we exposed IKKβ (−/−) mouse embryonic fibroblast (MEFs) to IL-1β and evaluated NF-κB-mediated function. As expected, IKKβ (−/−) cultures exhibited impaired NF-κB activation and were less responsive to IL-1β when compared to IKKβ (+/+) cultures (Li et al., 1999). In particular, zinc/pyrithione inhibited IκBα phosphorylation and degradation as well as IL-6 production in IKKβ (+/+) cells, while these effects were attenuated in IKKβ (−/−) cultures (Figure 4C and Figure S4C). Although recombinant IKKα was inhibited by zinc, IKKβ (−/−) MEFs that contain IKKα did not exhibit zinc-mediated inhibition, indicating that the primary effect of zinc occurs directly through inhibition of IKKβ and/or IKKβ-related kinase complex formation. We also conducted similar experiments in NEMO (−/−) MEFs but did not observe zinc-mediated effects because cultures were completely unresponsive to pro-inflammatory cytokines (data not shown) (Rudolph et al., 2000).
Figure 4. Zinc inhibits NF-κB activation through IKK.
(A) Zinc (10 µM)/pyrithione (10 µM), added 30 min prior to TNFα (10 ng/mL), inhibits NF-κB activation in A549 cells: Western blot analysis (Figure S4A shows the entire blot); Immunofluorescent staining of p65 at 30 min after TNFα treatment (Scale bar: 10 µm); IKK kinase activity following immunoprecipitation of the IKK complex. (B) Zinc inhibits human recombinant IKKα and IKKβ activity in a dose-dependent manner. (C) Effect of zinc on IKKβ (−/−) MEFs. IKKβ (−/−) and (+/+) MEFs were treated with zinc (10 µM)/pyrithione (2 µM) for 30 min, followed by IL-1β (200 ng/mL) treatment. IL-6 was measured in culture medium by ELISA at 6 hrs. Western analysis was performed on samples at 30 min after IL-1β treatment. (D) A 65Zn-blotting assay was performed to evaluate zinc binding with IKKβ. Samples include recombinant IKKβ (cleaved and intact GST-fusion) and native IKKβ that was immunoprecipitated from TNFα-treated A549 cells. (E) Confocal image analysis identified co-localization of reconstituted IKKβ-DsRed and zinc (FluoZin-3, green). IKKβ (−/−) MEFs were treated with IL-1β (100 ng/mL) for 4 hrs. Figure S4F shows the images in multiple channels. (F) Dose-response curves of zinc inhibition on IKKβ and corresponding mutants. The IC50 values are shown on the right. (G) Model of zinc coordination in the kinase domain of human IKKβ. Left: the computationally derived model of the human IKKβ protomer, including the N- and C-lobe of the kinase domain (pink and red, respectively), the ubiquitin-like domain (blue), and the scaffold/dimerization domain (dark green). Highlighted in bright green is the zinc binding site in the C-lobe of the kinase domain. Right: Magnified view of the zinc binding site and contributing binding residues.
Next, we observed that zinc directly binds to IKKβ (Figure 4D and Figure S4D). Based on this, we then immunoprecipitated the IKKβ–associated protein complex from LPS-stimulated cultures and treated immunoprecipitates with the zinc-specific chelator TPEN to remove protein-bound zinc. TPEN chelation resulted in an increase of kinase activity, substantiating that zinc directly binds to IKK intracellularly and inhibits kinase activity (Figure S4E). Further, using confocal microscopy, we observed that overexpressed DS-Red-tagged IKKβ partially co-localized with intracellular labile zinc, further supporting a direct interaction between labile zinc and IKKβ within the cytosol (Figure 4E and Figure S4F). The crystal structure of IKKβ (Xenopus laevis) has recently been reported (Xu et al., 2011). A human IKKβ protomer model was constructed based on homology modeling. Accordingly, we utilized a combination of In Silico modeling and site-directed mutagenesis and revealed a novel zinc coordination site located at His 143. Strikingly, this coordination site resides within the C-lobe of the kinase domain, that is critical for IKKβ catalytic activity and responsiveness to zinc-mediated inhibition (both human and mouse). The structural model predicts that the coordination site is comprised of the zinc binding residues H137, H143, D204 and I141 (Figure 4F–G and Figure S4G–I). In contrast, mutation of H313 located within the ubiquitin-like domain (ULD), at a potential alternative zinc coordination site identified by our screening approach, did not alter zinc-mediated kinase inhibition, indicating that zinc does not impact kinase activity through a ULD-related interaction.
Collectively, our findings reveal that ZIP8-mediated zinc transport is critical in attenuating NF-κB signaling through zinc-mediated inhibition of IKKβ kinase activity.
Zinc deficiency augments IKK activity and the inflammatory response in vivo
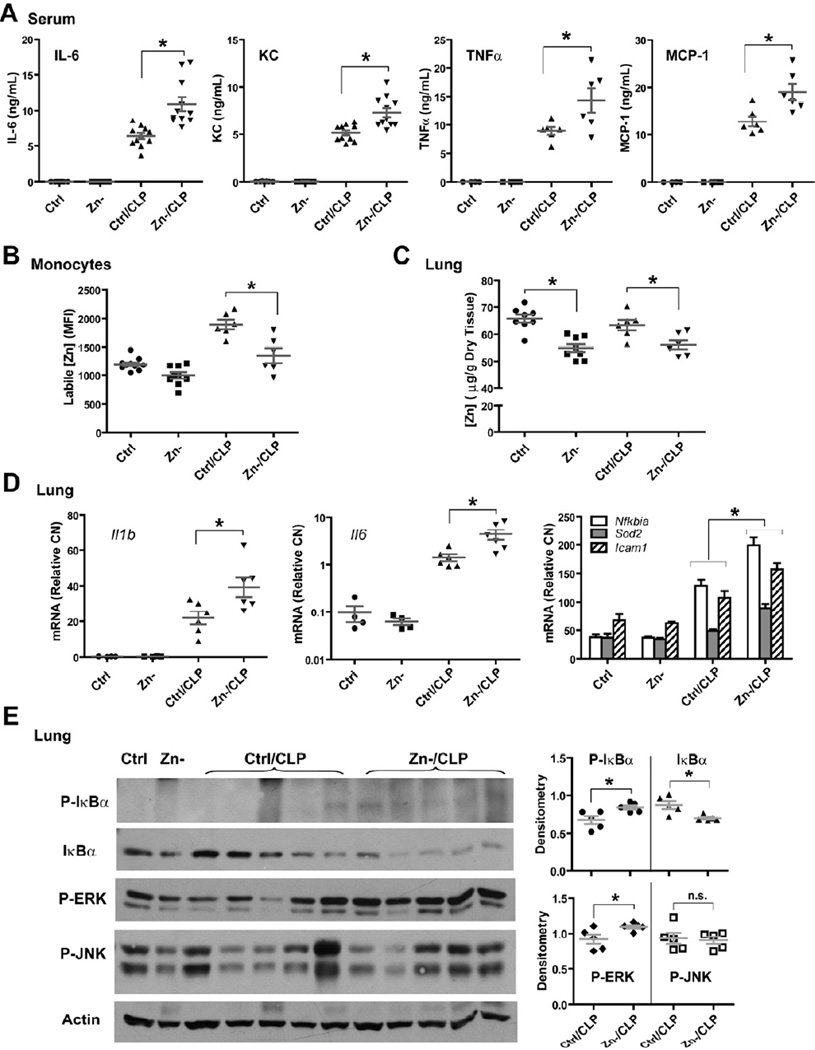
We recently reported that moderate zinc deficiency significantly increased systemic inflammation, tissue injury and mortality in response to polymicrobial sepsis (Bao et al., 2010; Knoell et al., 2009). Having established that zinc inhibits NF-κB activation through IKKβ in vitro, we postulated that systemic zinc deficiency in mice would result in elevated inflammation that would correlate with insufficient control of IKK signaling. To test this hypothesis, adult mice were first administered a zinc-deficient diet for 3 weeks, followed by LPS injection or cecal ligation and puncture (CLP) treatment for 2 hrs. Animals maintained on a zinc-deficient diet had a significant decrease in zinc levels in the serum, peripheral monocytes and lung (Figure 5B–C) (Knoell et al., 2009). In response to CLP, zinc-deficient mice also exhibited a significant increase in serum pro-inflammatory cytokines (IL-6, KC, TNFα and MCP-1) and lung transcripts of NF-κB target genes when compared to their normal dietary counterparts, indicative of an elevation in NF-κB activation (Figure 5A and 5D). A significant increase of phosphorylated IκBα and IκBα degradation was also observed in the lung, consistent with increased IKKβ activity in the setting of zinc deficiency. ERK phosphorylation was also increased whereas alterations in JNK phosphorylation were nominal (Figure 5E). Similar results were observed with LPS exposed animals (Figure S5A–C) and an in vitro monocyte cell line model in which cells were grown in zinc-deficient medium (Figure S5D).
Figure 5. Systemic zinc deficiency increases the pro-inflammatory response to sepsis.
(A) Cytokine analysis of serum obtained from mice that were maintained on zinc deficient or corresponding control diet for three weeks, followed by cecal ligation and puncture (CLP). Samples were obtained 2 hrs after CLP. Zn, zinc; Zn-, zinc deficient; Ctrl/CLP: control (normal) diet plus CLP; Zn-/CLP, zinc-deficient diet plus CLP. (B) Labile zinc levels in peripheral blood monocytes at 2 hrs after CLP. Figure 6F describes the methodology. (C) Total zinc levels in the lung at 2 hrs after CLP, as measured by AAS. (D) Gene expression analysis of NF-κB target genes in mouse lung tissues. (E) Total proteins were extracted from lung and subjected to Western analysis. Densitometry is presented as a histogram to the right.
ZIP8 is upregulated and correlates with altered zinc metabolism in vivo in response to LPS and sepsis
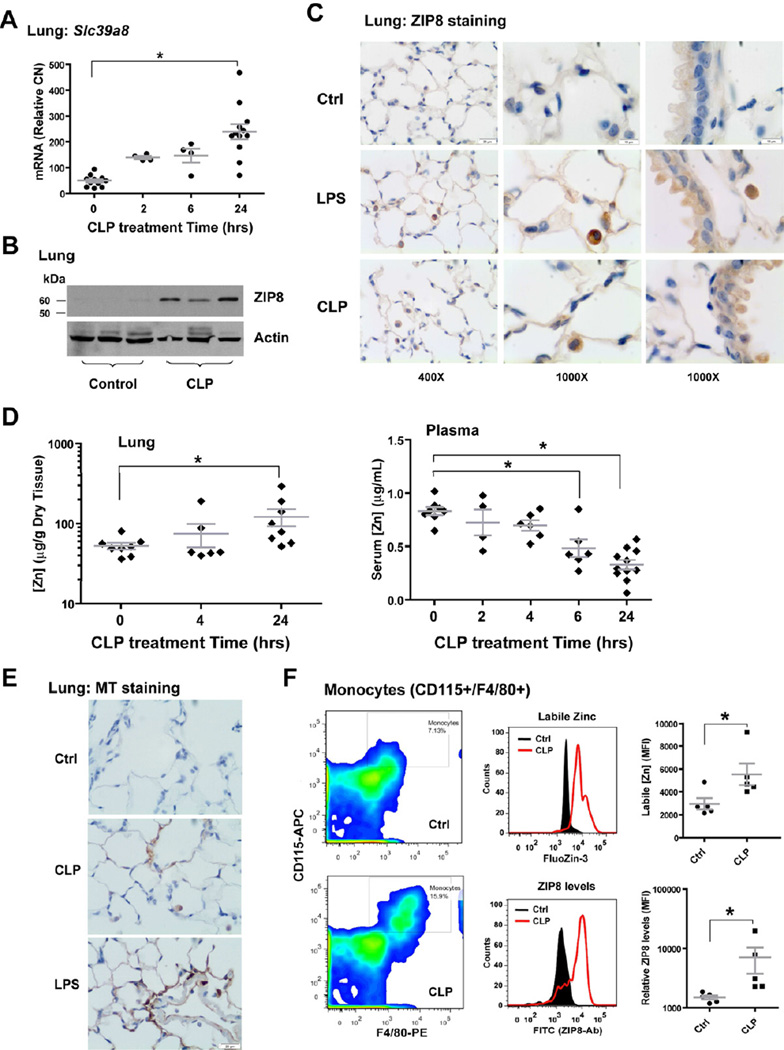
Based upon in vitro studies, we sought to determine whether the up-regulation of ZIP8 could be recapitulated in vivo. A significant increase in ZIP8 mRNA and protein expression was observed in lung tissue in response to both CLP and endotoxin. Expression was localized to alveolar epithelia, upper-airway epithelia, and alveolar macrophages (Figure 6A–C and Figure S6A). Slc39a8 expression did not change in the liver (data not shown); however, expression of Slc39a14 (ZIP14), the closest homologue to Slc39a8, was up-regulated in the liver (Figure S6B) (Liuzzi et al., 2005), but did not change in the lung (data not shown). At the same time, plasma zinc levels precipitously declined following LPS and CLP challenge, consistent with hypozincemia, in the absence of body zinc loss (Cousins and Leinart, 1988). In sharp contrast, zinc levels in the lung and liver increased. Also, consistent with human studies (Gaetke et al., 1997), plasma zinc levels began to recover toward baseline within 16 hrs following LPS challenge. CLP resulted in persistent hypozincemia and accumulation of zinc in the lung and liver, presumably as a consequence of increased and prolonged systemic inflammation (Figure 6D and Figure S6C–D). Accordingly, an increase in tissue metallothionein was observed in the lung and liver in response to LPS and CLP treatment, which could result from zinc influx, inflammation, or both (Figure 6E and Figure S6E). CLP also significantly increased both ZIP8 expression and intracellular zinc content in circulating monocytes (Figure 6F).
Figure 6. Endotoxin or sepsis induces Slc39a8 expression resulting in increased zinc levels in lung and monocytes.
(A) Slc39a8 expression was induced in mouse lung following CLP treatment. (B) Western analysis shows the induction of ZIP8 protein in whole lung homogenates 24 hrs after CLP. (C) Immunostaining shows that ZIP8 expression is increased at 24 hrs following LPS or CLP treatment in alveolar epithelia, upper airway epithelia, and alveolar macrophages (Scale bar: 20 µm for 400×; 10 µm for 1000× magnification). (D) Time-lapse analysis of zinc levels in serum and lung tissue, following CLP exposure. (E) Metallothionein (MT) staining in the lung 24 hrs after LPS or CLP (Scale bar: 20 µm). (F) Mouse peripheral leukocytes were stained with CD115-APC and F4/80-Pacific Blue, along with FluoZin-3 or anti-ZIP8 antibody and FITC-secondary conjugate. Monocytes were selected as CD115+/F4/80+ cells, followed by histogram analysis of FluoZin-3 or FITC-positive cells within this population. The relative levels of zinc or ZIP8 were compared, based on MFI.
Slc39a8 hypomorphic mouse fetal fibroblasts (MFFs) are more responsive to pro-inflammatory cytokines
Obliteration of ZIP8 is embryo-lethal precluding in vivo validation studies in Slc39a8-knockout mice. In lieu of this, we conducted studies in Slc39a8 hypomorphic mice that harbor a Slc39a8 (neo) allele that contains the neomycin-resistance gene (neo) in intron 3. Homozygous Slc39a8 (neo/neo) mice have a significant reduction in ZIP8 mRNA and protein levels (> 90%) and diminished zinc levels in vital tissues. Due to developmental defects, the Slc39a8 (neo/neo) homozygotes die between GD18.5 and 48 hrs postnatally (Galvez-Peralta et al., 2012; Wang et al., 2011).
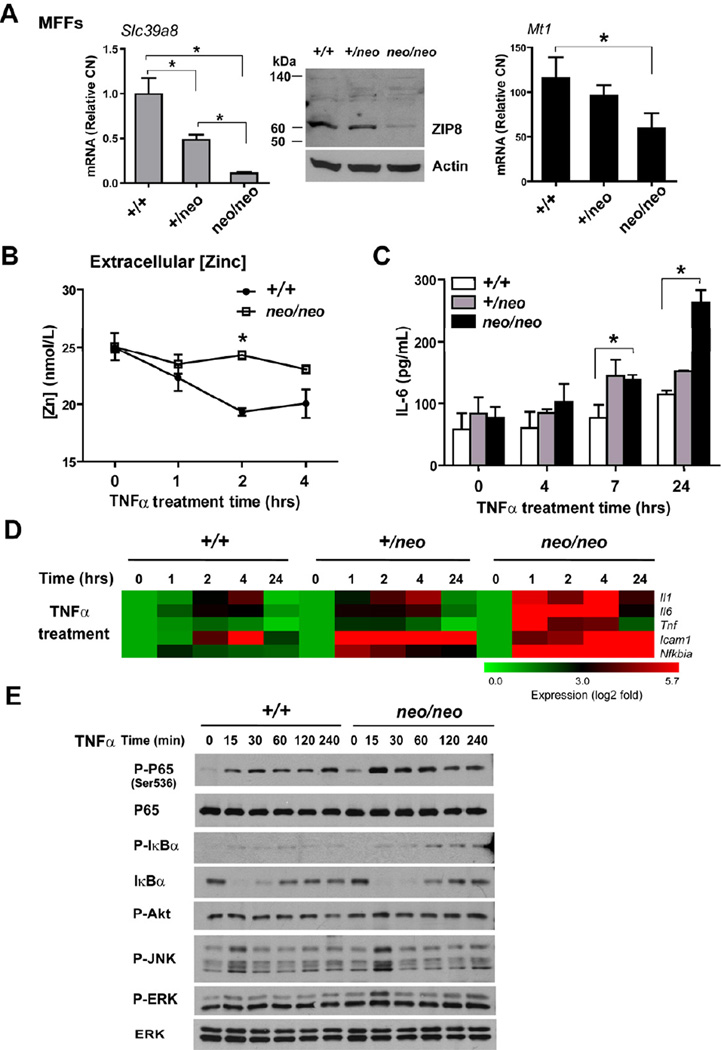
In order to examine the function of ZIP8, we generated MFFs from GD16.5 Slc39a8 (+/+), Slc39a8 (+/neo), and Slc39a8 (neo/neo) mice. As expected, Slc39a8 (neo/neo) MFFs had significantly diminished ZIP8 mRNA and protein levels compared to that in Slc39a8 (+/+) and Slc39a8 (+/neo) cells. Metallothionein (Mt1) expression was also decreased in Slc39a8 (neo/neo) cultures most likely as a consequence of decreased intracellular zinc levels (Figure 7A). A time-dependent analysis of zinc content in culture medium revealed that Slc39a8 (neo/neo) MFFs are impaired in their ability to uptake zinc in response to TNFα (Figure 7B). We then examined the inflammatory response of MFFs to TNFα and IL-1β. Slc39a8 (neo/neo) MFFs produced more IL-6, compared with that in Slc39a8 (+/neo) and Slc39a8 (+/+) cells (Figure 7C and Figure S7B) and consistently showed increased transcript levels of NF-κB-driven genes (Figure 7D and Figure S7A, S7C–D). Strikingly, the signaling events demonstrate that the presence of phosphorylated p65 and IκBα are elevated in Slc39a8 (neo/neo) MFFs in tandem with prolonged IκBα degradation. These findings are also consistent with elevated IKKβ activity, thereby further corroborating that ZIP8 negatively regulates NF-κB through IKKβ (Figure 7E).
Figure 7. Slc39a8 hypomorphic mouse fetal fibroblasts (MFFs) have an elevated pro-inflammatory response to TNFα.
(A) Real-time PCR quantified the mRNA levels of ZIP8 and MT1 in Slc39a8 (+/+), Slc39a8 (+/neo) and Slc39a8 (neo/neo) primary MFFs cultures. Western analysis shows ZIP8 protein levels. (B) Total zinc levels in culture medium were determined by AAS. (C) Slc39a8 (+/+), Slc39a8 (+/neo) and Slc39a8 (neo/neo) MFFs were treated with mouse TNFα (50 ng/mL) for the indicated time. IL-6 levels were measured in the culture supernatants. (D) Analysis of pro-inflammatory gene expression profiles in Slc39a8 (+/+), Slc39a8 (+/neo) and Slc39a8 (neo/neo) MFFs that were treated with TNFα for the indicated time points. Data are presented as a representative heatmap following normalization to untreated group. Gene designation is to the right of each row. (E) Western analysis of signaling pathways in Slc39a8 (+/+) and Slc39a8 (neo/neo) MFFs treated with TNFα.
DISCUSSION
Overwhelming bacterial infection quickly activates the innate immune response and triggers inflammation in order to enable host defense. The magnitude of the initial response must be agile, relative to the type and extent of infection, to facilitate pathogen removal, extinguish inflammation, and ultimately restore homeostasis. Indeed, in the words of Lewis Thomas, “Our arsenals for fighting off bacteria are so powerful, and involve so many different defense mechanisms, that we are in more danger from them than from the invaders”. Consistent with his postulate, sepsis patients typically do not succumb to the initial infection, but rather succumb later to immune dysfunction, endorgan damage, and secondary infection. Mortality from sepsis leading to septic shock is strongly associated with over-activation of the initial inflammatory response (Abraham and Singer, 2007). Increased expression of NF-κB-driven cytokines, including TNFα, IL-1β, and IL-6, is associated with increased risk of vital organ failure and a worse prognosis (Hotchkiss and Karl, 2003), but it is still not known why immune imbalance occurs.
Multiple negative feedback pathways have evolved to control the extent of innate immune activation (Ruland, 2011). Herein, we report the discovery of a unique negative feedback regulator ZIP8 that directly couples zinc metabolism to the regulation of innate immunity. ZIP8 shares a similar feature with other established NF-κB negative feedback regulators, including IκBα (Chiao et al., 1994) and A20 (Boone et al., 2004), in that its expression requires activation by the very pathway it controls; however, ZIP8 is unique in that its function is coupled to the importation of zinc. Further, ZIP8 functions by guiding zinc into the cytosol to inhibit IKKβ, thereby regulating immune balance. Zinc directly binds to a novel coordination site in IKKβ that includes H143 within the kinase domain. Because H143 together with D145 and Mg2+ are known to coordinate ATP binding, we predict that zinc interaction with H143 will result in a rotameric switch that weakens ATP binding, thereby reducing or inactivating IKKβ enzyme activity, although this remains to be established (Figure 4G). IKKβ also serves as a critical switch for activating the ERK-signaling pathway (Banerjee and Gerondakis, 2007). Consistent with this, we observed that ZIP8-mediated zinc transport inhibited ERK signaling. The function of zinc in concert with ZIP8 on NF-κB is conserved in different cell types, including monocytes, macrophages and lung epithelial cells. Consistent with this, loss of the zinc exporter Slc30a5 (Znt5) in mast cells resulted in increased labile zinc and suppressed NF-κB signaling in response to FcεRI stimulators (Nishida et al., 2009). However, it is important to recognize that zinc function and trafficking is highly dependent on the cell type within the context of cell activation. For example, TLR4 stimulation in dendritic cells decreases cytosolic free zinc through the suppression of ZIP6 (Kitamura et al., 2006). T cell (CD3+) activation up-regulates ZIP8, which promotes labile zinc release from the lysosome and enhances IFN-γ production (Aydemir et al., 2009). TCR activation in CD4+ T cells induces zinc influx through ZIP6, resulting in augmentation of TCR signaling (Yu et al., 2011). These important findings underscore the complexity of zinc metabolism relative to immune function.
Humans and bacteria, in many respects, are engaged in a “tug of war” at the host-microbial interface and compete for vital resources to maintain essential biological functions (Kehl-Fie and Skaar, 2010). We speculate that zinc, typically abundant within the extracellular milieu, is a vital commodity at the onset of infection and that ZIP8 plays a vital role to uptake zinc into host cells thereby providing a competitive advantage. Zinc metabolism and turnover in humans is relatively high, with approximately 1% of total body zinc content replenished daily by the diet (King et al., 2000). In cells, a labile zinc pool is rapidly exchangeable and altered in response to zinc conditions as well as to extracellular stimuli. Labile zinc regulates signaling, through both kinases and phosphatases, and its levels are maintained by zinc transporters (Haase and Rink, 2007). Recent elucidation of ZIP protein structure has revealed that zinc transport is a nonsaturable and electrogenic process consistent with features of a zinc permeable channel (Lin et al., 2010), a model distinct from conventional ATP or voltage-gated transport, indicating that expression of ZIPs is coupled to functional control. Moreover, as we reveal, ZIP8 is directly regulated by NF-κB at the transcriptional level, making it unique and highly specialized to allow the rapid sequestration of zinc in response to infection. Similar to ZIP8, ZIP14 is regulated by IL-6-dependent signaling in the liver (Liuzzi et al., 2005). Phylogenetic analysis of SLC39 family proteins reveals that ZIP8 and ZIP14 are related and distinct from the other 12 ZIPs (Girijashanker et al., 2008). This is intriguing when taking into account that both genes emerged in land animals from a single gene in sea animals approximately 420 million years ago. This evolutionary divergence supports the notion that specialized zinc transporter function(s) may have evolved as a consequence of new environmental pressures. As driven in part by ZIP8 and ZIP14, hypozincemia is a well characterized phenomenon that occurs in response to systemic infection and inflammation (Cousins and Leinart, 1988; Gaetke et al., 1997; Sobocinski et al., 1978). Based on our findings that correlate with in vivo observations, we propose that ZIP8 and ZIP14 are unique zinc transporters that are rapidly induced in a tissue-specific manner, thereby channeling zinc to fundamentally important intracellular checkpoints that help to coordinate and balance host defense.
Zinc deficiency is a significant health care problem (Hambidge and Krebs, 2007) and a leading cause of infections including pneumonia, diarrhea and malaria (Caulfield et al., 2004). Rapid changes in zinc metabolism that lead to hypozincemia are common in critically ill patients. Septic shock non-survivors have lower serum zinc levels, compared with survivors who are able to recover from hypozincemia (Wong et al., 2007). In adult septic subjects, lower plasma zinc levels correlated with higher ZIP8 expression levels in monocytes, higher cytokine levels, and increased organ damage within the first 24 hrs following ICU admission (Besecker et al., 2011). Based upon these observations, it is plausible that prolonged hypozincemia and elevated ZIP8 expression in monocytes might be useful prognostically as a guide for zinc supplementation in sepsis patients. Zinc supplementation has been shown to prevent the incidence of serious infections in children (Brooks et al., 2005). We also observed that zinc supplementation significantly improves survival in an animal model of combined zinc-deficiency and sepsis (Knoell et al., 2009). Further investigation is needed to understand the complexity of zinc metabolism throughout the entire course of sepsis before we are able to determine the impact of zinc supplementation on sepsis patients. Accurate biomarkers that are predictive of zinc status will also be required since plasma zinc levels are a poor indicator in the setting of inflammation.
In conclusion, we report the novel discovery that the zinc transporter SLC39A8 is a negative regulator of the NF-κB signaling pathway through zinc-mediated suppression of IKK activity in monocytes, macrophages, and lung epithelia. In vivo, ZIP8 expression is induced following innate immune activation and correlates with intracellular zinc sequestration. We contend that this discovery reveals a new biology that bridges a fundamental gap in our understanding of how zinc metabolism, or deficits therein, may critically influence the initial host response to infection. In addition, it provides a framework to understand how inadequate zinc consumption can increase risk of immune dysfunction, thereby compromising the host’s capability to battle pathogens at the front line of defense.
EXPERIMENTAL PROCEDURES
Animal studies
C57BL/6 mice (The Jackson Laboratory) were either injected (5 µg/g body weight) intraperitoneally (i.p.) with LPS (Escherichia coli serotype 055:B5; Sigma-Aldrich) or treated with cecal ligation and puncture (CLP). To establish systemic zinc-deficiency model, mice were randomly placed on a zinc-deficient diet (0.5–1.5 ppm zinc) or a matched control diet (50.5–51.5 ppm zinc) for three weeks (Knoell et al., 2009). All animal studies were conducted in accordance with the terms and conditions of prior approval as set forth by The Ohio State University Institutional Animal Care and Use Committee.
Real-time PCR
Quantitative PCR was performed using SYBR® Green reagent (Applied Biosystems). Relative copy numbers (RCN) of selected genes were normalized to the expression of housekeeping genes (GAPDH or cyclophilin), then calculated with the following equation: RCN = 2−ΔCt × 100, where ΔCt is the Ct(target) − Ct(reference).
Promoter cloning and luciferase assay
SLC39A8 luciferase reporter constructs were generated from human genomic DNA using PCR amplification of an approximately 2k bp promoter fragment, followed by insertion into pGL3 vector. Deletion constructs were generated using a PCR-based subcloning strategy. Sequence analysis confirmed the fidelity of all the constructs. Luciferase assay was performed with Dual-Glo™ system (Promega).
Western blot, immunoprecipitation (IP) and kinase assay
Standard western blot and IP were performed. Kinase assay was performed using the HTScan® Kinase Assay Kit, with IκBα (Ser32) peptide as the specific substrate (Cell signaling technology).
65Zinc-blotting assay
Protein samples were resolved by SDS-PAGE and transferred to a PVDF membrane. Immunoblotting (IB) was first conducted using anti-IKKβ antibody, followed by intense stripping. Proteins on PVDF filter were re-natured and incubated with 100 µCi of 65ZnCl2. Autoradiography was then performed.
Zinc measurement by atomic absorption spectroscopy (AAS) or inductively-coupled plasma optical-emission spectrometry (ICP-OES)
Solid tissue were dried, weighted and digested in 1 mL mixed acid solution (nitric acid: perchloric acid (1:2)) at 80°C for 4–6 hrs. Liquid samples were digested with nitric acid (1%) for overnight. Samples were subjected to AAS (AAnalyst 400, Perkin Elmer) or a Vista Pro ICP-OES (Varian, Inc).
Statistics
Data are representative of three independent experiments (mean ± s.d. or s.e.m.). Statistical comparisons among multiple groups were performed using one-way ANOVA with Tukey’s or Dunnett’s post-hoc test. Comparison among four groups with two factors was performed using 2 × 2 factorial ANOVA. Pairs of treatment groups were compared using two-tailed unpaired Student’s t-test. Significance was assumed at p-value of less than 0.05 (* p < 0.05; n.s., not significantly different).
Supplementary Material
Highlights.
NF-κB regulates the expression of the zinc transporter SLC39A8 (ZIP8).
ZIP8 negatively regulates NF-κB through Zn-mediated down-modulation of IKK activity.
Zn inhibits IKKβ upon binding to a specific site located in the kinase domain.
MFFs from Slc39a8 hypomorphic mice lack the ability to control NF-κB activation.
ACKNOWLEDGMENTS
Dr. Denis Guttridge (The Ohio State University) provided the p65, p50 and IκBα-SR plasmids. Dr.s Michael Karin (University of California at San Diego) and Ying Xia (University of Cincinnati) provided IKKβ and NEMO knockout MEFs.
Technical support from Catherine Powell, Dara Burris, Chuan Yu, David Vera, and Bryan Lee.
Supported by Lifeline of Ohio Tissue Procurement Agency.
Supported by National Institutes of Health (HL R01 HL086981-01 to D.L.K., T32 ES016646 to M.G.-P., and P30 ES06096 and R01 ES010416 to D.W.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- Aiba I, Hossain A, Kuo MT. Elevated GSH level increases cadmium resistance through down-regulation of Sp1-dependent expression of the cadmium transporter ZIP8. Mol Pharmacol. 2008;74:823–833. doi: 10.1124/mol.108.046862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Aydemir TB, Liuzzi JP, McClellan S, Cousins RJ. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J Leukoc Biol. 2009;86:337–348. doi: 10.1189/jlb.1208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Gerondakis S. Coordinating TLR-activated signaling pathways in cells of the immune system. Immunol Cell Biol. 2007;85:420–424. doi: 10.1038/sj.icb.7100098. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Gugasyan R, McMahon M, Gerondakis S. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci U S A. 2006;103:3274–3279. doi: 10.1073/pnas.0511113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Liu MJ, Lee B, Besecker B, Lai JP, Guttridge DC, Knoell DL. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2010;298:L744–L754. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum NA, Kobayashi M, Moriwaki Y, Matsumoto M, Toyoshima K, Seya T. Mycobacterium bovis BCG cell wall and lipopolysaccharide induce a novel gene, BIGM103, encoding a 7-TM protein: identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures. Genomics. 2002;80:630–645. doi: 10.1006/geno.2002.7000. [DOI] [PubMed] [Google Scholar]
- Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1127–L1136. doi: 10.1152/ajplung.00057.2008. [DOI] [PubMed] [Google Scholar]
- Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD, Knoell DL. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr. 2011;93:1356–1364. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- Brooks WA, Santosham M, Naheed A, Goswami D, Wahed MA, Diener-West M, Faruque AS, Black RE. Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, low-income population in Bangladesh: randomised controlled trial. Lancet. 2005;366:999–1004. doi: 10.1016/S0140-6736(05)67109-7. [DOI] [PubMed] [Google Scholar]
- Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263–268. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield LE, de Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–198. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- Chiao PJ, Miyamoto S, Verma IM. Autoregulation of I kappa B alpha activity. Proc Natl Acad Sci U S A. 1994;91:28–32. doi: 10.1073/pnas.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins RJ, Leinart AS. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. FASEB J. 1988;2:2884–2890. doi: 10.1096/fasebj.2.13.2458983. [DOI] [PubMed] [Google Scholar]
- Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, Ji J, Shen P, Zheng S, Chen ZJ, Wang RF. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI. Effects of endotoxin on zinc metabolism in human volunteers. Am J Physiol. 1997;272:E952–E956. doi: 10.1152/ajpendo.1997.272.6.E952. [DOI] [PubMed] [Google Scholar]
- Galvez-Peralta M, He L, Jorge-Nebert LF, Wang B, Miller ML, Eppert BL, Afton S, Nebert DW. ZIP8 zinc transporter: indispensable role for both multiple-organ organogenesis and hematopoiesis in utero. PLoS One. 2012;7:e36055. doi: 10.1371/journal.pone.0036055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol. 2008;73:1413–1423. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H, Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007;20:579–585. doi: 10.1007/s10534-006-9029-8. [DOI] [PubMed] [Google Scholar]
- Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- Hambidge KM, Krebs NF. Zinc deficiency: a special challenge. J Nutr. 2007;137:1101–1105. doi: 10.1093/jn/137.4.1101. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Jeon KI, Jeong JY, Jue DM. Thiol-reactive metal compounds inhibit NF-kappa B activation by blocking I kappa B kinase. J Immunol. 2000;164:5981–5989. doi: 10.4049/jimmunol.164.11.5981. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130:1360S–1366S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, DiSilvestro RA, Crouser ED. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med. 2009;37:1380–1388. doi: 10.1097/CCM.0b013e31819cefe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Lin W, Chai J, Love J, Fu D. Selective electrodiffusion of zinc ions in a Zrt-, Irt-like protein, ZIPB. J Biol Chem. 2010;285:39013–39020. doi: 10.1074/jbc.M110.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843–6848. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Nishida K, Hasegawa A, Nakae S, Oboki K, Saito H, Yamasaki S, Hirano T. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. J Exp Med. 2009;206:1351–1364. doi: 10.1084/jem.20082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- Ruland J. Return to homeostasis: downregulation of NF-kappaB responses. Nat Immunol. 2011;12:709–714. doi: 10.1038/ni.2055. [DOI] [PubMed] [Google Scholar]
- Sobocinski PZ, Canterbury WJ, Jr, Mapes CA, Dinterman RE. Involvement of hepatic metallothioneins in hypozincemia associated with bacterial infection. Am J Physiol. 1978;234:E399–E406. doi: 10.1152/ajpendo.1978.234.4.E399. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Wang B, He L, Dong H, Dalton TP, Nebert DW. Generation of a Slc39a8 hypomorph mouse: Markedly decreased ZIP8 Zn(2+)/(HCO(3)(−))(2) transporter expression. Biochem Biophys Res Commun. 2011;410:289–294. doi: 10.1016/j.bbrc.2011.05.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren HS. Strategies for the treatment of sepsis. N Engl J Med. 1997;336:952–953. doi: 10.1056/NEJM199703273361311. [DOI] [PubMed] [Google Scholar]
- Waterfield M, Jin W, Reiley W, Zhang M, Sun SC. IkappaB kinase is an essential component of the Tpl2 signaling pathway. Mol Cell Biol. 2004;24:6040–6048. doi: 10.1128/MCB.24.13.6040-6048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HR, Shanley TP, Sakthivel B, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, Kalyanaraman M, Tofil NM, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics. 2007;30:146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Lo YC, Li Q, Napolitano G, Wu X, Jiang X, Dreano M, Karin M, Wu H. Crystal structure of inhibitor of kappaB kinase beta. Nature. 2011;472:325–330. doi: 10.1038/nature09853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Lee WW, Tomar D, Pryshchep S, Czesnikiewicz-Guzik M, Lamar DL, Li G, Singh K, Tian L, Weyand CM, Goronzy JJ. Regulation of T cell receptor signaling by activation-induced zinc influx. J Exp Med. 2011;208:775–785. doi: 10.1084/jem.20100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.