Abstract
The PPARγ nuclear receptor regulates the expression of genes involved in lipid and carbohydrate metabolism, and it has protective effects in some patients with type 2 diabetes. Nevertheless, the therapeutic value of the PPARγ nuclear receptor protein is limited due to the secondary effects of some PPARγ ligands. Because the downstream effects of PPARγ are determined by the binding of specific cofactors that are mediated by ligand-induced conformational changes, we evaluated the differential effects of various ligands on the binding of certain cofactors associated with PPARγ. The ligands used were rosiglitazone for treating type 2 diabetes and telmisartan for treating arterial hypertension. Functional, phenotypic, and molecular studies were conducted on pre-adipocyte 3T3-L1 and functional studies in U2OS cells. The moderating influence of various cofactor families was evaluated using transient transfection assays. Our findings confirm that telmisartan has a partial modulating effect on PPARγ activity compared to rosiglitazone. The cofactors SRC1 and GRIP1 mediate the activity of telmisartan and rosiglitazone and partially determine the difference in their effects. Studying the modulating activity of these cofactors can provide interesting insights for developing new therapeutic approaches for certain metabolic diseases.
Keywords: DNA binding proteins co-activator, gene expression, transcription, telmisartan
Introduction
PPARγ (peroxisome proliferator-activated receptor-gamma) is a transcription factor and a member of the nuclear receptor protein family, which regulates gene expression via binding at concrete DNA sites known as PPARγ response elements (PPREs). The role of PPARγ includes regulating carbohydrate and lipid metabolism and cellular differentiation processes (Ferre 2004; Lehrke and Lazar, 2005; Shulman and Mangelsdorf, 2005). At the structural level, PPARγ has several functional domains. The ligand binding domain (LBD) at the C-terminus modulates the receptor’s structural conformation, thus permitting the capture of transcriptional cofactors that assist in reactions required to induce greater transcription of specific genes (Nolte et al., 1998; Bähr et al., 2011). These cofactors may act through direct changes in the structural conformation of chromatin, as is the case with the p160 family of steroid receptors (SRC) and the p300 (E1A-associated 300-kDa binding protein)/CBP (cyclic AMP response element-binding protein) complex (Aoyagi and Archer, 2008; Bähr et al., 2011).
PPARγ is a key regulator in the differentiation of adipose cells. Gene expression resulting from complete activation of PPARγ by certain synthetic ligands can increase insulin sensitivity (Rosenfeld and Glass, 2001; Staels and Fruchart, 2005). Notwithstanding these beneficial effects, the pharmacological potential of these ligands is limited by adverse effects, including weight gain, water retention and increased concentrations of lipoproteins (Guan et al., 2005; Lago et al., 2007; Nissen and Wolski, 2007). A partial activation of PPARγ remains an interesting theoretical approach if the metabolic benefits can be maintained and the secondary effects be eliminated. This could be achieved through the use of partial agonists, known as selective PPARγ modulators (SPPARMs) (Toyoma et al., 2011). Telmisartan, an angiotensin receptor blocker (ARB), was characterized as a potential partial modulator of PPARγ, although data on telmisartan’s effects are not entirely conclusive (Schupp et al., 2005).
In this study, we evaluate the activation of PPARγ in the presence of rosiglitazone and telmisartan, and further investigate PPARγ’s ability to incorporate specific cofactors in pre-adipocyte cells. We find that both rosiglitazone and telmisartan differentially reduce triglyceride accumulation in 3T3-L1 pre-adipocyte cells, and show that the magnitude of the effect is dependent on the presence of certain cofactors. These findings are likely due to a dissimilar effect of these two ligands on the PPARγ receptor.
Materials and Methods
Plasmids
The PPARγ coding sequence was cloned into the SalI site of the pSV.SPORT1 vector (Tontonoz et al., 1994). The Gal4-PPARγ LBD and PPRE-Luc plasmids were provided by Dr. Bruce Spiegelman of the Dana-Farber Cancer Institute, Boston, MA. DNA encoding p300 was inserted between the NotI and HindIII restriction sites in the CMV-NHA vector. The cloning procedures for SRC1 (steroid receptor coactivator-1), GRIP1 (glucocorticoid receptor interacting protein 1) and PRIP (PPARγ interacting protein) have been described previously (Onate et al., 1995; Hong et al., 1996; Zhu et al., 2000). The VP16-SRC1 vector was constructed by inserting the polymerase chain reaction (PCR)-amplified SRC1 gene fragment into the EcoRI site of the VP16 activation domain (residues 409–490), The 5XUAS reporter gene was inserted between the BamHI and HindIII restriction sites in the pT109 vector, which has a TK promoter coupled with luciferase (Takeshita et al., 1998).
Transient transfection and double-hybrids
The U2OS and 3T3-L1 cell lines were obtained from the American Tissue Culture Collection (ATCC) and grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal calf serum and 1% penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere. When the cells reached 70% confluence, they were transiently transfected using a lipofectamine 2000 (Invitrogen) protocol in 12-well culture dishes. Transfections were done with 0.85 μg of PPRE-Luc (reporter plasmid), 0.5 μg of CMVβ-Gal (control plasmid), 0.2 μg of PPARγ and 0.1 μg of each of the co-activators (p300, SRC1 GRIP1 and PRIP) added to each well. The total amount of transfected DNA was normalized using an empty pcDNA3.1 vector. After 24 h, the medium was replaced with DMEM with 10% fetal calf serum treated with activated carbon and a resin to eliminate endogenous ligands plus either 1 μM or 10 μM of the ligand to be tested (rosiglitazone or telmisartan). Dimethyl sulfate (Me2SO4) was used as a vehicle at a maximum concentration of 0.2%. After an additional 24 h of incubation, the cells were washed and lysed with Triton X-100 buffer, and the activity levels of both luciferase and β-Galactosidase were quantified.
For the double-hybrid study, the above protocol was used to transfect U2OS cells with 0.85 μg of the 5XUAS-TK-Luc reporter plasmid, 0.1 μg of the Gal4 PPARgamma plasmid and 0.35 μg of the VP16-SRC1 and the CMVβ-Galactosidase plasmid was used as an internal control. The fold-change in luciferase or β-Galactosidase expression in the cells containing the experimental vectors was calculated relative to expression in the presence of only the control expression vector pcDNA3.1 The data was expressed as the mean ± the standard error of the mean (SEM) and represent a minimum of three independent experiments, with each data point run in triplicate for each experiment.
Adipose cell differentiation assay
Pre-adipocyte 3T3-L1 cells were maintained in DMEM medium supplemented with 10% fetal calf serum and 1% antibiotics. Differentiation was induced 48 hours after the cells reached confluence using media containing 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine and either telmisartan (1 μM or 10 μM), rosiglitazone (1 μM) or a combination of both ligands, as shown in the results. After 48 h, the medium was changed, and only the ligands were added; the medium was replaced every 2 days during the 8 days of treatment. Differentiated cells were then washed and fixed with 10% formaldehyde and stained with 0.6% red oil in 60% isopropanol for 2 h at room temperature. For the quantification step, the stained single layer was washed extensively to remove leftover dye, and 1 mL of isopropyl alcohol was added to differentiate the disks. After 5 min, the absorbance was measured by spectrophotometry at 510 nanometers.
Western blot analysis
The 3T3-L1 cells were lysed in RIPA buffer [1X PBS, 1% Nonidet P-40, 0.1% SDS and protease inhibitors (Roche)]. Samples were collected at 0, 4 and 8 days after differentiation. After centrifugation at 4000 g x 4 m, 200 μg of total protein was mixed with an equal volume of sample buffer, and the mixture was denatured at 95 °C for 3 min. The proteins were separated by electrophoresis in an SDS polyacrylamide gel and then transferred to a nitrocellulose membrane. The membrane was incubated in 5% non-fat powdered milk in 0.1% v/v Tween-20 in PBS for 1 h at 4 °C to block nonspecific binding. The membrane was then incubated with antibodies against fatty acid-binding protein 4/adipocyte lipid-binding protein (Fabp4/aP2) (ABCAm, Cambridge MA, USA) at a 1:1000 dilution. The membrane was then washed and incubated with HRP goat anti-rabbit antibody (1:2000) (ABCAm, Cambridge MA, USA) for 1 h. The membrane was then developed with ECL solution (Amersham Pharmacia Biotech) and visualized by autoradiography. Quantitative analysis was performed by means of densitometry analyses of western blots from three different experiments. Results are expressed as means ± SEM.
Statistical analyses
Analysis of Variance (ANOVA) tests were performed using StatView software and differences were considered statistically significant when p < 0.05. In the transfection studies, co-transfections with β•galactosidase were performed so as to normalize the data and avoid dispersion of the results. β•galactosidase has a different promoter reporter vector and thus does not interfere with the results. Assays were performed in triplicate. The Student t-test was used for comparisons of two treatments.
Results
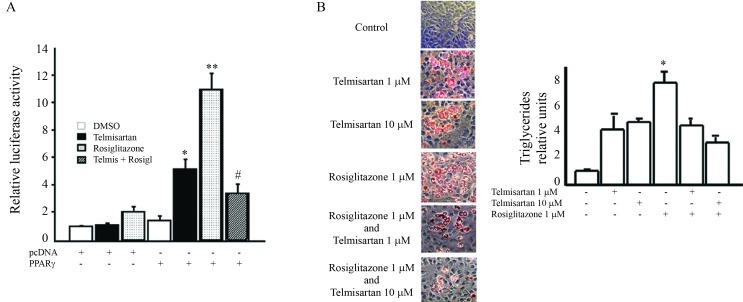
To measure the transcriptional activation of PPARγ by telmisartan, U2OS cells were transiently transfected with a plasmid encoding for PPARγ and a reporter vector encoding for natural PPRE. Treatment of transfected cells with telmisartan significantly increased the luciferase activity. However, large doses of telmisartan (10 μM) did not result in a greater increase in PPARγ compared to that observed when the cells were treated with rosiglitazone. We next investigated a potential synergistic effect between the two ligands on PPARγ transactivation. Treatment of transfected cells with telmisartan combined with rosiglitazone significantly reduced the activation of PPARγ compared to the level of activation observed with rosiglitazone alone (Figure 1A).
Figure 1.
Telmisartan and rosiglitazone induce PPARγ transcriptional activation and differentiation of adipose cells. A. U2OS cells were transiently transfected with a reporter plasmid along with luciferase PPRE-Luc and PPARγ vectors. The cells were then treated with 10 μM telmisartan or 1 μM rosiglitazone. The luciferase activity was normalized using β-Galactosidase expression, and the results were calculated from assays performed in triplicate. B. Differentiation of pre-adipocyte 3T3-L1 cells was induced with a medium containing 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine and either telmisartan (1 μM or 10 μM) or rosiglitazone (1 μM). Triglyceride accumulation was quantified as described in the Materials and Methods section. The data are expressed as the mean ± SE, and represent a minimum of three independent experiments, with each data point run in triplicate for each experiment. Statistical analysis were done by Analysis of Variance (ANOVA) For A: * fold-change in luciferase expression relative to expression in the presence of only the control expression vector p < 0.05; ** fold-change in luciferase expression relative to expression in the presence of only the control expression vector p < 0.01; # fold-change in luciferase expression relative to Rosiglitazone activation in the presence of PPARγ expression vector p < 0.01. For B: *p < 0.05 Rosiglitazone vs. Telmisartan.
Keeping in mind that PPARγ plays a fundamental role in establishing the adipocyte phenotype, we analyzed the effect of agonists on the differentiation of 3T3-L1 pre-adipocytes to see if the data were in concordance with the data obtained from the functional assays. We found that rosiglitazone induces a greater accumulation of triglycerides, thus implying a higher level of differentiation, compared to high doses of telmisartan. When telmisartan was added to the differentiation medium at doses of 1 or 10 μM in the presence of rosiglitazone, a reduction in pre-adipocyte differentiation was observed (Figure 1B).
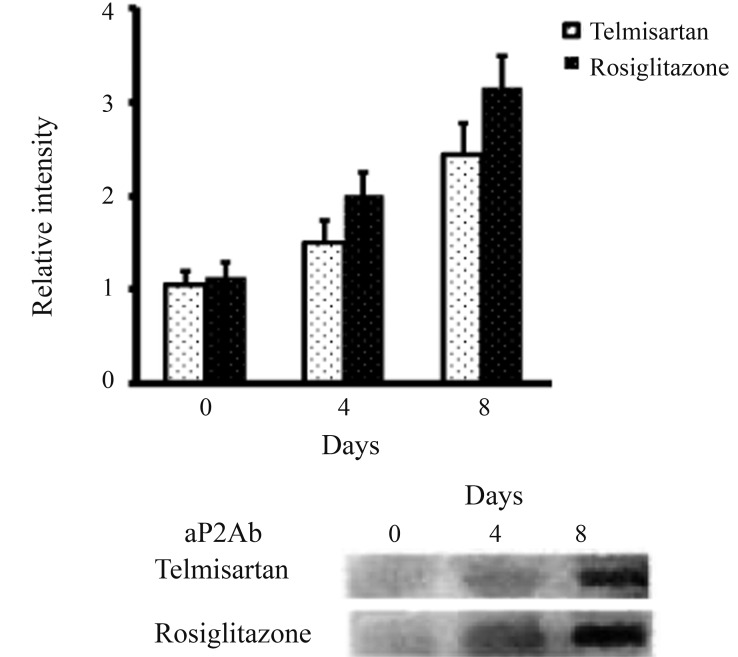
A molecular evaluation of PPARγ transcriptional activity was performed by measuring the production of Fabp4/aP2, (fatty acid binding protein 4) and a higher level of expression was observed when the cells were treated with rosiglitazone rather than telmisartan (Figure 2).
Figure 2.
Expression of aP2 after telmisartan or rosiglitazone stimulation. Pre-adipocyte 3T3-L1 cells were differentiated and treated with telmisartan or rosiglitazone. The expression levels of aP2 were analyzed on the days shown (day 0 was defined as 48 hours after the start of differentiation) and analyzed by western blotting. Quantitative analysis was performed via densitometric analysis of the western blots in three different experiments.
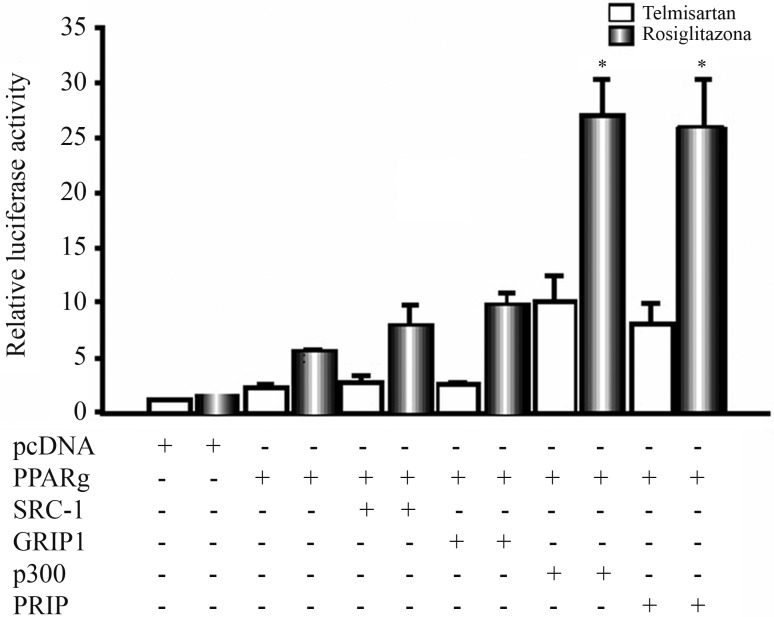
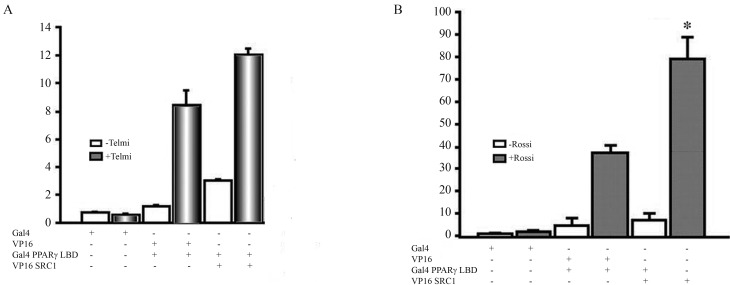
To determine whether the observed effects were due to a difference in the incorporation of cofactors by rosiglitazone and telmisartan, we tested whether the incorporation of various cofactors resulted in different effects on PPARγ activity. Co-transfection with p300 and PRIP significantly increased activation of PPARγ by both agonists, whereas the p160 family cofactors (SRC1 and GRIP) did not result in a significant increase in PPARγ activity after the addition of telmisartan (Figure 3). To evaluate the interaction between PPARγ and SRC1 in the presence of telmisartan and rosiglitazone, we performed a double-hybrid assay in mammalian cells. When telmisartan was tested, the luciferase activity increased approximately 9-fold compared to the basal level. When the Gal4-PPARγ LBD and VP16-SRC1 plasmids were co-transfected, there was a slight increase in the luciferase activity. This is in contrast to the results observed when rosiglitazone was added, which resulted in a 40-fold increase in luciferase activity; moreover, co-transfection with both SRC1 and Gal4-PPARγ LBD led to a 100% increase in luciferase activity relative to transfection with Gal4-PPARγ LBD alone (Figure 4).
Figure 3.
PPARγ activation in the presence of different activating cofactors. U2OS cells were cotransfected as in Figure 1, and plasmids with different activating cofactors were cotransfected in the presence of telmisartan or rosiglitazone. Statistical evaluation was performed based on three different experiments. Test was performed Analysis of Variance (ANOVA) and differences were considered statistically significant when the mean value with standard error was p ≤ 0.05.
Figure 4.
Evaluation of the interaction of SRC1 cofactors with PPARγ in the presence of telmisartan or rosiglitazone. U2OS cells were cotransfected using the double-hybrid technique, and SRC1 interaction with PPARγ after treatment with telmisartan or rosiglitazone was evaluated. The data are expressed as the mean ± SEM, and represent a minimum of three independent experiments, with each data point run in triplicate for each experiment. Test was performed Analysis of Variance (ANOVA) and differences were considered statistically significant when the mean value with standard error was p ≤ 0.05. As in the transfection studies are performed co-transfection with β-galactosidase, to normalize the data and avoids the dispersion of the results. β-galactosidase has a different promoter reporter vector to thereby does not interfere with the results.
Discussion
PPARγ agonists have been good help to treat diabetes type 2 by increasing insulin sensitivity and decreasing proinflammatory adipocytokines. However, adverse effects, such as water accumulation and lightweight gain, are associated with some of these agonists (Guan et al., 2005; Lago et al., 2007). Studies of PPARγ expression in murine and human models have suggested that neither total agonism nor antagonism of PPARγ offer an optimal treatment approach for metabolic disorders (Barroso et al., 2005; Kintscher, 2012). Still, the identification of molecules that moderately activate PPARγ could be an important object of study.
SPPARMs are a group of ligands that interact selectively with cofactors, resulting in partial activation of PPARγ function and thus theoretically avoiding the adverse effects associated with total agonists (Yamauchi et al., 2001). Previous studies have shown that telmisartan, a specific and selective agonist of angiotensin II receptors, acts as a SPPARM and promotes the differentiation of adipose cells via the partial activation of PPARγ (Gelman et al., 2007; Destro et al., 2011). In the current study, we found that telmisartan can stimulate the differentiation of 3T3-L1 pre-adipocytes both phenotypically and at the molecular level. However, the effect of telmisartan is less than the one observed after treatment with rosiglitazone (Figure 2). Our results show that the differential coupling of activating cofactors could explain the lower activation of PPARγ by telmisartan (Figure 4).
We furthermore found that p300 plays an important role in the coactivation of PPARγ as mediated by telmisartan and rosiglitazone. However, the p160 family of cofactors (SRC1 and GRIP1) did not induce higher activation in the presence of telmisartan (Figure 3). It is likely that these cofactors do not associate with the transcription complex in the presence of telmisartan. To further assess this hypothesis, we performed double-hybrid assays to evaluate the interaction of these proteins. We found an increase of nearly 40% in luciferase activity relative to basal levels when Gal4-PPARγ LBD and SRC1-VP16 were co-transfected in the presence of telmisartan. However, this same assay done in the presence of rosiglitazone showed a greater than 100% increase in luciferase activity (Figure 4).
Structural studies have shown less stabilized binding of telmisartan with Helix 12 of PPARg, which may explain the reduced recruitment of coactivators (Amano et al., 2012). Thus, we conclude that there is a decreased interaction of SRC1 with the transcriptional machinery when telmisartan is present compared to the total agonist rosiglitazone. Tagami et al., 2009, found scarce recruitment of SRC1 from PPARγ in mammalian two-hybrid assay with telmisartan. However, as they found an increase in PPARγ transactivation after GRIP1 cotransfection, one can argue that a possibly different PPARγ promoter might be the cause of the differences observed. Consistent with the functional data, we found that cells treated with telmisartan showed lower accumulation of lipids compared to those treated with rosiglitazone, which had a high concentration of triglycerides.
In conclusion, this study compared the functional and molecular effects of telmisartan and rosiglitazone on the PPARγ nuclear receptor complex. We found that the selective recruitment of cofactors could explain the moderate activation of PPARγ gene expression and low accumulation of triglycerides induced by telmisartan. The characterization of substances that act as SPPARMs is of great importance, given that these compounds could result in fewer adverse effects compared to total PPARγ activators, while maintaining the beneficial metabolic effects associated with moderate activation of PPARγ.
Acknowledgments
We thank Dr. Akira Takeshita, Toshi Iwasaki and Nori Kibuchi for the vector plasmids of SRC1. We also thank Ivan Martinez, Carolina Romero and Luis Celis for their technical support. This study was sponsored by La Sabana University through an internal research-funding request. The code assigned to this project by the Research Office of La Sabana University is MED-134-2010. This work was supported in part by Grant 123051929210 from Co-lombian Department of Science, Technology and Innovation, Colciencias. Companies that distribute telmisartan or rosiglitazone did not support this study financially.
Footnotes
Associate Editor: Carlos F.M. Menck
References
- Amano Y, Yamaguchi T, Ohno K, Niimi T, Orita M, Sakashita H, Takeuchi M. Structural basis for telmisartan-mediated partial activation of PPAR gamma. Hypertens Res. 2012;35:715–719. doi: 10.1038/hr.2012.17. [DOI] [PubMed] [Google Scholar]
- Aoyagi S, Archer TK. Dynamics of co-activator recruitment and chromatin modifications during nuclear receptor mediated transcription. Mol Cell Endocrinol. 2008;280:1–5. doi: 10.1016/j.mce.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähr IN, Tretter P, Krüger J, Stark RG, Schimkus J, Unger T, Kappert K, Scholze J, Parhofer KG, Kintscher U. High-dose treatment with telmisartan induces monocytic peroxisome proliferator-activated receptor-γ target genes in patients with the metabolic syndrome. Hypertension. 2011;58:725–732. doi: 10.1161/HYPERTENSIONAHA.111.173542. [DOI] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Willimas TDM, Lewis H, Schafer AJ, et al. Dominant negative mutations in human PPAR gamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- Destro M, Cagnoni F, Dognini GP, Galimberti V, Taietti C, Cavalleri C, Galli E. Telmisartan: Just an anti-hypertensive agent? A literature review. Expert Opin Pharmacother. 2011;12:2719–2735. doi: 10.1517/14656566.2011.632367. [DOI] [PubMed] [Google Scholar]
- Ferre P. The biology of PPAR receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004;53(Suppl 1):S43–S50. doi: 10.2337/diabetes.53.2007.s43. [DOI] [PubMed] [Google Scholar]
- Gelman L, Feige JN, Desvergne B. Molecular basis of selective PPARgamma modulation for the treatment of type 2 diabetes. Biochim Biophy Acta. 2007;1771:1094–1007. doi: 10.1016/j.bbalip.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absortion. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- Hong H, Kohli K, Trivedi A, Johnson D, Stallcup M. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintscher U. And in the end - Telmisartan directly binds to PPARγ. Hypertens Res. 2012;35:704–705. doi: 10.1038/hr.2012.35. [DOI] [PubMed] [Google Scholar]
- Lago R, Singh P, Nesto R. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: A meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARγ. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Nissen S, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Nolte RT, Wisley GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, Rosenfeld MG, Wilson TM, Christopher KG, Milburn MV. Ligand binding and co-activator assembly of the peroxisome proliferators receptor-gamma. Nature. 1998;395:137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- Onate S, Tsai S, Tsai M, O’Malley B. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M, Glass CK. Coregulator codes of transcriptional regulation by nuclear receptors. J Biol Chem. 2001;40:36865–36868. doi: 10.1074/jbc.R100041200. [DOI] [PubMed] [Google Scholar]
- Schupp M, Clemenz M, Gineste R, Witt H, Janke J, Helleboid S, Hennuyer N, Ruiz P, Unger T, Staels B, et al. Molecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activity. Diabetes. 2005;54:3442–3452. doi: 10.2337/diabetes.54.12.3442. [DOI] [PubMed] [Google Scholar]
- Shulman AI, Mangelsdorf DJ. Retinoid X receptor heterodimers in the metabolic syndrome. N Engl J Med. 2005;353:604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- Staels B, Fruchart JC. Therapeutic roles of peroxisome proliferator activated receptor agonists. Diabetes. 2005;54:2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- Tagami T, Yamamoto H, Moriyama K, Sawai K, Usui T, Shimatsu A, Naruse M. A selective peroxisome proliferator-activated receptor-gamma modulator, telmisartan, binds to the receptor in a different fashion from thiazolidinediones. Endocrinology. 2009;150:862–870. doi: 10.1210/en.2008-0502. [DOI] [PubMed] [Google Scholar]
- Takeshita A, Yen P, Ikeda M, Cardona G, Liu Y, Koibuchi N, Norwitz ER, Chin WW. Thyroid hormone response elements differentially modulate the interactions of thyroid hormone receptors with two receptor binding domains in the steroid receptor coactivator-1. J Biol Chem. 1998;273:21554–21562. doi: 10.1074/jbc.273.34.21554. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Toyama K, Nakamura T, Kataoka K, Yasuda O, Fukuda M, Tokutomi Y, Dong YF, Ogawa H, Kim-Mitsuyama S. Telmisartan protects against diabetic vascular complications in a mouse model of obesity and type 2 diabetes, partially through peroxisome proliferator activated receptor-γ-dependent activity. Biochem Biophys Res Commun. 2011;410:508–513. doi: 10.1016/j.bbrc.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Murakami K, Matojima K, Komeda K, Ide T, Kubota N, Terauchi Y, Tobe K, et al. The mechanism by which both hetetozygous peroxisome proliferator-activated receptor gamma (PPAR gamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem. 2001;276:41245–41254. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Kan L, Qi C, Kanwar Y, Yeldandi A, Rao M, Reddy J. Isolation and characterization of peroxisome proliferator-activated receptor (PPAR) interacting protein (PRIP) as a co-activator for PPAR. J Biol Chem. 2000;275:13510–13516. doi: 10.1074/jbc.275.18.13510. [DOI] [PubMed] [Google Scholar]