Pregnant women have long been orphaned from drug studies. The application of physiologically based pharmacokinetic modeling to quantitatively describe drug disposition and effects during pregnancy is an attractive approach that is now actively pursued as it allows optimal use of available information for both efficient study designs and prediction of maternal–fetal exposure.
Pregnant women have long been orphaned from drug studies, similar to the situation for children before the Best Pharmaceuticals Act for Children and the Pediatric Research Equity Act.1 Yet drugs are being prescribed for pregnancy-related conditions (gestational diabetes, preeclampsia, premature labor) as well as part of chronic disease management (e.g., asthma, depression, diabetes, epilepsy, and hypertension). For many of these drugs, good dosing guidelines during pregnancy are lacking while pharmacokinetics are likely altered as a result of significant physiological changes such as increased plasma volume, higher glomerular filtration, and increased activity of phase I and II drug metabolizing enzymes. Following the success of the Pediatric Pharmacology Research Units network as an infrastructure for high quality pediatric labeling and translational studies, the National Institute of Child Health and Development now supports an active network of Obstetric-Fetal Pharmacology Research Units (http://opru.rti.org) to carry out pharmacology research to enhance understanding of obstetrical pharmacokinetics and pharmacodynamics, and improve appropriate therapeutics during pregnancy. Ongoing research within the Obstetric-Fetal Pharmacology Research Unit network focuses on systems approaches to understanding pharmacokinetics and pharmacodynamics of oral hypoglycemics for treatment of gestational diabetes, agents thought to alter uterine activity, and several other drugs used during pregnancy (such as antibiotics and antidepressants). The application of physiologically based pharmacokinetic modeling to quantitatively describe drug disposition and effects during pregnancy is a highly attractive approach that is now actively pursued as it allows optimal use of available information for both efficient study designs and prediction of maternal–fetal exposure. These scientific activities are in line with what has been advocated by the US Food and Drug Administration's (FDA) Critical Path Initiative as the “systems pharmacology” approach and apply innovative computational techniques to integrate the effects of pregnancy-induced changes in physiology to describe and predict drug disposition as associated with response to therapy and adverse events. Physiologically based pharmacokinetics (PBPK) is a modeling technique that attempts to mathematically represent an organism, all of the organism's components, and time-dependent changes that are important for describing the absorption, distribution, metabolism, and excretion of a drug in humans.2 The physiological representation through organ-specific models has the advantage to incorporate elements that reflect true clearance mechanisms as well as changes over time. This can be represented as disease progression or as physiological changes such as the one that occur during pregnancy from prepregnancy, through the continuum of first, second, and third trimester and postpartum.3 PBPK modeling is increasingly used in pharmaceutical research and drug development.2,4 Earlier this year, the FDA's Pharmaceutical Science and Clinical Pharmacology Advisory Committee voted in support of extending the use PBPK modeling for pediatric drug development although several members of the committee emphasized the need for more data and prospective evaluation and validation of the models with observed data (http://www.fda.gov). In addition, a recent FDA guidance on drug–drug interaction studies posted for comments also includes an in-depth discussion on PBPK.5 Therefore mechanistic PBPK models that use organs and tissues with physiologic volumes has seen a tremendous increase in application, initially for environmental toxins and chemicals but now also for drugs. This is evidenced by more than 800 PBPK-related publications and over 40 publications of pregnancy physiologically based pharmacokinetic models developed for theoretical assessment of the kinetics of drugs during pregnancy, all trying to take into account relevant dynamic changes of the maternal and embryonic/fetal physiological functions.6
Increasing Interest in PBPK—Advantages of Bottom–Up Over Top–Down Compartmental Analysis
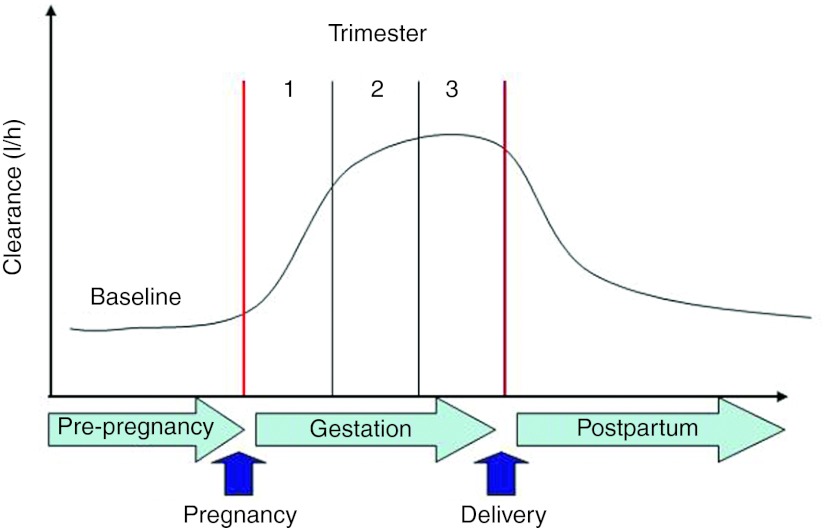
Most of the early pharmacokinetic data in pregnant women have been obtained from small parallel studies. In recent years, for some drugs, our knowledge base has been supplemented with data derived from population pharmacokinetic analyses. These data, typically collected in the third trimester have been our primary sources for the identification of factors that would explain pregnancy-induced changes in drug disposition. For instance, nifedipine is a calcium channel blocker and one of the few therapeutic options available for the treatment of chronic and gestational hypertensive disorders that are a major complication during pregnancy. Current recommendations for acute and maintenance doses of nifedipine are based on expert opinion rather than on evidence-based data from well-designed pharmacokinetic–pharmacodynamic studies. Clearance during the 3rd trimester has been reported to be increased by a factor of 2–3 as compared with that in patients and healthy subjects.7 Figure 1 shows a simulation of anticipated effects of gestation and trimester on maternal nifedipine clearance. These changes may markedly alter the pharmacodynamics of nifedipine. In addition, pharmokinetics/pharmacodynamics changes may be different in subjects with chronic hypertension or patients with preeclampsia. A comprehensive evaluation of these changes is an important first step in optimizing drug therapy during pregnancy, with special consideration to the disease state. Of note, nifedipine belongs to the Pregnancy Category C drug classification by the US FDA, which implies that “Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.” Despite FDA Category C, nifedipine remains one of most commonly used drugs in pregnancy. Beside ethical constraints, there are challenges for performing informative pharmokinetics/pharmacodynamics studies during pregnancy including unfriendly designs burdened by intense sampling schedules and long waiting times (at least one dosing interval) that further complicate enrollment. These barriers are somewhat mitigated by population pharmokinetics/pharmacodynamics analysis and modeling. Such “top–down” population analysis identifies important covariates or patient characteristics such as age, body weight, weeks of pregnancy, and organ function, and their association with pharmacokinetic behavior of the drug. Despite the level of sophistication of some of the top–down modeling, analysis is based primarily on pharmacostatistical considerations, and there is an unmet clinical need to improve our understanding of key sources of variability in a mechanism-based fashion. Fundamental to the PBPK approach is the separation of information on the system (i.e., human body) from that of the drug (e.g., physicochemical characteristics determining permeability through membranes, partitioning to tissues, binding to plasma proteins, or affinities toward certain metabolizing enzymes and transporter proteins) and the study design (e.g., dose, route and frequency of administration, concomitant drugs, and food intake). This paradigm or “bottom–up” approach includes physiologically based in vitro–in vivo extrapolation and has in recent years gained momentum due to our increased understanding of the contributing factors (physical chemistry, systems biology, physiology, and pharmacogenetics) and advances in quantitative modeling using mechanistic models.
Figure 1.
Simulation of anticipated effects of gestation and trimester on maternal nifedipine clearance. Clearance is predicted to gradually increase during the first two trimesters to peak in the 3rd trimester at two to three times the postpartum clearance estimate.7 Courtesy Samer Mouksassi, Pharsight, Montreal, Canada.
Recent Advances
In the September issue of CPT-PSP, two studies described the applications of physiologically based pharmacokinetic modeling of CYP3A substrates in pregnant women.8,9 The studies are noteworthy as they highlight several important developments in the analysis of pharmacokinetic data in pregnant women that will aid in the design of more informative studies and the development of evidence-based dosing algorithms. These two research teams developed PBPK models using distinct approaches to predict the disposition of the CYP3A substrates midazolam, nifedipine, and indinavir. The semimechanistic pregnancy compartment model developed by Quinney et al. incorporated the intestinal lumen, intestinal wall, liver, portal circulation, and systemic circulation as elements to represent the key organs involved in the absorption, metabolism, and distribution of the investigated drugs midazolam and nifedipine. Physiological changes associated with pregnancy were modeled through the addition of placental and fetal compartments with metabolic capability, increased volume of distribution, decreased plasma protein binding, and increased CYP3A activity. Physiological compartments, not considered key determinants of drug clearance, were collapsed into the central and peripheral compartment.
The model used by Ke and colleagues represents a time-varying full-PBPK model based on the 13-compartment Simcyp model extended with a lumped compartment to represent placental–fetal organs including the fetus, placenta, and the amniotic fluid. Systemic clearance was considered to occur in the maternal liver and kidney, and presystemic metabolism was considered to occur in both maternal small intestine and the liver. Both studies used sensitivity analyses to interrogate known and potential pregnancy-related effects on absorption and bioavailability, such as hepatic blood flow, protein binding, CYP3A activity in the gut and liver, and fetal and maternal drug metabolism on pharmacokinetic parameters to predict mean area under the curve, peak plasma concentration, and trough plasma concentration and compare the results with published data in pregnant and nonpregnant women. Sensitivity analysis is the technique used to quantify the influences associated with specific input parameters, interactions between parameters and any nonlinear processes on model predictions. Of note, both studies conclude that PBPK modeling successfully predicted the disposition of CYP3A substrates midazolam, nifedipine, and indinavir during the third trimester. In addition, the models indicate that the two- to threefold increase in clearance can be attributed to increased CYP3A activity in the liver but not in the gut.
Future Directions
Despite the promise of PBPK as a powerful tool, there still are significant knowledge gaps, and a great amount of work will need to go into developing fully predictive pregnancy PBPK models. For now, available models quantitatively may give us a good starting point as to what to expect in terms of drug exposure in the mother. Positively, with the increasing efforts including recent systemic overviews of available data on the pregnancy-induced anatomical, physiological, and pharmacokinetic changes during pregnancy, this will definitely contribute to further model optimization. However, current limitations in PBPK modeling in particular, the need for prospective validation of PBPK models for their intended use in pregnancy need to be addressed. Given the limited pool of experts in PBPK modeling and the lack of formal comparisons of existing PBPK software, it would be desirable to develop guidance or best practice documents for PBPK modeling using existing experience and databases. Another challenge is the prediction of exposure-based fetal toxicities and the limitation to discern drug disposition parameters for the placenta and fetus without directly sampling these compartments.
Nevertheless, the potential of the PBPK approach is exiting, and to paraphrase Malcolm Rowland, one of the founding fathers: it is here and it can only get better.10
Conflict of Interest
The author declared no conflict of interest.
References
- van Hasselt J.G., Andrew M.A., Hebert M.F., Tarning J., Vicini P., &, Mattison D.R. The status of pharmacometrics in pregnancy: highlights from the 3(rd) American conference on pharmacometrics. Br. J. Clin. Pharmacol. 2012;74:932–939. doi: 10.1111/j.1365-2125.2012.04280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M., Peck C., &, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu. Rev. Pharmacol. Toxicol. 2011;51:45–73. doi: 10.1146/annurev-pharmtox-010510-100540. [DOI] [PubMed] [Google Scholar]
- Abduljalil K., Furness P., Johnson T.N., Rostami-Hodjegan A., &, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin. Pharmacokinet. 2012;51:365–396. doi: 10.2165/11597440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Zhao P.et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review Clin. Pharmacol. Ther. 89259–267.2011 [DOI] [PubMed] [Google Scholar]
- Grillo J.A.et al. Utility of a physiologically-based pharmacokinetic (PBPK) modeling approach to quantitatively predict a complex drug-drug-disease interaction scenario for rivaroxaban during the drug review process: implications for clinical practice Biopharm. Drug Dispos. 3399–110.2012 [DOI] [PubMed] [Google Scholar]
- Lu G., Abduljalil K., Jamei M., Johnson T.N., Soltani H., &, Rostami-Hodjegan A. Physiologically-based pharmacokinetic (PBPK) models for assessing the kinetics of xenobiotics during pregnancy: achievements and shortcomings. Curr. Drug Metab. 2012;13:695–720. doi: 10.2174/138920012800840374. [DOI] [PubMed] [Google Scholar]
- Prevost R.R., Akl S.A., Whybrew W.D., &, Sibai B.M. Oral nifedipine pharmacokinetics in pregnancy-induced hypertension. Pharmacotherapy. 1992;12:174–177. [PubMed] [Google Scholar]
- Ke A.B., Nallani S.C., Zhao P., Rostami-Hodjegan, A., &, Unadkat J.D. A PBPK model to predict disposition of CYP3A-metabolized drugs in pregnant women: verification and discerning the site of CYP3A induction e-pub ahead of print 26 September 2012; . CPT: Pharmacometrics & Systems Pharmacology. 2012;1 doi: 10.1038/psp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinney S.K.et al. A semi-mechanistic metabolism model of CYP3A Substrates in pregnancy: predicting changes in midazolam and nifedipine pharmacokinetics CPT: Pharmacometrics & Systems Pharmacology 12012e-pub ahead of print 26 September 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M.Addressing drug development questions with physiologically based pharmacokinetics 2012 . < http://www.rosaandco.com/webinarArchive.html >