Abstract
Proteins are the dominant cellular target for oxidative reactions because they comprise the majority of macromolecules. Post-translational oxidative protein modifications include fragmentation, aggregation and alteration of specific amino acid residues. The amino acids and amino acid residues most susceptible to oxidative modification are those containing sulfur and those with aromatic rings. Tryptophan reacts with radicals, ozone and singlet oxygen to form the end-product N-formylkynurenine (NFK). We recently described a novel anti-NFK antiserum and validated its use in immunological assays for the specific detection of NFK in isolated proteins and protein mixtures. Here we photooxidize rose bengal-containing HaCaT keratinocyte cells and examine the results using fluorescent confocal microscopy and staining with anti-NFK antiserum and markers for both Golgi and mitochondria. We show that photosensitization mediates the accumulation of NFK and that NFK can be detected in photosensitized cells with only slightly decreased viability. Additionally, we detect NFK modified proteins in both Golgi and mitochondria of photosensitized cells. These experiments demonstrate that we have developed a tool for the specific detection of oxidized tryptophan residues in cells and suggest that this tool could be useful in tracking the fate of these oxidized proteins.
INTRODUCTION
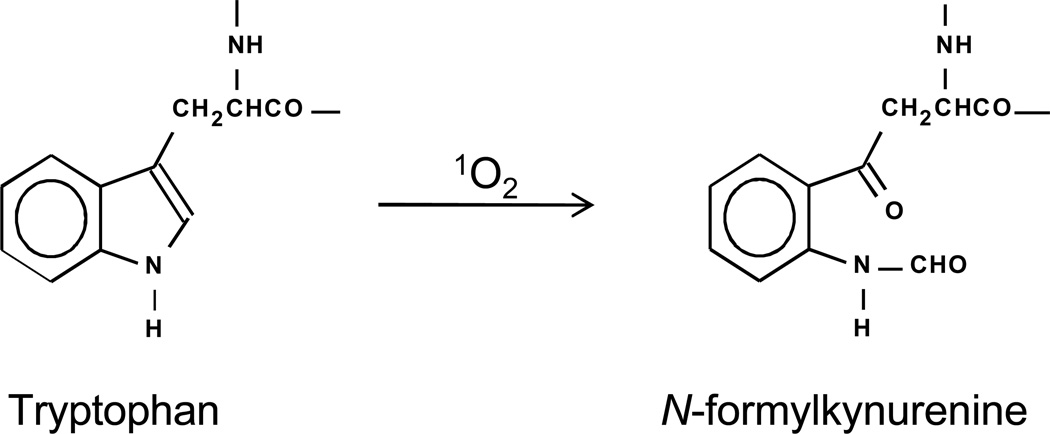
Cells can contain photosensitizers of either endogenous or exogenous origin which, upon illumination by UV and/or visible light, produce singlet oxygen (1O2). All cellular components can react with 1O2, but because proteins comprise the majority of cellular macromolecules, they are the most frequent targets of all oxidative species including 1O2 (1–3). Photosensitization of proteins can result in their aggregation into larger and sometimes insoluble multimeric forms, in their fragmentation due to breakage of the polypeptide backbone, and in alteration of specific amino acid residues. The amino acids and amino acid residues that are most reactive with 1O2 are the aromatics and the sulfur-containing ones (1–3). Tryptophan reacts with 1O2 resulting in the production of hydroperoxides, hydrotryptohans, kynurenenine and N-formylkynurenine (NFK) (1) (Fig. 1). Because tryptophan hydroperoxides are relatively unstable at physiological temperatures (4) the end-products of tryptophan reaction with 1O2 are a complex mixture of hydrotryptohans, kynurenenine and NFK, with the relative amounts of the different end products varying from protein to protein (4, 5). We recently reported a new method for detection of NFK residues in oxidized proteins using an NFK-specific polyclonal antiserum. In this previous work (6) we used western blotting for the detection of NFK in rose bengal-photooxidized myoglobin, in riboflavin-photosensitized milk and resulting from the reaction of carbonate radical with human SOD.
Figure 1.
Photochemical production of NFK from tryptophan.
In the current study, we use our anti-NFK antiserum to visualize the results of photosensitization in human keratinocyte cell culture. Because the skin and eyes are the organs most exposed to light, they are more likely than other tissues to suffer 1O2 damage, making skin cell and eye cell cultures highly suitable for photooxidation experiments. Both physical and biological techniques are routinely used to study and detect 1O2 itself and 1O2 reaction products in cells (7, 8). Singlet oxygen exhibits characteristic IR phosphorescence at 1268 nm, and Bilski et al. (7) used HaCaT keratinocyte monolayers to demonstrate that it is possible to detect 1O2 IR phosphorescence in irradiated rose bengal-containing cells. Another study (8) used immuno-spin trapping and confocal microscopy to identify 1O2 targets in rose bengal-containing HeCaT cells. He et al. (8) were able to trap protein radicals produced as a consequence of the long-lived hydroperoxides present in 1O2-exposed cells (9, 10) and show that these protein radical adducts partially colocalized with Golgi. More recently, Breitenbach et al. (11) described using focused irradiation of photosensitizer-containing HeLa cells to produce and detect 1O2 in single cells.
The aim of the present study was to determine the feasibility of using our recently developed anti-NFK antiserum to localize the products of 1O2-mediated tryptophan residue oxidation in cells. Here we used rose bengal photosensitization of HaCaT keratinocytes to show that NFK accumulates only in rose bengal-containing cells, and only after they are subjected to irradiation. Experiments were also performed to determine if we could colocalize oxidized proteins with cellular organelles. We exposed HaCaT keratinocyte cells to increasing concentrations of rose bengal and used fluorescent confocal microscopy to image NFK-containing proteins in both Golgi bodies and mitochondria of irradiated cells. We could detect NFK in cellular proteins in cells irradiated in the presence of as low as 1 µM rose bengal and could also visualize NFK-containing proteins in both Golgi and mitochondria. These experiments demonstrate that we have developed a tool which enables us to visualize oxidized proteins within cells and that should allow us to track the fate of these oxidized proteins using the NFK modification they contain.
MATERIALS AND METHODS
Cells
HaCaT keratinocytes, a transformed epidermal human cell line, were grown at 37°C in Dulbecco’s modified Eagle medium with 10% fetal calf serum in an atmosphere of 95% air-5% CO2. Experiments used cells at 80–90% confluence.
Chemicals
Rose bengal was obtained from Sigma Chemical Co. (St. Louis, MO), and the concentration of solutions was determined optically at λ=559 (ε = 90.4 mM−1 cm−1).
Light treatment
Before incubation with rose bengal, cells were washed twice in PBS at 37° C and after rose bengal was added, they were incubated for 1 h in the dark at 37°C. The cells were then washed twice with PBS at 37°C and exposed to cool white visible light (Phillips F40 AX50 5000 K advantage) for 20 min. Fluence measured using a YSI-Kettering Model 65A Radiometer (Yellow Springs Instrument Co., Yellow Springs, OH) was 1.8 J cm−2. Control samples were kept in the dark under the same conditions. All manipulations beginning with the addition of rose bengal were performed under dim red lighting. Cells were fixed and processed for confocal microscopy immediately (within 2 to 5 min) after light treatment.
Confocal microscopy
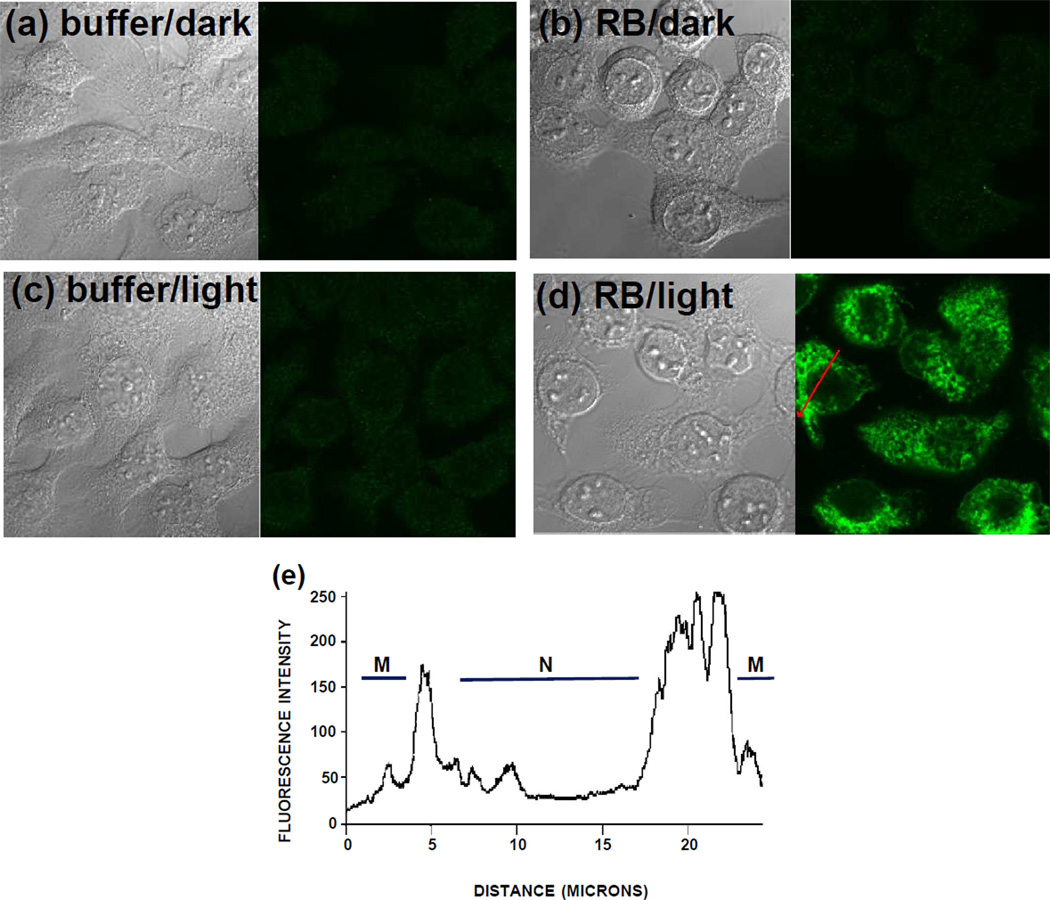
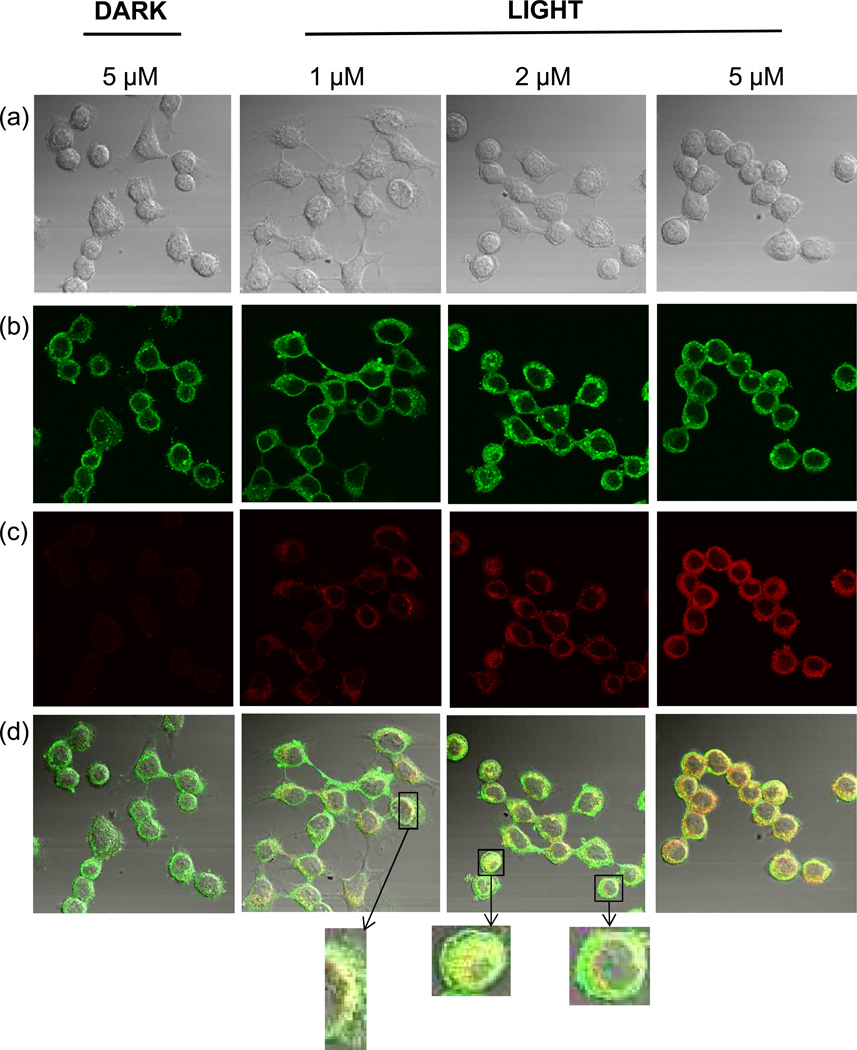
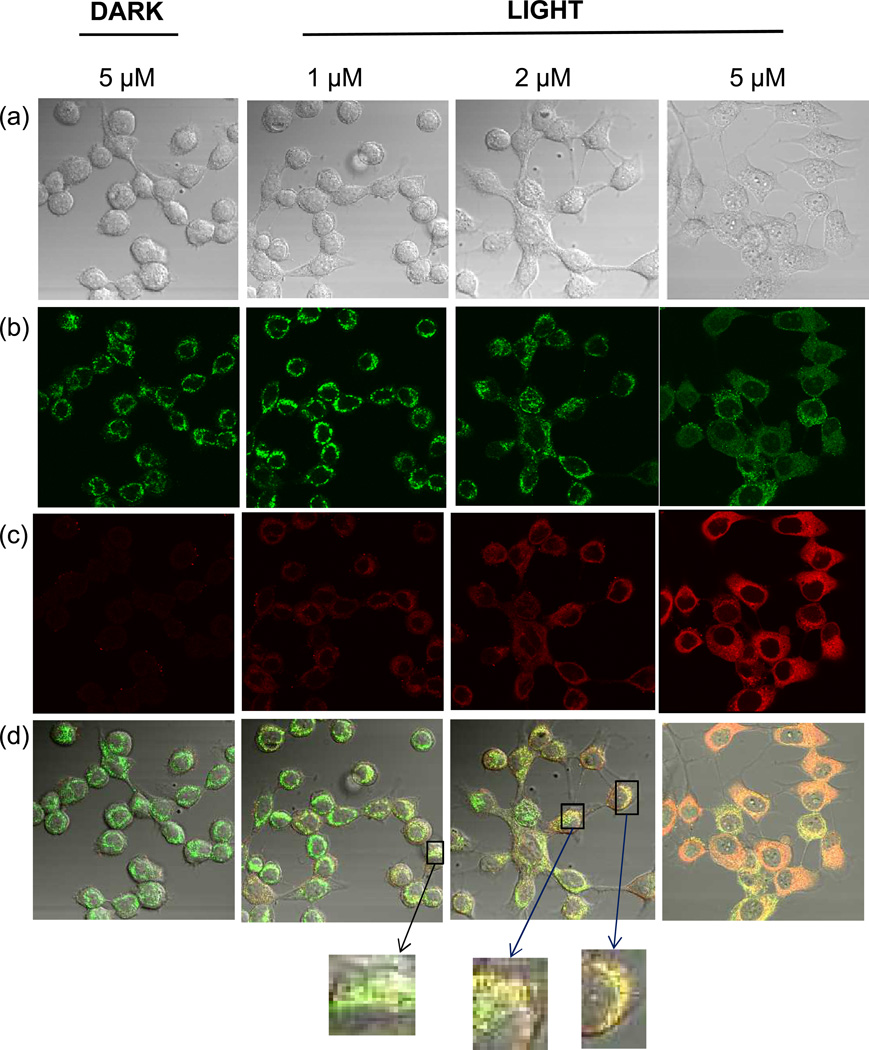
HaCaT keratinocytes were seeded onto 35 mm plates containing 1.5 mm thick coverslips (MatTek, Ashland MA). Treated cells were fixed and then stained with rabbit polyclonal anti-NFK (6) antibody followed by either Alexa Fluor 488 (Fig. 2) or Alexa Fluor 568 anti-rabbit antibody (Figs. 3 and 4.) For colocalization experiments, cells were simultaneously stained with either mouse monoclonal anti-golgin 97 or mouse monoclonal anti-mitochondrial complex III followed by Alexa Fluor 568 anti-mouse antibody. Pixel intensity in Fig. 2 was determined using the Zeiss LSM Image Browser.
Figure 2.
Confocal images of HaCaT cells stained with anti-NFK antibody and Alexa 488 secondary antibody and visualized using a 63X oil immersion lens. Cells incubated with 10 µM rose bengal (RB) (b, d) or buffer (a, c) for 1 hour in the dark were washed free of the dye and were then exposed to 20 min incubation under cool white fluorescent lights (c, d) or were maintained in the dark (a, b). Paired images show transmission microscopy on the left side and confocal fluorescence microscopy on the right side. (e) Pixel intensity across a cell in (d) as indicated by red arrow. M, membrane; N, nucleus.
Figure 3.
Partial colocalization of NFK with Golgi in HaCaT cells after photosensitization with rose bengal. HaCaT cells were incubated with 1, 2, or 5 µM rose bengal as indicated. After washing, cells were exposed to 20 min incubation under cool white fluorescent lights or were maintained in the dark as indicated. Cells were stained with anti-NFK antibody and anti-Golgi-97 antibody followed by Alexa 488 and Alexa 568 antibody. (a) DIC image (transmission microscopy), (b) anti-golgin-97 (green), (c) anti-NFK (red), and (d) merge of (a), (b) and (c).
Figure 4.
Partial colocalization of NFK with mitochondria in HaCaT cells after photosensitization with rose bengal. HaCaT cells were incubated with 1, 2, or 5 µM rose bengal as indicated. After washing, cells were exposed to 20 min incubation under cool white fluorescent lights or were maintained in the dark as indicated. Cells were stained with anti-NFK antibody and anti-mitochondrial complex III antibody followed by Alexa 488 and Alexa 568 antibody. (a) DIC image (transmission microscopy), (b) anti-mitochondrial complex III (green), (c) anti-NFK (red), and (d) merge of (a), (b) and (c).
Cell viability assays
Cell viability was determined using CellTiter 96 (Promega, Madison, WI) which measures the production of a formazan product produced only by metabolically active mitochondria. Cells were seeded onto the wells of microtiter plates and allowed to grow until 80 to 90% confluence. Cells washed twice in PBS were then incubated in the dark for 1 h at 37°C with rose bengal or with PBS followed by washing twice with PBS. For visible light treatment, cells were treated as described above, and control samples were kept in the dark under the same conditions. All manipulations beginning with the addition of rose bengal were performed under dim red lighting. Cell viability was determined following light treatment by removal of buffer and addition of the assay substrate in medium without phenol red. Accumulation of the formazan product was determined by its absorbance at 490 nm after 2h incubation with substrate at 37°C. The data presented are the averages and standard deviations from 8 wells.
RESULTS
An initial experiment was performed to determine whether we could visualize NFK-containing proteins in cells using anti-NFK antiserum and confocal fluorescence microscopy. HaCaT keratinocyte cells were incubated in the dark with either 10 µM rose bengal or with buffer. The cells were then washed and exposed to light or were maintained in the dark. Staining with anti-NFK revealed that only the photosensitized, light-exposed rose bengal-treated cells (Fig. 2d) accumulated NFK and that NFK was undetectable in the other treatments (Fig. 2a, b, and c). Analysis of the pixel intensity (Fig. 2e) in the light-exposed RB-treated cells (Fig. 2d) indicated that the tryptophan oxidation product NFK accumulated in the perinuclear region.
In order to more closely define the area of NFK accumulation, we then incubated HaCaT cells with either 1, 2, or 5 µM rose bengal and exposed them to light or incubated them in the dark as indicated. In addition to staining with anti-NFK, the cells were also stained with either anti-golgin-97 (Fig. 3) or with anti-mitochondrial complex III (Fig. 4). As in Fig. 2, NFK was undetected in rose bengal-containing cells maintained in the dark. In contrast, light-exposed rose bengal-containing cells accumulated increasing amounts of NFK with increasing concentrations of the photosensitizer. Staining with either anti-golgin-97 (Fig. 3) or anti-mitochondrial complex III (Fig. 4) showed colocalization of NFK with both Golgi and mitochondria in photosensitized cells. With increasing photosensitization, colocalization became more apparent.
Cell viability assays were also performed to determine the effect of photosensitization on cell survival (Table 1). These assays indicated that rose bengal was not toxic to the HaCaT cells in the absence of light and that toxicity increased with increasing rose bengal concentration with a high (90.3%) survival rate in cells treated with 1 µM rose bengal to a less than 50% survival rate in cells treated with 10 µM rose bengal.
Table 1.
Cell viability
| Light or Dark | µM rose bengal | Abs 490 | % cell viability* |
|---|---|---|---|
| Dark | 0 | .403 ±.038 | 100 |
| Dark | 10 | .389 ±.023 | 96.5 |
| Light | 0 | .418 ±.035 | 103.8 |
| Light | 1 | .364 ±.020 | 90.3 |
| Light | 2 | .310 ±.035 | 76.9 |
| Light | 5 | .235 ±.022 | 58.3 |
| Light | 10 | .161 ±.012 | 40.1 |
As percentage of dark, 0 µM rose bengal-treated cells
DISCUSSION
A previous paper showed that immunoassays with anti-NFK antiserum were both a specific and sensitive measure of tryptophan residue oxidation to NFK in in vitro experiments with single proteins and protein mixtures (6). This current study has now shown that anti-NFK is also an effective tool for the detection of many reactive oxygen species in cells because it allows not only a clear distinction between photooxidized and non-oxidized cells (Fig. 2) but is also able to detect NFK-containing proteins in cells subject to low, non-lethal levels of oxidation due to photostress (Figs. 3 & 4, Table 1). The cell viability assays indicated that illumination in the presence of 1 µM rose bengal caused little loss of viability. The confocal analysis of the cells, however, showed NFK levels clearly above those found in non-photooxidized controls.
This ability to detect oxidative modification of proteins under conditions that allow cell survival provides a tool to map the fate of oxidized proteins in healthy cells. The range of photooxidation observed in this study (90% to 40% viability) also indicates that NFK is a useful tag for oxidized proteins in cells experiencing increasing degrees of oxidation and could be used to provide valuable information on how the level of oxidation contributes to cell fate.
A recent study (8) undertaken prior to the development of the anti-NFK antiserum used a creative but somewhat indirect approach to detect the site of 1O2 production in photosensitized HaCaT cells. He et al. (8) based their experimental design on the observation (9, 10) that exposing cells to 1O2 results in the generation of long-lived protein hydoperoxides. Rose bengal-photooxidized HaCaT cells were subsequently treated with both copper chloride to promote the production of protein radicals from the protein hydroperoxides and DMPO to trap the protein radicals. Staining of the cells with anti-DMPO antiserum and visualization with confocal fluorescence microscopy allowed them to localize the DMPO protein adducts and, hence, where the 1O2 had been. The results from this study showed that DMPO protein radical adducts localized in the perinuclear region, coincident with the site of rose bengal and 1O2 localization, and that radical adducts could be partially colocalized with Golgi.
Our experiments also examined HaCaT cells photosensitized with rose bengal, but using a more direct approach. Because anti-NFK antiserum recognizes a direct product of photosensitization, these experiments could be performed without the addition of exogenous substances such as DMPO. This approach avoids the possible interference and scavenging that can result from the addition of probes which act as either antioxidants or prooxidants. Our work confirms and also extends the findings of He et al. (8). We not only report the partial colocalization of 1O2 oxidation in the Golgi but describe detection of NFK-containing proteins colocalizing with mitochondria.
Most reports of rose bengal-mediated subcellular effects describe localization in and photooxidation of Golgi and plasma membranes (12–14). Because rose bengal is anionic, it is generally accepted that it does not localize to mitochondria, which more readily take up cationic dyes (15). Previous reports of rose bengal-mediated photosensitization of mitochondria used either isolated submitochondrial particles (16) or stained the cells with rose bengal acetate (17), a derivative which is more hydrophobic and more readily cell permeable than the parent molecule. Our data, however, clearly show colocalization of NFK-containing proteins with a protein of mitochondrial complex III. There is more than one potential explanation for this. It is possible that a low concentration of rose bengal may diffuse into mitochondria and produce sufficient 1O2 to oxidize tryptophan residues to NFK and that our assay is sensitive enough to detect even a low level of this oxidation. It is also possible that during the time course of the experiment, proteins photooxidized elsewhere have translocated either into or very close to the mitochondria.
There has been growing recognition of the role that 1O2-mediated oxidation plays in development, cell death and signal transduction in both plants and animals (18–24). Studies aimed at detection of singlet oxygen and singlet oxygen effects frequently employ physical/chemical methods to detect 1O2 in vivo (7, 11, 25) and indirect methods to assess where singlet oxygen has been produced in cells by detecting end products of singlet oxygen chemistry (8, 9). We have now demonstrated that the use of anti-NFK in immunological assays enables us to detect and measure a specific product of protein oxidation in cells. This immunological approach, therefore, will allow us to first, map the site(s) of oxidative protein modification and then to chronicle the fate of those altered proteins.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. We are grateful to Jeff Tucker and Holly Rutledge for their expert help with confocal microscopy. We thank Ann Motten, B. Jean Corbett and Mary J. Mason and for valuable help in the preparation of this manuscript. We dedicate this paper to the memory of Colin F. Chignell.
References
- 1.Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003;305:761–770. doi: 10.1016/s0006-291x(03)00817-9. [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ. The oxidative environment and protein damage. Biochim. Biophys. Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 4.Gracanin M, Hawkins CL, Pattison DI, Davies MJ. Singlet-oxygen-mediated amino acid and protein oxidation: Formation of tryptophan peroxides and decomposition products. Free Rad. Biol. Med. 2009;47:92–102. doi: 10.1016/j.freeradbiomed.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Ronsein GE, de Oliveira MCB, de Medeiros MHG, Di Mascio P. Characterization of O2 (1Δg)-derived oxidation products of tryptophan: A combination of tandem mass spectrometry analyses and isotopic labeling studies. J. Am. Soc. Mass Spectrom. 2009;20:188–197. doi: 10.1016/j.jasms.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Ehrenshaft M, de Oliveira Silva S, Perdivara I, Bilski P, Sik RH, Chignell CF, Tomer KB, Mason RP. Immunological detection of N-formylkynurenine in oxidized proteins. Free Rad. Biol. Med. 2009;46:1260–1266. doi: 10.1016/j.freeradbiomed.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilski P, Kukielczak BM, Chignell CF. Photoproduction and direct spectral detection of singlet molecular oxygen (1O2) in keratinocytes stained with rose bengal. Photochem. Photobiol. 1998;68:675–678. [PubMed] [Google Scholar]
- 8.He Y-Y, Council SE, Feng L, Bonini MG, Chignell CF. Spatial distribution of protein damage by singlet oxygen in keratinocytes. Photochem. Photobiol. 2008;84:69–74. doi: 10.1111/j.1751-1097.2007.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies MJ. Reactive species formed on proteins exposed to singlet oxygen. Photochem. Photobiol. Sci. 2004;3:17–25. doi: 10.1039/b307576c. [DOI] [PubMed] [Google Scholar]
- 10.Wright A, Hawkins CL, Davies MJ. Photo-oxidation of cells generates long-lived intracellular protein peroxides. Free Rad. Biol. Med. 2003;34:637–647. doi: 10.1016/s0891-5849(02)01361-8. [DOI] [PubMed] [Google Scholar]
- 11.Breitenbach T, Kuimova MK, Gbur P, Hatz S, Schack NB, Pedersen BW, Lambert JDC, Poulsen L, Ogilby PR. Photosensitized production of singlet oxygen: spatially-resolved optical studies in single cells. Photochem. Photobiol. Sci. 2009;8:442–452. doi: 10.1039/b809049a. [DOI] [PubMed] [Google Scholar]
- 12.Kochevar IE, Bouvier J, Lynch M, Lin C-W. Influence of dye and protein location on photosensitization of the plasma membrane. Biochim. Biophys. Acta. 1994;1196:172–180. doi: 10.1016/0005-2736(94)00236-3. [DOI] [PubMed] [Google Scholar]
- 13.Soldani C, Bottone MG, Croce AC, Fraschini A, Bottiroli G, Pellicciari C. The Golgi apparatus is a primary site of intracellular damage after photosensitization with Rose Bengal acetate. European J. Histochem. 2004;48:443–448. doi: 10.4081/919. [DOI] [PubMed] [Google Scholar]
- 14.Tseng SCG, Feenstra RPG, Watson BD. Characterization of photodynamic actions of rose bengal on cultured cells. Invest. Ophthalmol. Vis. Sci. 1994;35:3295–3307. [PubMed] [Google Scholar]
- 15.Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. Targeting mitochondria. Acc. Chem. Res. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- 16.Giulivi C, Sarcansky M, Rosenfeld E, Boveris A. The photodynamic effect of rose bengal on proteins of the mitochondrial inner membrane. Photochem. Photobiol. 1990;52:745–751. doi: 10.1111/j.1751-1097.1990.tb08676.x. [DOI] [PubMed] [Google Scholar]
- 17.Bottone MG, Soldani C, Fraschini A, Alpini C, Croce AC, Bottiroli G, Pellicciari C. Enzyme-assisted photosensitization with rose Bengal acetate induces structural and functional alteration of mitochondria in HeLa cells. Histochem. Cell Biol. 2007;127:263–271. doi: 10.1007/s00418-006-0235-9. [DOI] [PubMed] [Google Scholar]
- 18.Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana . Proc. Natl. Acad. Sci. USA. 2007;104:672–677. doi: 10.1073/pnas.0609063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baruah A, Simkova K, Apel K, Laloi C. Arabidopsis mutants reveal multiple singlet oxygen signaling pathways involved in stress response and development. Plant Mol. Biol. 2009;70:547–563. doi: 10.1007/s11103-009-9491-0. [DOI] [PubMed] [Google Scholar]
- 20.Grimbaldeston MA, Geczy CL, Tedla N, Finlay-Jones JJ, Hart PH. S100A8 induction in keratinocytes by ultraviolet A irradiation is dependent on reactive oxygen intermediates. J. Invest. Derm. 2003;121:1168–1174. doi: 10.1046/j.1523-1747.2003.12561.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim C, Meskaukiene R, Apel K, Laloi C. No single way to understand singlet oxygen signalling in plants. EMBO Reports. 2008;9:435–439. doi: 10.1038/embor.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochevar IE, Lynch MC, Zhuang S, Lambert CR. Singlet oxygen, but not oxidizing radicals, induces apoptosis in HL-60 cells. Photochem. Photobiol. 2000;72:548–553. doi: 10.1562/0031-8655(2000)072<0548:sobnor>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Otsu K, Sato K, Ikeda Y, Imai H, Nakagawa Y, Ohba Y, Fujii J. An abortive apoptotic pathway induced by singlet oxygen is due to the suppression of caspase activation. Biochem. J. 2005;389:197–206. doi: 10.1042/BJ20042067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soldani C, Croce AC, Bottone MG, Fraschini A, Biggiogera M, Bottiroli G, Pellicciari C. Apoptosis in tumour cells photosensitized with Rose Bengal acetate is induced by multiple organelle photodamage. Histochem. Cell. Biol. 2007;128:485–495. doi: 10.1007/s00418-007-0333-3. [DOI] [PubMed] [Google Scholar]
- 25.Baier J, Maisch T, Maier M, Landthaler M, Baumler W. Direct detection of singlet oxygen generated by UVA irradiation in human cells and skin. J. Invest. Derm. 2007;127:1498–1506. doi: 10.1038/sj.jid.5700741. [DOI] [PubMed] [Google Scholar]