Abstract
The MUC1 oncoprotein is overexpressed by diverse human cancers; however, it has remained largely unclear how MUC1 contributes to tumorigenesis. In this issue of Oncogene and in concert with published work, Behrens et al. report that the MUC1 receptor subunit activates genes involved in invasion, angiogenesis and metastasis.
Keywords: MUC1, MUC1-C subunit, nuclear localization, transcriptional regulation, Wnt/β-catenin, p53
Mucin 1 (MUC1) is a heterodimeric protein that is aberrantly expressed by most human carcinomas and certain hematologic malignancies (Kufe, 2009). Estimates indicate that about 900,000 of the 1.4 million tumors diagnosed in the US each year have aberrant MUC1 expression. MUC1 has therefore emerged as an attractive target for the development of anticancer agents. However, progress in the identification of drugs that target MUC1 has been limited by the lack of understanding of how MUC1 contributes to malignant progression. In this context, MUC1 consists of two subunits; an extracellular mucin subunit and a transmembrane receptor subunit. Early research was predominantly focused on the shed extracellular mucin subunit. By contrast, more recent studies of the MUC1 transmembrane receptor have revealed a function for this subunit as an oncoprotein that is targeted to the nucleus and regulates gene expression. In this issue of Oncogene, Behrens et al. provide more insights into the transcriptional effects of MUC1 in cancer cells. Importantly, the findings that MUC1 regulates gene expression have provided the basis for developing direct inhibitors of the MUC1 cytoplasmic domain as anti-cancer agents.
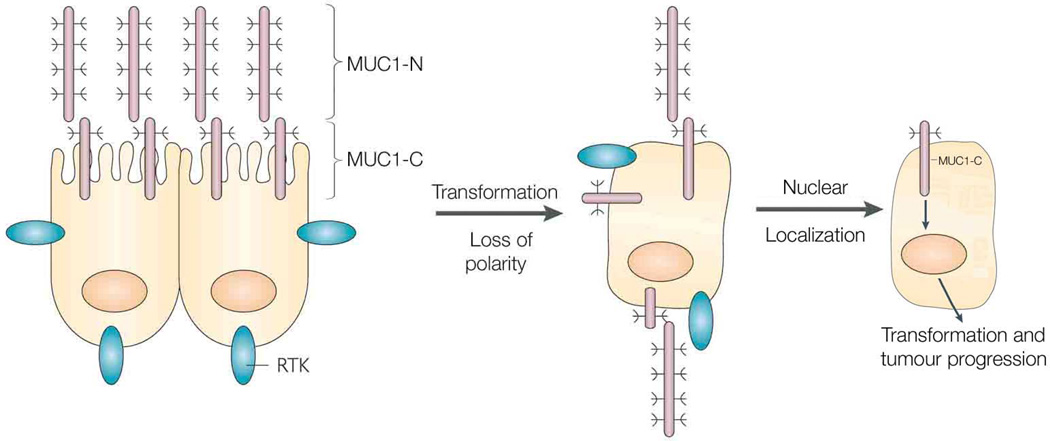
MUC1 is translated as a single polypeptide that undergoes autocleavage into two subunits that then form a noncovalent heterodimer. The MUC1 N-terminal subunit (MUC1-N) is a highly glycosylated mucin that is expressed at the cell surface in a complex with the transmembrane MUC1 C-terminal subunit (MUC1-C) (Fig. 1). The MUC1 heterodimer is positioned at the apical border of normal epithelial cells where it contributes to a protective mucous barrier (Fig. 1). With loss of polarity as a result of transformation, the MUC1-C subunit interacts with receptor tyrosine kinases, such as ErbB1–4, that otherwise reside at the basolateral borders, and participates in their downstream signaling pathways (Kufe, 2009) (Fig. 1). In this regard, the MUC1-C cytoplasmic domain serves as a substrate for phosphorylation in the response to activation of EGFR, FGFR3, PDGFRβ and Met (Kufe, 2009).
Figure 1. Localization of the MUC1 subunits in normal epithelial cells and carcinoma cells.
The MUC1 heterodimer consisting of the MUC1-N mucin subunit and the MUC1-C transmembrane subunit are positioned at the apical membrane of polarized epithelial cells. With loss of polarity associated with the epithelial stress response or transformation, MUC1 is repositioned over the entire cell membrane and interacts with receptor tyrosine kinases (RTKs). In addition, with overexpression of MUC1 in carcinoma cells, MUC1-C accumulates in the cytoplasm and is targeted to the nucleus where it regulates the expression of genes associated with tumorigenesis, angiogenesis and ECM remodeling. Reproduced and modified with permission from reference (Kufe, 2009).
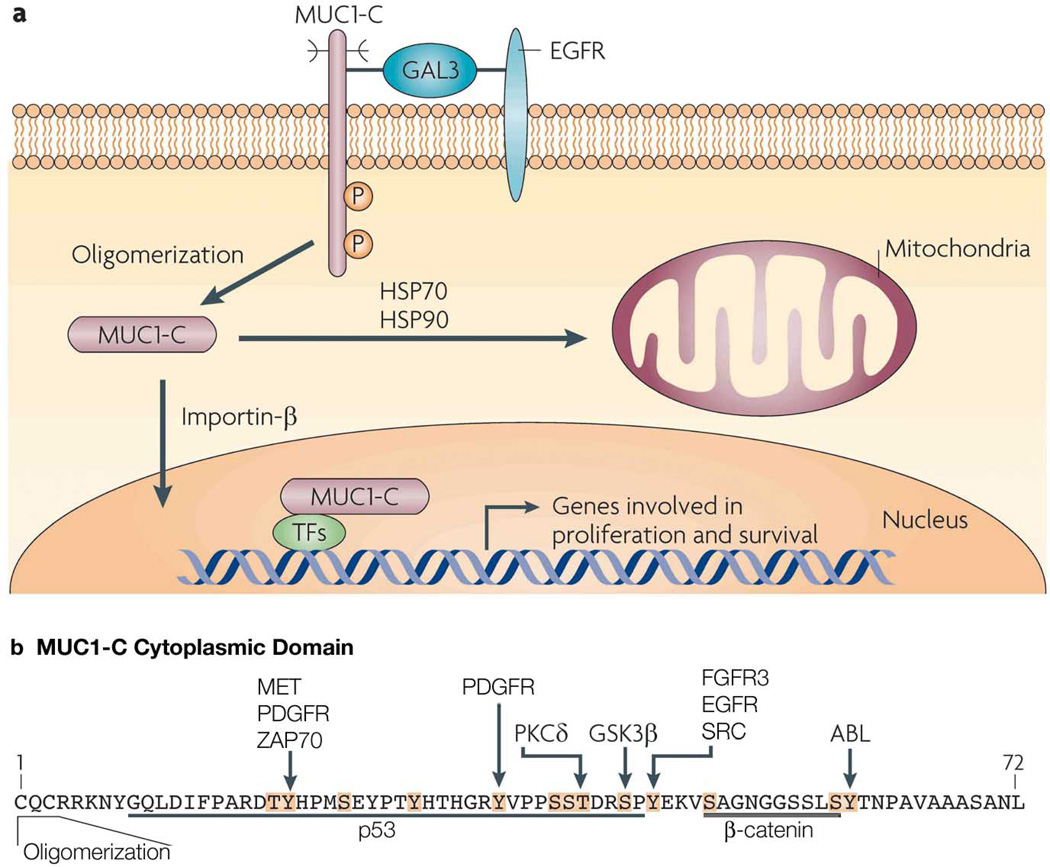
The MUC1-C subunit consists of a 58 amino acid extracellular domain that is glycosylated on Asn-36 and then serves as a binding site for the galectin-3 ligand (Fig. 2a). Galectin-3 functions as a bridge to associate MUC1-C with EGFR and potentially other cell surface receptors (Ramasamy et al., 2007) (Fig. 2a). The MUC1-C cytoplasmic domain or tail consists of 72 amino acids that include a CQC sequence adjacent to the transmembrane domain (Fig. 2b). The CQC motif is necessary for the formation of MUC1-C oligomers (Kufe, 2009) (Figs. 2a and b). The MUC1-C cytoplasmic domain also contains 13 documented and putative tyrosine and serine/threonine phosphorylation sites that contribute to interactions with various effectors linked to transformation (Fig. 2b). For example, phosphorylation of the YEKV site induces binding of the MUC1-C cytoplasmic SAGNGGSSLS sequence to β-catenin (Fig. 2b), that in turn stabilizes β-catenin and promotes activation of Wnt target genes, such as cyclin D1 (Huang et al., 2005) and, as now shown by Behrens et al., connective tissue growth factor (CTFG) (Behrens et al., 2010).
Figure 2. Activation of the MUC1-C subunit in carcinoma cells.
(a) The MUC1-C subunit forms complexes with EGFR that are mediated at least in part by galectin-3 (GAL3). The MUC1-C cytoplasmic domain is phosphorylated by EGFR on the YEKV motif. Localization of MUC1-C to the cytoplasm is associated with its oligomerization and transport to the nucleus by an importin-β-mediated mechanism. The association of MUC1-C with certain transcription factors (TFs) promotes the expression of genes involved in proliferation, survival, angiogenesis and ECM remodeling. MUC1-C is also transported to the mitochondrial outer membrane in a complex with HSP70 and HSP90. (b) Amino acid sequence of the MUC1-C cytoplasmic domain which contains documented and potential phosphorylation sites (highlighted in orange with the known kinases indicated). The CQC motif is necessary for MUC1-C oligomerization and targeting to the nucleus and mitochondria. Also highlighted are the p53 and β-catenin binding sites. Reproduced and modified with permission from reference (Kufe, 2009).
In carcinoma cells, MUC1-C accumulates in the cytoplasm as a result of release from the cell membrane and/or redirection from the endoplasmic reticulum. MUC1-C is also targeted to the nucleus by a mechanism that is dependent on its oligomerization and thereby interaction with importin β (Kufe, 2009) (Fig. 2a). Importantly, in this regard, blocking the MUC1-C CQC motif abrogates oligomerization and thus has provided a druggable approach to inhibit localization of MUC1-C to the nucleus (Raina et al., 2009). In the nucleus, MUC1-C associates with certain transcription factors that include β-catenin/TCF4, p53, estrogen receptor α (ERα), NF-κB p65 and STATs (Kufe, 2009). Moreover, as determined by chromatin immunoprecipitation (ChIP) analysis, MUC1-C associates with these transcription factors on the respective response elements in their target genes (Kufe, 2009). Behrens et al. now extend these findings to the promoter of the CTGF gene and, specifically, demonstrate MUC1-induced increases in β-catenin and p53 occupancy (Behrens et al., 2010).
Overexpression of MUC1 is sufficient for the induction of anchorage-independent growth and tumorigenicity (Kufe, 2009). Moreover, studies have shown that the MUC1-C cytoplasmic domain confers transformation and that the MUC1-N subunit is not required for this response (Huang et al., 2005). The available evidence indicates that targeting of MUC1-C to the nucleus and the induction of specific gene expression is responsible, at least in part, for the transformed phenotype. MUC1-C is also targeted to the mitochondrial outer membrane where it blocks induction of apoptosis in the response to genotoxic, oxidative, hypoxic and other forms of stress (Kufe, 2009) (Fig. 2a). The focus of this commentary relates to the nuclear function of MUC1-C and the reader is referred to a recent review that summarizes the effects of MUC1-C on mitochondrial death pathways, with the perspective that targeting of MUC1-C to the nucleus and mitochondria is integrated in promoting growth and survival (Kufe, 2009).
As noted above, MUC1-C forms complexes with certain transcription factors that in turn occupy response elements on promoters of target genes (Fig. 2a). Whether these complexes originate in the cytoplasm or form in the nucleus may vary depending on the transcription factor. With regard to p53, the MUC1-C cytoplasmic domain interacts constitutively with p53 and the interaction is increased in the response to genotoxic stress (Wei et al., 2005). The MUC1-C cytoplasmic domain (amino acids 9–46) binds directly to the p53 regulatory domain (amino acids 363–393) (Fig. 2b). ChIP and re-ChIP analyses of ZR-75-1 breast cancer cells that overexpress endogenous MUC1 demonstrated that MUC1-C occupies the p53-responsive p21 gene promoter in complexes with the p53 protein. MUC1-C occupancy with the p53 transcription complex was associated with (i) recruitment of the CBP histone acetytransferase, (ii) a decrease in the HDAC1 histone deacetylase, and (iii) an increase in histone H4 acetylation (Wei et al., 2005). In concert with these findings, MUC1-C occupancy of the p21 promoter was associated with increased expression of p21 in the growth arrest and survival response to genotoxic stress (Wei et al., 2005).
In the studies by Behrens et al., forced overexpression of exogenous MUC1 in pancreatic cancer cells was associated with MUC1 cytoplasmic tail (MUC1.CT) occupancy of the CTGF gene promoter (Behrens et al., 2010). The MUC1.CT nomenclature apparently refers to the MUC1-C subunit based on the detection of the 20–25 kDa MUC1-C in the immunoblots shown in Fig. 1b in their paper. Indeed, to date, there has been no evidence that MUC1-C is cleaved such that the 72 amino acid cytoplasmic tail/domain is released for intracellular targeting. The authors show that MUC1.CT occupies regions within the CTGF gene, the proximal promoter (−859/−658) and several regions, including −2345/−1996, upstream in the CTGF promoter (Fig. 3 in their paper). MUC1 overexpression was also associated with increased occupancy of (i) p53 on the −2345/−1996 promoter region, and (ii) β-catenin on the −859/−658 and −2345/−1996 promoter regions. Notably, a TCF binding site (CTTTGTT) is located at −640 the CTGF proximal promoter (Deng et al., 2007) and the locations of the potential p53 and TCF binding sites within −2345/−1996 are not identified. Nonetheless, MUC1 overexpression is clearly associated with activation of CTGF gene transcription and protein expression.
As reported last year, the MUC1-C cytoplasmic domain activates specific gene families involved in oncogenesis, angiogenesis, and extracellular matrix (ECM) remodeling (Khodarev et al., 2009). In addition, a set of MUC1-C-induced genes associated with tumorigenesis was applied to the analysis of breast and lung adenocarcinoma databases. A 35-gene MUC1-C-induced tumorigenesis signature predicted significant decreases in both disease-free and overall survival in patients with breast (n=295) and lung (n=442) cancers (Khodarev et al., 2009). In subsequent studies, MUC1-C-induced gene signatures have been significantly associated with (i) failure of ER+ breast cancers to respond to tamoxifen treatment (Pitroda et al., 2009), and (ii) poor prognosis breast cancers (Khodarev et al., 2010). These findings have collectively established a relationship between MUC1-C-induced gene expression profiles and aggressive behavior of breast and lung cancers.
In the paper by Behrens et al., the authors report that overexpression of exogenous MUC1 in pancreatic cancer cells is also associated with targeting of genes related to invasion, angiogenesis and metastasis (Behrens et al., 2010). Specifically, they show that MUC1 induces expression of CTGF, a mediator of ECM remodeling and angiogenesis. These findings indicate that MUC1 induces similar sets of gene signatures in breast, lung and pancreas cancers. The findings further indicate that blocking the MUC1-C subunit and its localization to the nucleus could inhibit the induction of genes that contribute to the malignant phenotype. Indeed, a peptide drug that directly blocks MUC1-C oligomerization has been shown to be effective against human breast cancer cells growing in vitro and as tumor xenografts in mouse models (Raina et al., 2009). What is needed now, at least in part, is thus to effectively exploit the involvement of MUC1-C-induced gene expression in tumorigenesis with the development of agents that inhibit its nuclear localization and function.
References
- Behrens ME, Grandgenett P, Bailey J, Singh P, Yi C, Yu F, et al. The reactive tumor microenvironment: MUC1 signaling directly reprograms transcription of CTGF. Oncogene. 2010 doi: 10.1038/onc.2010.327. (e-pub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YZ, Chen PP, Wang Y, Yin D, Koeffler HP, Li B, et al. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J. Biol. Chem. 2007;282:36571–36581. doi: 10.1074/jbc.M704141200. [DOI] [PubMed] [Google Scholar]
- Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks GSK3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005;65:10413–10422. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- Khodarev N, Ahmad R, Rajabi H, Pitroda S, Kufe T, McClary C, et al. Cooperativity of the MUC1 oncoprotein and STAT1 pathway in poor prognosis human breast cancer. Oncogene. 2010;29:920–929. doi: 10.1038/onc.2009.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodarev N, Pitroda S, Beckett M, MacDermed D, Huang L, Kufe D, et al. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res. 2009;69:2833–2837. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe D. Mucins in cancer: function, prognosis and therapy. Nature Reviews Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitroda S, Khodarev N, Beckett M, Kufe D, Weichselbaum R. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc. Natl. Acad. Sci. USA. 2009;106:5837–5841. doi: 10.1073/pnas.0812029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina D, Ahmad R, Joshi M, Yin L, Wu Z, Kawano T, et al. Direct targeting of the MUC1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133–5141. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol. Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7:167–178. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]