Abstract
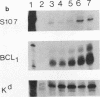
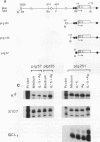
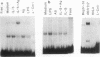
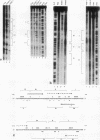
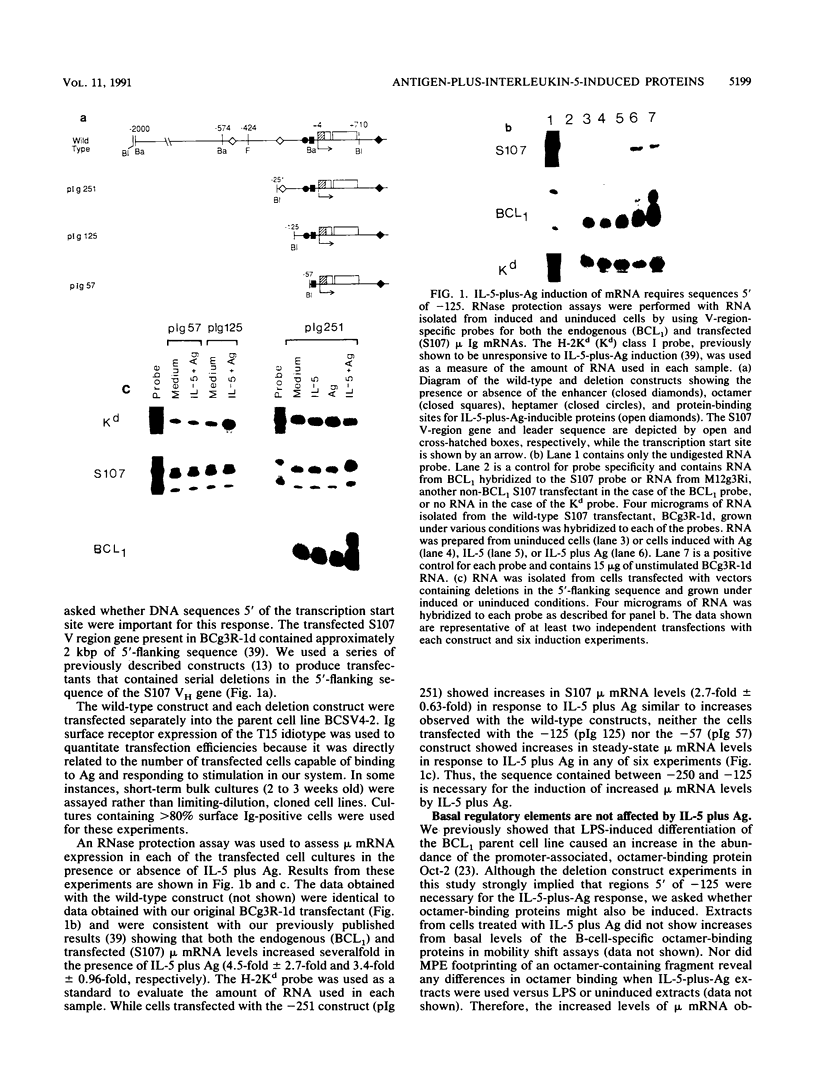
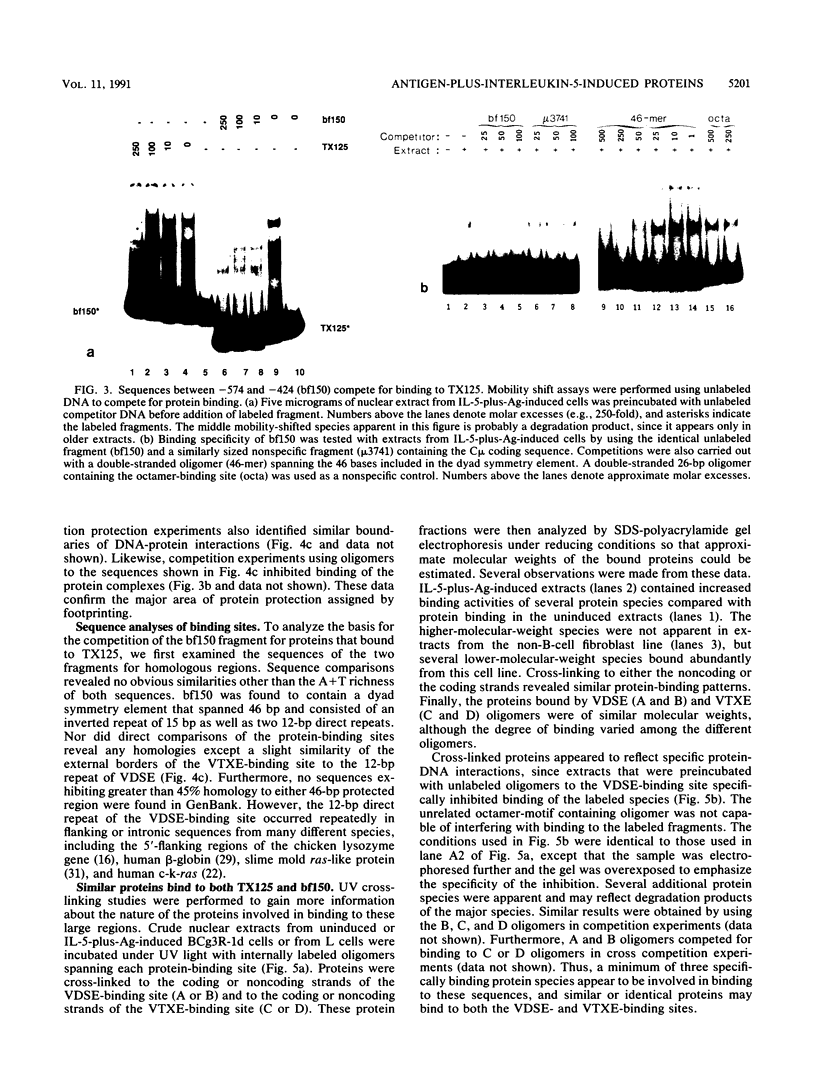
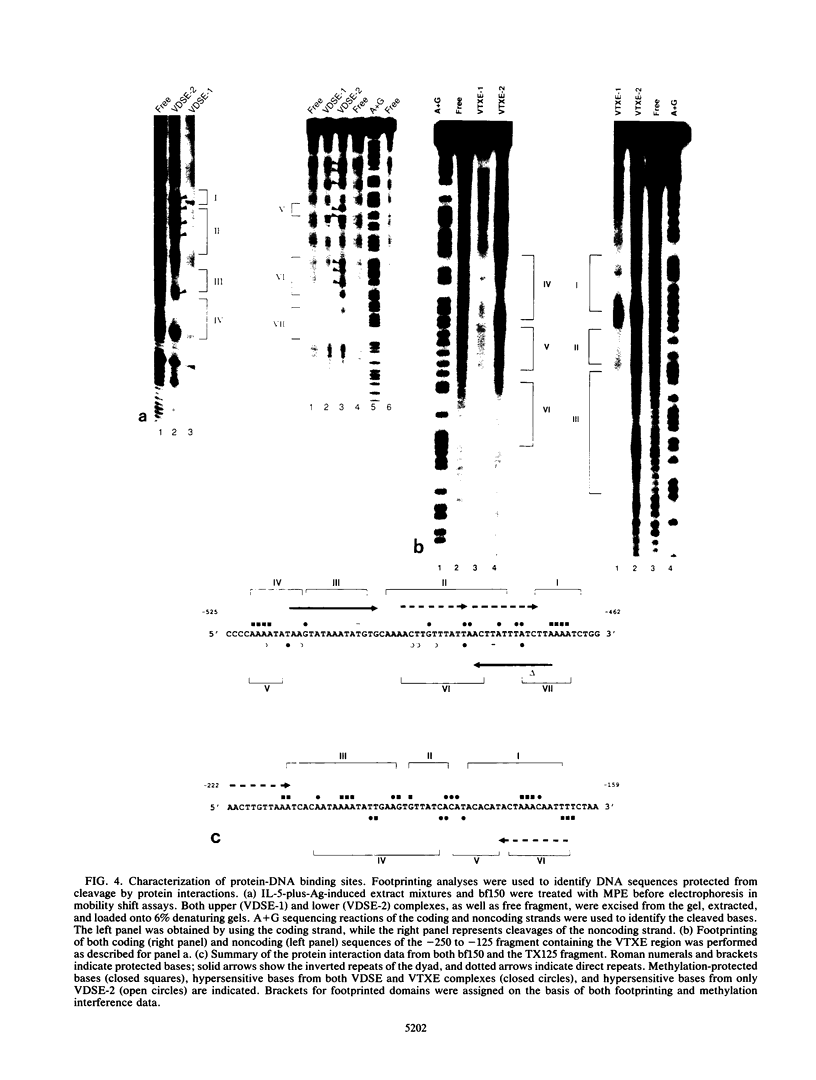
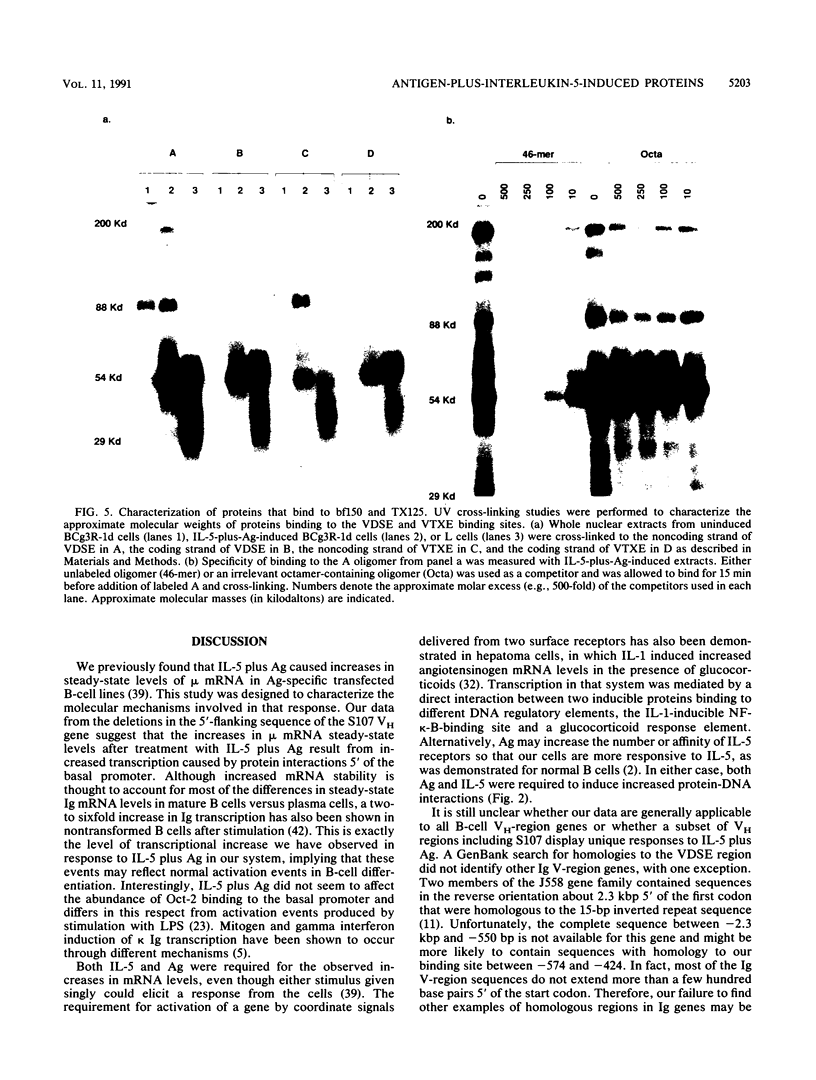
Although much has been learned about basal levels of immunoglobulin (Ig) transcription, the regulatory effects of cytokines and antigen (Ag) upon Ig expression in lymphocytes have not been fully characterized. We previously reported that Ag plus interleukin-5 (IL-5) caused increased steady-state Ig mRNA levels in Ag-specific cell lines. In this study, we have identified a region between -250 and -125 bp 5' of the Ig transcription start site that is necessary for the induction of increased mu mRNA levels by Ag plus IL-5. Mobility shift and UV cross-linking studies indicated that IL-5 plus Ag induced increased protein binding to this region. Furthermore, this sequence was found to be closely related to another A + T-rich sequence at -525 bp 5' of the transcription start site. Both sequences exhibited similar B-cell-specific and inducible protein binding. Our data suggest that treatment with IL-5 plus Ag induces several DNA-binding proteins, some of which may participate in increasing Ig transcription above basal levels by binding to sequences 5' of the octamer motif.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson M. R., Pike B. L., Nossal G. J. Effects of antigens and lymphokines on early activation of single hapten-specific B lymphocytes. J Immunol. 1987 Feb 15;138(4):1056–1063. [PubMed] [Google Scholar]
- Allison K. C., Strober W., Harriman G. R. Induction of IL-5 receptors on normal B cells by cross-linking surface Ig with anti-Ig-dextran. J Immunol. 1991 Jun 15;146(12):4197–4203. [PubMed] [Google Scholar]
- Ballard D. W., Bothwell A. Mutational analysis of the immunoglobulin heavy chain promoter region. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9626–9630. doi: 10.1073/pnas.83.24.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Briskin M., Kuwabara M. D., Sigman D. S., Wall R. Induction of kappa transcription by interferon-gamma without activation of NF-kappa B. Science. 1988 Nov 18;242(4881):1036–1037. doi: 10.1126/science.3143155. [DOI] [PubMed] [Google Scholar]
- Calame K., Eaton S. Transcriptional controlling elements in the immunoglobulin and T cell receptor loci. Adv Immunol. 1988;43:235–275. doi: 10.1016/s0065-2776(08)60367-3. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Carthew R. W., Sharp P. A. A single polypeptide possesses the binding and transcription activities of the adenovirus major late transcription factor. Mol Cell Biol. 1986 Dec;6(12):4723–4733. doi: 10.1128/mcb.6.12.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C., Berenson J., Goverman J., Boyer P. D., Crews S., Siu G., Calame K. An immunoglobulin promoter region is unaltered by DNA rearrangement and somatic mutation during B-cell development. Nucleic Acids Res. 1982 Dec 11;10(23):7731–7749. doi: 10.1093/nar/10.23.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. B., Effron K., Rechavi G., Ben-Neriah Y., Zakut R., Givol D. Simple DNA sequences in homologous flanking regions near immunoglobulin VH genes: a role in gene interaction? Nucleic Acids Res. 1982 Jun 11;10(11):3353–3370. doi: 10.1093/nar/10.11.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton S., Calame K. Multiple DNA sequence elements are necessary for the function of an immunoglobulin heavy chain promoter. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7634–7638. doi: 10.1073/pnas.84.21.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Hedley M. L., Drake B. L., Head J. R., Tucker P. W., Forman J. Differential expression of the class I MHC genes in the embryo and placenta during midgestational development in the mouse. J Immunol. 1989 Jun 1;142(11):4046–4053. [PubMed] [Google Scholar]
- Hendrickson W., Schleif R. A dimer of AraC protein contacts three adjacent major groove regions of the araI DNA site. Proc Natl Acad Sci U S A. 1985 May;82(10):3129–3133. doi: 10.1073/pnas.82.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen D. R., Kassavetis G. A., Barry J., Alberts B. M., Geiduschek E. P. Enhancement of bacteriophage T4 late transcription by components of the T4 DNA replication apparatus. Science. 1989 Sep 1;245(4921):952–958. doi: 10.1126/science.2672335. [DOI] [PubMed] [Google Scholar]
- Imler J. L., Lemaire C., Wasylyk C., Wasylyk B. Negative regulation contributes to tissue specificity of the immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1987 Jul;7(7):2558–2567. doi: 10.1128/mcb.7.7.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. G., Carayannopoulos L., Capra J. D., Tucker P. W., Hanke J. H. The ubiquitous octamer-binding protein(s) is sufficient for transcription of immunoglobulin genes. Mol Cell Biol. 1990 Mar;10(3):982–990. doi: 10.1128/mcb.10.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981 Sep;25(3):753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Jordano J., Perucho M. Initial characterization of a potential transcriptional enhancer for the human c-K-ras gene. Oncogene. 1988 Apr;2(4):359–366. [PubMed] [Google Scholar]
- Landolfi N. F., Capra J. D., Tucker P. W. Interaction of cell-type-specific nuclear proteins with immunoglobulin VH promoter region sequences. Nature. 1986 Oct 9;323(6088):548–551. doi: 10.1038/323548a0. [DOI] [PubMed] [Google Scholar]
- Landolfi N. F., Yin X. M., Capra J. D., Tucker P. W. Protection analysis (or "footprinting") of specific protein-DNA complexes in crude nuclear extracts using methidiumpropyl-EDTA-iron (II). Biotechniques. 1989 May;7(5):500–504. [PubMed] [Google Scholar]
- Lewin B. Commitment and activation at pol II promoters: a tail of protein-protein interactions. Cell. 1990 Jun 29;61(7):1161–1164. doi: 10.1016/0092-8674(90)90675-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. L., Calame K. L. Complex protein binding within the mouse immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1987 Dec;7(12):4194–4203. doi: 10.1128/mcb.7.12.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Schwartz E., Ballantine M., Surrey S. Nucleotide sequence analysis of the delta beta-globin gene region in humans. J Biol Chem. 1983 Oct 10;258(19):11599–11609. [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Reymond C. D., Gomer R. H., Mehdy M. C., Firtel R. A. Developmental regulation of a Dictyostelium gene encoding a protein homologous to mammalian ras protein. Cell. 1984 Nov;39(1):141–148. doi: 10.1016/0092-8674(84)90199-5. [DOI] [PubMed] [Google Scholar]
- Ron D., Brasier A. R., Wright K. A., Habener J. F. The permissive role of glucocorticoids on interleukin-1 stimulation of angiotensinogen gene transcription is mediated by an interaction between inducible enhancers. Mol Cell Biol. 1990 Aug;10(8):4389–4395. doi: 10.1128/mcb.10.8.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Heguy A., Roeder R. G. Identification and purification of a human lymphoid-specific octamer-binding protein (OTF-2) that activates transcription of an immunoglobulin promoter in vitro. Cell. 1987 Dec 4;51(5):783–793. doi: 10.1016/0092-8674(87)90101-2. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Identification of a novel lymphoid specific octamer binding protein (OTF-2B) by proteolytic clipping bandshift assay (PCBA). EMBO J. 1988 Dec 20;7(13):4221–4229. doi: 10.1002/j.1460-2075.1988.tb03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Dutton R. W., McKenzie D., Helstrom H., English M. Role of antigen in the B cell response. Specific antigen and the lymphokine IL-5 synergize to drive B cell lymphoma proliferation and differentiation to Ig secretion. J Immunol. 1988 Jun 15;140(12):4224–4230. [PubMed] [Google Scholar]
- Vitetta E. S., Fernandez-Botran R., Myers C. D., Sanders V. M. Cellular interactions in the humoral immune response. Adv Immunol. 1989;45:1–105. doi: 10.1016/s0065-2776(08)60692-6. [DOI] [PubMed] [Google Scholar]
- Webb C. F., Das C., Coffman R. L., Tucker P. W. Induction of immunoglobulin mu mRNA in a B cell transfectant stimulated with interleukin-5 and a T-dependent antigen. J Immunol. 1989 Dec 15;143(12):3934–3939. [PubMed] [Google Scholar]
- Webb C. F., Das C., Eneff K. L., Tucker P. W. Identification of a matrix-associated region 5' of an immunoglobulin heavy chain variable region gene. Mol Cell Biol. 1991 Oct;11(10):5206–5211. doi: 10.1128/mcb.11.10.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth T., Staudt L., Baltimore D. An octamer oligonucleotide upstream of a TATA motif is sufficient for lymphoid-specific promoter activity. Nature. 1987 Sep 10;329(6135):174–178. doi: 10.1038/329174a0. [DOI] [PubMed] [Google Scholar]
- Yang C. C., Nash H. A. The interaction of E. coli IHF protein with its specific binding sites. Cell. 1989 Jun 2;57(5):869–880. doi: 10.1016/0092-8674(89)90801-5. [DOI] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Transcriptional regulation of the mu-delta heavy chain locus in normal murine B lymphocytes. J Exp Med. 1984 Aug 1;160(2):564–583. doi: 10.1084/jem.160.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]