Abstract
Background
To investigate the prognostic importance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer (NSCLC).
Patients and methods
Using a prospective design, 118 consecutive participants with histologically confirmed metastatic (inoperable) NSCLC and Eastern Cooperative Oncology group (ECOG) 0–3 completed a six-minute walk test to assess functional capacity and questionnaire that assessed self-reported exercise behavior. Cox proportional models were used to estimate the risk of all-cause mortality according to six-minute walk distance (6MWD) (<358.5 m, 358.5–450 m, ≥450 m) and exercise behavior (MET-hrs wk−1) categories with adjustment for important covariates.
Results
Median follow-up was 26.6 months; 77 deaths were reported during this period. Functional capacity was an independent predictor of survival (Ptrend = 0.003) and added incremental prognostic value beyond that provided by PS plus other traditional markers of prognosis (Ptrend = 0.025). Compared with patients achieving a 6MWD <358.5 m, the adjusted hazard ratio (HR) for all-cause mortality was 0.61 (95% CI, 0.34–1.07) for a 6MWD of 358.5–450 m, and 0.48 (95% CI, 0.24–0.93) for a 6MWD >450 m. In unadjusted analysis, there was a borderline significant effect of exercise behavior on survival (p = 0.052). Median survival was 12.89 months (95% CI, 9.11–21.05 months) for those reporting <9 MET-hrs wk−1 compared with 25.63 months (95% CI, 11.28 to ∞ months) for those reporting ≥9 MET-hrs wk−1.
Conclusions
Functional capacity is a strong independent predictor of survival in advanced NSCLC that adds to the prediction of survival beyond traditional risk factors. This parameter may improve risk stratification and prognostication in NSCLC.
Keywords: Prognosis, Functional assessment, Exercise, Cardiorespiratory fitness, Survival
1. Introduction
Lung cancer continues to be the leading cause of cancer-related mortality in both men and women in North America and Western Europe [1]. Approximately 80% of these patients will be diagnosed with non-small cell lung cancer (NSCLC) and the majority will present with metastatic (inoperable) disease. Despite significant advancements in therapy and supportive care, concomitant improvements in survival have been slight [2–4]. For inoperable NSCLC, the median survival rate is approximately 8–10 months[5,6], and two, three, and five-year overall survival rates are only 20%, 12%, and 7%, respectively [7,8]. As such, identifying strong prognostic factors to optimize treatment and survival outcomes are of principal importance.
The considerable interest in identifying robust prognostic markers in metastatic NSCLC has led to the identification of more than 150 risk factors, the majority of which are inconsistently reliable [9]. Outside of tumor stage, performance status (PS) scoring, either assessed by the Karnofsky Performance Scale (KPS) or Eastern Cooperative Oncology Group (ECOG) scoring systems, is the most consistent independent predictor of survival in multivariate analysis [10]. Furthermore, PS has been identified as one of five factors comprising a pragmatic mortality prognostic index in NSCLC. The other four factors identified were: age (>70 years); sex (male); histological type (large-cell carcinoma), and tumor node metastasis [9]. Despite the prognostic importance of PS, these scoring systems fail to fully characterize functional capacity and lack the sensitivity to accurately discriminate prognosis for individuals categorized as ‘good’ PS (i.e., KPS > 70; ECOG 0–1) [11]. Alternative measures that provide a more sensitive and objective assessment of physical functioning may allow for more accurate prognostication, assist in discriminating prognosis for high PS patients, and suggest new targets for therapeutic intervention [12].
There are several methods available to clinicians that provide an objective determination of physical functioning. Recently our group found that peak oxygen consumption (VO2peak), the gold standard assessment of functional (aerobic) capacity, was a strong independent predictor of death in surgical candidates with NSCLC after adjustment for KPS, age, gender, and pulmonary function (FEV1) [13]. Despite the stark advantages, however, assessment of VO2peak is contraindicated in a proportion of metastatic NSCLC patients due to the extent of comorbid disease; these tests also require specialized equipment and trained personnel, limiting clinical feasibility beyond major tertiary hospitals. In contrast, 6MWD is a simple, clinically feasible and objective assessment of functional capacity that is practical even in severely deconditioned clinical populations (e.g., chronic heart failure, chronic obstructive pulmonary disease (COPD), and organ transplant recipients). 6MWD is a robust predictor of mortality in non-cancer settings [14–17]; only two studies to date have investigated the prognostic importance of 6MWD in the oncology setting [12,18].
Exercise behavior is a major determinant of functional capacity. Several landmark epidemiological studies suggest that, in general, self-reported regular exercise (e.g., >brisk walking for 30 min, 5 d wk−1) is associated with substantial reductions in the risk of cancer-specific death following a diagnosis cancer [19–22]. Exercise behavior and functional capacity are correlated but provide distinct information. Prior studies examining the association between exercise behavior and survival in cancer patients have not objectively evaluated functional capacity. Therefore it is not known whether a functional capacity assessment such as 6MWD provides prognostic information beyond exercise behavior.
Against this background, we sought to investigate the prognostic importance of functional capacity and exercise behavior in patients with inoperable NSCLC. We also investigated whether these parameters provided additional prognostic information beyond traditional markers (e.g. PS, age, gender) in this population.
2. Method
2.1. Patients and setting
Patients were adults with histologically confirmed Stage IIIB, IV, or recurrent metastatic (i.e., inoperable) NSCLC presenting to Duke University Health System (DUHS), who were able to read and understand English, consent to participate, and whose primary attending oncologist approved participation. Specifically, data was pooled from two studies in which patients were recruited to either a cross-sectional study (one time assessment of 6MWD) or prospective study (repeated assessment of 6MWD across time; only baseline data is included in this report). Both studies were approved by the DUHS Institutional Review Board; written informed consent was obtained prior to initiation of any study procedures.
2.2. Functional capacity
Functional capacity was assessed via 6MWD in a measured corridor according to the American Thoracic Society (ATS) guidelines [23]. Briefly, patients were instructed to walk at their fastest pace and to cover the longest possible distance over a six-minute period while under the supervision of trained clinical research assistant. 6MWD was recorded in meters. Age and sex-predicted 6MWD was calculated from Gibbons et al. [24].
2.3. Exercise behavior
Self-reported exercise behavior was assessed using the leisure score index (LSI) of the Godin Leisure-Time Exercise Questionnaire (GLTEQ) [25]. The LSI contains three questions that assess the average frequency of mild, moderate, and strenuous intensity exercise during free time in a typical week. Participants reported their average weekly exercise since their primary adjuvant treatment consultation, as well as average duration within each level of exercise intensity. To calculate total exercise behavior, the frequency of exercise sessions per week within each intensity category was multiplied by the average reported duration, weighted by an estimate of the metabolic equivalent (MET), summed across all intensities, and expressed as average MET-hours per week. The standard MET weightings and examples for each level of exercise intensity are as follows: mild (3 METs, e.g., easy walking, yoga), moderate (5 METs, e.g., brisk walking, tennis), and strenuous (9 METs, e.g., running, vigorous swimming).
2.4. Clinical parameters and follow-up
Patient characteristics and clinical information were abstracted from electronic medical records. PS was assessed using the ECOG scale at the time of study enrollment by the attending oncologist. Follow-up survival data was obtained through multiple confirmatory sources: DUHS vital statistics; the social-security death index, electronic medical record (up to December, 2010); tumor registry; and clinical notes. When vital status was unclear, the patient and/or family were contacted by telephone.
2.5. Statistical analysis
Descriptive statistics were reported for clinical parameters and study outcomes. The Cox proportional hazards model was used to examine the association between functional capacity and exercise behavior on survival. A likelihood ratio (LR) test was used in the context of the Cox model to assess the contribution of functional capacity (6MWD) and exercise behavior in predicting survival beyond that provided by ECOG (<2 vs. ≥2) alone as well as the combination of age, sex, cohort (when applicable), and ECOG. Functional capacity was categorized via an unbiased tertile split as defined by 6MWD (i.e., <358.5, 358.5–450 m, >450 m). The median value of 6MWD within each category was used as a predictor for linear trend in analyses. Exercise behavior was analyzed as MET-hrs wk−1 (<9 MET-hrs wk−1 vs. ≥9 MET-hrs wk−1) based on prior work [26]. Survival was defined as the time between assessment of functional capacity and death; for patients remaining alive, survival was censored at the time of last follow-up. A two-sided significance level of 0.05 was used for all statistical tests. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
3. Results
Participant recruitment took place between December, 2007 and August, 2010. In brief, 1137 patients were screened for eligibility during the study period (across both studies). Of these, 277 (24%) met inclusion criteria and 118 (10%) agreed to participate and completed all study procedures. Major reasons for non-eligibility were histological type (n = 343), diagnosis of early stage NSCLC (n = 326), and inability to complete 6MWT (n = 70). Major reasons for study refusal were not interested (n = 34), no time (n = 62), and not feeling well (n = 60).
3.1. Clinical characteristics
Patient characteristics are presented in Table 1. Mean age was 61 ± 10 yr (range, 28–84 yr) and 60% were male. Sixty-two percent were diagnosed with adenocarcinoma and 13% were ECOG 0 (range, 0–3). The median time to study enrollment from the diagnosis of metastatic NSCLC was 13.4 ± 16.7 months. Mean 6MWD was 396 ± 116 m (range, 90–640 m), equivalent to 38 ± 17% below that predicted for age and sex. No adverse events were observed during functional capacity testing. Mean exercise behavior (n = 68 patients) was 28 ± 45 MET-hrs wk−1; 26% were currently meeting national exercise guidelines (i.e., ≥150 min wk−1 of moderate to vigorous intensity exercise), and 21% reported no exercise behavior.
Table 1.
Characteristics of the participants (n = 118).
| Variable | No. (%) | Mean ± SD |
|---|---|---|
| Age (yr) | 61 ± 10 | |
| Range | 28–84 | |
| BMI (kg m−2) | 26 ± 5 | |
| Range | 16–42 | |
| Male | 71 (60) | |
| ECOG | ||
| 0 | 15 (13) | |
| 1 | 79 (67) | |
| 2 | 19 (16) | |
| 3 | 5 (4) | |
| 6MWD (m) | 396 ± 116 | |
| Range | 90–640 | |
| 6MWD, below age-sex predicted (%) | −38 ± 17 | |
| Exercise behavior* | ||
| Total exercise (min wk−1) | 402 ± 695 | |
| Total exercise (MET-hrs wk−1) | 28 ± 45 | |
| Meeting ACSM guidelines (%) | 18 (26) | |
| Reporting no exercise behavior (%) | 14 (21) | |
| Time since inoperable diagnosis (months) | 13.4 ± 16.7 | |
| Histologic features – no. (%) | ||
| Adenocarcinoma | 73 (62) | |
| Squamous | 11 (9) | |
| Other (bronchoalveolar, unclassified) | 34 (29) | |
| Current therapy | ||
| Chemotherapy | 70 (59) | |
| Radiation | 10 (8) | |
| Prior therapy for operable disease (if applicable) | ||
| Surgery | 27 (23) | |
| Chemotherapy | 55 (47) | |
| Radiation | 54 (46) |
Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; 6MWD, six-minute walk distance; ACSM, American College of Sports Medicine.
n = 68.
3.2. Univariate survival analysis
Median follow-up was 26.6 months. During this period, 77 deaths were recorded (65% of the total sample). The median time from the functional capacity assessment to death was 12.9 months (95% CI, 11.3–18.4).
3.2.1. Functional capacity
Median 6MWD across tertiles was 283 m (90–356.8 m), 416 m (358.5–450 m), and 510 m (452–640 m). 6MWD was an independent predictor of survival (unadjusted Ptrend = 0.003; Table 2; Fig. 1) The corresponding median survival across tertiles was 8.98 months (6.09–12.70), 14.38 months (11.38–21.05), and 20.26 months (11.78–28.91), respectively. Each 50-m improvement in 6MWD was associated with a 13% reduction in the risk of death. Functional capacity provided incremental prognostic information beyond PS (Ptrend = 0.036; Table 2) and beyond PS plus other traditional markers of prognosis (Ptrend = 0.025; Table 2). Compared with patients achieving a 6MWD <358.5 m, the adjusted hazard ratio (HR) for all-cause mortality was 0.61 (95% CI, 0.34–1.07) for a 6MWD of 358.5–450 m, and 0.48 (95% CI, 0.24–0.93) for a 6MWD >450 m (Table 2).
Table 2.
Association between six-minute walk distance and survival (n = 118).
| Analysis | Six-minute walk distance (m) |
Likelihood ratio Ptrend | |||||
|---|---|---|---|---|---|---|---|
| <358.5 | 358.5–450 | >450 | |||||
| No. of events | 31 | 25 | 21 | ||||
| No. at risk | 39 | 42 | 37 | ||||
| Median, 6MWD | 283 | 90–356.8 | 416 | 358.5–450 | 510 | 452–640 | |
| Median, months | 8.98 | 6.09–12.70 | 14.38 | 11.38–21.05 | 20.26 | 11.78–28.91 | |
| Unadjusted, HR | Referent | 0.53 | 0.31–0.90 | 0.44 | 0.25–0.78 | 0.003 | |
| Adjusted,a HR | Referent | 0.60 | 0.35–1.04 | 0.54 | 0.30–0.99 | 0.036 | |
| Adjusted,b HR | Referent | 0.61 | 0.34–1.07 | 0.48 | 0.24–0.93 | 0.025 | |
Abbreviations: 6MWD, six-minute walk distance; HR, hazard ratio.
Adjusted for performance status (ECOG).
Adjusted for age, gender, cohort, and performance status (ECOG).
Fig. 1.
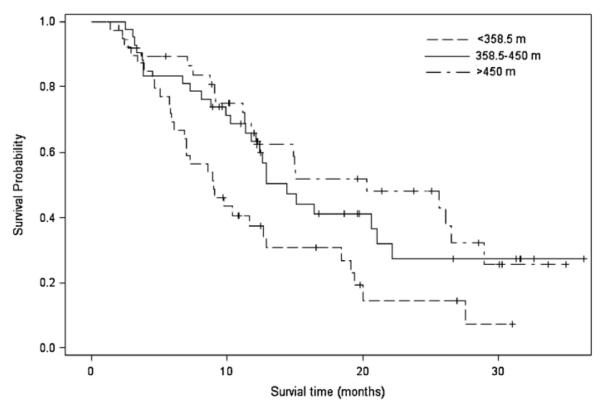
Association between six-minute walk distance and survival.
3.2.2. Exercise behavior
The association between exercise behavior (i.e., <9 MET-hrs wk−1 vs. ≥9 MET-hrs wk−1) and survival is presented in Table 3. In the unadjusted analysis, there was a borderline significant effect of exercise behavior on survival (p = 0.052; Table 3; Fig. 2). Median survival was 12.89 months (95% CI, 9.11–21.05 months) for those reporting <9 MET-hrs wk−1 compared with 25.63 months (95% CI, 11.28 to ∞ months) for those reporting ≥9 MET-hrs wk−1. After adjustment for covariates, including PS, there was no longer a borderline significant effect of exercise behavior on survival (p = 0.324; Table 3). Compared with patients reporting <9 MET-hrs wk−1, the adjusted HR for mortality was 0.67 (95% CI, 0.31–1.48) for patients reporting ≥9 MET-hrs wk−1.
Table 3.
Association between exercise behavior and survival (n = 68).
| Analysis | MET-hrs wk−1 |
||||
|---|---|---|---|---|---|
| <9 MET-hrs wk−1 | ≥9 MET-hrs wk−1 | Likelihood ratio P | |||
| No. of events | 19 | 16 | |||
| No. at risk | 31 | 37 | |||
| Median, months | 12.89 | 9.11–21.05 | 25.63 | 11.28–∞* | |
| Unadjusted, HR | Referent | 0.51 | 0.26–1.01 | 0.052 | |
| Adjusted,a HR | Referent | 0.60 | 0.30–1.22 | 0.159 | |
| Adjusted,b HR | Referent | 0.67 | 0.31–1.48 | 0.324 | |
Abbreviations: HR, hazard ratio.
Adjusted for performance status (ECOG).
Adjusted for age, gender, and performance status (ECOG).
The upper confidence limit of the median could not be calculated due to insufficient follow-up time.
Fig. 2.
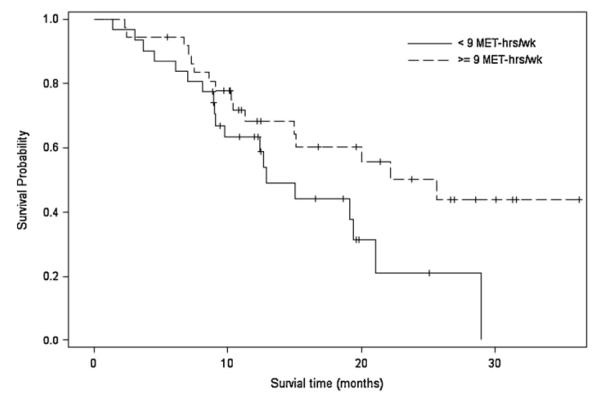
Association between exercise behavior [MET-hrs wk−1(<9 MET-hrs wk−1 vs. >9 MET-hrs wk−1)] and survival.
4. Discussion
Our findings demonstrate that functional capacity, as measured by 6MWD, is a strong, independent predictor of prognosis that adds to the prediction of survival beyond traditional risk factors in patients with metastatic NSCLC. Specifically, relative to patients in the lowest 6MWD category, higher functional capacity was associated with an adjusted 39% to 52% reduction in the risk death. Each 50-m improvement in 6MWD was associated with a 13% reduction in the risk of death. The magnitude of risk reduction observed in the present study is comparable to that reported by the only other two studies investigating the prognostic importance of objective measures of physical functioning in patients with NSCLC. Kasymjanova et al. [18] investigated the association between 6MWD and survival in 64 patients with inoperable NSCLC. Relative to <400 m, a 6MWD ≥400 m was associated with a 56% reduction in the risk of death after adjustment for important covariates. Similarly, our group found that VO2peak, as measured by a maximal cardiopulmonary exercise test, was associated with a 21% to 24% reduction in the risk of death, relative to the lowest VO2peak category, in 398 surgical candidates with NSCLC. Intriguingly, the magnitude of risk reduction was stronger for patients determined as inoperable with a 21% to 61% [13]. Interestingly, in further work by our group we found that 6MWD was not an independent predictor of prognosis in 243 patients with grade III/IV recurrent malignant glioma [12], suggesting that 6MWD may not be an appropriate clinical tool for objectively assessing physical functioning in all cancer populations.
Our findings may have important implications for mortality risk prediction in metastatic NSCLC. Here we found that 6MWD added incremental mortality risk prediction beyond established markers including PS. In universal oncology practice and clinical trials, physical functioning is evaluated using subjective PS scoring systems. In general, patients with a PS score ≥70% (KPS) or 0–1 (ECOG) are considered ‘physically capable’ of receiving cytotoxic therapy. Furthermore, PS is used to classify patients into prognostic risk categories and routinely used clinically in the planning, randomization, eligibility, and evaluation of clinical trials as well as in decisions regarding the ‘optimal’ therapeutic approach. However, PS scoring systems are subjective and do not provide a sensitive assessment of physical functioning [11]. To this end, objective tools such as 6MWD provide a simple and sensitive assessment of physical functioning that, in turn, may allow for more accurate prognostication. This is especially important among patients with good PS (i.e., KPS > 70; ECOG 0–1) where discrimination of prognosis can be difficult [10]. Furthermore, maximal and/or submaximal functional capacity (exercise) testing may be particularly valuable for several additional reasons: (1) exercise tests assess the integrative capacity of the cardiovascular and musculoskeletal system to transport and utilize oxygen (O2) for ATP resynthesis under physiologic stress [27]. The efficiency to transport O2 is arguably one of the most important indices of health and longevity in humans – a process that is not captured by current measures, (2) exercise test endpoints (e.g., VO2peak, time to exhaustion, and 6MWD) are strong predictors of cardiovascular and all-cause mortality in diverse clinical settings [28,29]. As such, prognostic, validated clinical cut-points are available, and (3) exercise tests can inform therapeutic intervention as well as evaluate therapeutic efficacy [30]. In our opinion, there is now sufficient supportive evidence to initiate large, adequately powered prospective studies to systematically evaluate the clinical utility and prognostic importance of physical functioning in the oncology setting.
Another novel finding of our investigation is that exercise behavior is also an independent predictor of survival, although it did not add to the prediction beyond PS. Regular exercise behavior (i.e., ≥9 MET-hrs wk−1) was associated with a statistically significant 33% reduction in the risk of death, relative to inactive patients (<9 MET-hrs wk−1) after adjustment for important covariates. To our knowledge, this is the first study to investigate the prognostic significance of exercise behavior in NSCLC. Findings of the present study add to an increasing evidence base indicating that, in general, regular self-reported exercise is inversely associated with survival in several populations with solid tumors [12,21,31,32]. Of importance, our study is the first in patients with NSCLC to assess functional capacity and exercise behavior in the same cohort, thus permitting prognostic comparison of these measures. Results indicated that only functional capacity was a significant, independent predictor of prognosis, and remained significant after adjustment for exercise behavior (analysis not presented). These findings support prior work in which cardiorespiratory fitness was a stronger predictor of mortality than self-reported exercise behavior in more than 40,000 asymptomatic men and women [33]. These findings imply that interventions designed to specifically augment functional capacity may be required to optimally improve clinical outcomes in metastatic NSCLC.
To this end, a growing number of research groups, including our own, have started to investigate the feasibility, safety, and preliminary efficacy of exercise training to improve functional capacity/cardiorespiratory fitness, as well as other pertinent endpoints, in patients with NSCLC [34]. Of particular relevance, Quist et al. investigated the effects of a six-week supervised exercise training intervention on functional capacity (VO2peak and 6MWD) in 25 patients with metastatic NSCLC. Nineteen patients completed the study (76%); VO2peak and 6MWD increased by 0.09 L min−1 (6%) and 39.3 m (7%), respectively [35]. In an earlier study examining the effects of 4–6 weeks of supervised aerobic training prior to surgical resection, VO2peak and 6MWD increased by 2.4 mL kg−1 min−1 (14.6%) and 40 m (8.4%), respectively [36]. Phase III trials are required to definitively determine whether exercise training-induced changes in functional capacity can modify prognosis together with correlative science studies to elucidate the systemic and molecular mechanisms underpinning such effects.
Several limitations need to be considered when interpreting the findings of this study. The most important limitation is that we have only demonstrated correlation, and not causation. Another important limitation is patient selection bias because of the transparent purpose of the investigation and exclusion of patients with significant comorbid disease. As such, patients with better physical functioning, less advanced disease, and experiencing less treatment-related complications were likely to participate. We only had information on death from any cause; the specific cause of death is not known. In summary, functional capacity is an independent predictor of survival in metastatic NSCLC that may complement traditional markers of prognosis to improve risk stratification and prognostication.
Acknowledgements
LWJ is supported by NIH CA143254, CA142566, CA138634, CA133895, CA125458 and funds from George and Susan Beischer.
Footnotes
Conflict of interest The authors declare no conflicts of interest.
References
- [1].Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- [2].Kuten A, Anacak Y, Abdah-Bortnyak R, et al. Neoadjuvant radiotherapy concurrent with weekly Paclitaxel and Carboplatin and followed by surgery in locally advanced non-small-cell lung cancer. Am J Clin Oncol. 2003;26:184–7. doi: 10.1097/00000421-200304000-00017. [DOI] [PubMed] [Google Scholar]
- [3].Niho S, Kubota K, Goto K, et al. Combination second-line chemotherapy with gemcitabine and docetaxel for recurrent non-small-cell lung cancer after platinum-containing chemotherapy: a phase I/II trial. Cancer Chemother Pharmacol. 2003 doi: 10.1007/s00280-003-0618-8. [DOI] [PubMed] [Google Scholar]
- [4].Chen YM, Perng RP, Chen MC, et al. A phase II trial of vinorelbine plus gemcitabine in previously untreated inoperable (stage IIIb/IV) non-small-cell lung cancer patients aged 80 or older. Lung Cancer. 2003;40:221–6. doi: 10.1016/s0169-5002(03)00031-x. [DOI] [PubMed] [Google Scholar]
- [5].Scott HR, McMillan DC, Forrest LM, et al. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87:264–7. doi: 10.1038/sj.bjc.6600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jeremic B, Milicic B, Dagovic A, et al. Pretreatment clinical prognostic factors in patients with stage IV non-small cell lung cancer (NSCLC) treated with chemotherapy. J Cancer Res Clin Oncol. 2003;129:114–22. doi: 10.1007/s00432-002-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002;360:1131–5. doi: 10.1016/S0140-6736(02)11199-8. [DOI] [PubMed] [Google Scholar]
- [8].Firat S, Bousamra M, Gore E, et al. Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52:1047–57. doi: 10.1016/s0360-3016(01)02741-9. [DOI] [PubMed] [Google Scholar]
- [9].Blanchon F, Grivaux M, Asselain B, et al. 4-year mortality in patients with non-small-cell lung cancer: development and validation of a prognostic index. Lancet Oncol. 2006;7:829–36. doi: 10.1016/S1470-2045(06)70868-3. [DOI] [PubMed] [Google Scholar]
- [10].Abernethy AP, Shelby-James T, Fazekas BS, et al. The Australia-modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice [ISRCTN81117481] BMC Palliat Care. 2005;4:7. doi: 10.1186/1472-684X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jones LW, Cohen RR, Mabe SK, et al. Assessment of physical functioning in recurrent glioma: preliminary comparison of performance status to functional capacity testing. J Neurooncol. 2009;94:79–85. doi: 10.1007/s11060-009-9803-x. [DOI] [PubMed] [Google Scholar]
- [12].Ruden E, Reardon DA, Coan AD, et al. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011 doi: 10.1200/JCO.2011.34.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jones LW, Watson D, Herndon JE, 2nd, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116:4825–32. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lederer DJ, Arcasoy SM, Wilt JS, et al. Six-minute walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:659–64. doi: 10.1164/rccm.200604-520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lettieri CJ, Nathan SD, Browning RF, et al. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med. 2006;100:1734–41. doi: 10.1016/j.rmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- [16].Flaherty KR, Andrei AC, Murray S, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute walk test. Am J Respir Crit Care Med. 2006;174:803–9. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cote CG, Pinto-Plata V, Kasprzyk K, et al. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest. 2007;132:1778–85. doi: 10.1378/chest.07-2050. [DOI] [PubMed] [Google Scholar]
- [18].Kasymjanova G, Correa JA, Kreisman H, et al. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:602–7. doi: 10.1097/JTO.0b013e31819e77e8. [DOI] [PubMed] [Google Scholar]
- [19].Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–65. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- [20].Meyerhardt JA, Giovannucci EL, Ogino S, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–8. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–34. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- [22].Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–41. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- [23].ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- [24].Gibbons WJ, Fruchter N, Sloan S, et al. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001;21:87–93. doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- [25].Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- [26].Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- [27].Jones LW, Eves ND, Haykowsky M, et al. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- [28].Gupta S, Rohatgi A, Ayers CR, et al. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 2011;123:1377–83. doi: 10.1161/CIRCULATIONAHA.110.003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- [30].Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9:757–65. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- [31].Moorman PG, Jones LW, Akushevich L, et al. Recreational physical activity and ovarian cancer risk and survival. Ann Epidemiol. 2011;21:178–87. doi: 10.1016/j.annepidem.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kenfield SA, Stampfer MJ, Giovannucci E, et al. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–32. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee DC, Sui X, Ortega FB, et al. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med. 2011;45:504–10. doi: 10.1136/bjsm.2009.066209. [DOI] [PubMed] [Google Scholar]
- [34].Jones LW, Eves ND, Waner E, et al. Exercise therapy across the lung cancer continuum. Curr Oncol Rep. 2009;11:255–62. doi: 10.1007/s11912-009-0036-0. [DOI] [PubMed] [Google Scholar]
- [35].Quist M, Rorth M, Langer S, et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: a pilot study. Lung Cancer. doi: 10.1016/j.lungcan.2011.07.006. submitted for publication. [DOI] [PubMed] [Google Scholar]
- [36].Jones LW, Peddle CJ, Eves ND, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110:590–8. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]


