Abstract
Since hypercalciuria is a common feature of idiopathic calcium oxalate (CaOx) nephrolithiasis, renal epithelial cells of stone patients are exposed to various crystals in the presence of high calcium. This study was performed to determine the effect of high calcium levels on CaOx crystal-induced cell injury. We exposed human renal epithelial cell line, HK2 in vitro to CaOx monohydrate crystals at a concentration of 133 μg/cm2 for 1, 3, 6 or 12 h in the presence or absence of 5 or 10 mM/L calcium Ca++. We determined the release of lactate dehydrogenase as marker of injury and hydrogen peroxide (H2O2) and 8-isoprostane (8-IP) as sign of oxidative stress. Cells were also examined after trypan blue and nuclear DNA staining with 4′,6-diamidino-2-phenylindole to determine their membrane integrity and apoptosis respectively. Exposure of cells to 5 or 10 mM/L of Ca++, for up-to 6 h, resulted in increased trypan blue and DAPI staining and production of H2O2. Similarly an exposure to CaOx crystals also resulted in increased trypan blue and DAPI staining and H2O2 production. An exposure to 5 mM/L Ca or CaOx crystals also resulted in increased production of 8-IP. A combination of the two treatments, Ca and CaOx crystals, did not show anymore changes than exposure to high Ca or CaOx crystals alone, except in the case of a longer exposure of 12 h. Longer exposures of 12 h resulted in cells sloughing from the substrate. These results indicate that exposure to high levels of Ca or CaOx crystals is injurious to renal epithelial cells but the two do not appear to work synergistically. On the other hand, results of our earlier studies suggest that oxalate and CaOx crystals work in synergy, i.e., CaOx crystals are more injurious in the presence of high oxalate. Perhaps Ox and CaOx crystals activate different biochemical pathways while Ca and CaOx crystals affect the identical pathways.
Keywords: Calcium, Reactive oxygen species, Oxidative stress, Nephrolithiasis, Calcium oxalate
Introduction
Nephrolithiasis is a debilitating urological disorder with approximately 12% of white men and 6% of white women experiencing at least one stone episode by their seventh decade [1]. Crystalluria on the other hand is common in both normal individuals and nephrolithic patients. It is our hypothesis that persistent crystalluria resulting, for the most part, from hypercalciuria, hyperoxaluria and/or hypocitraturia leads to prolonged contacts between crystals and renal epithelial cells. The crystal cell interactions produce a variety of physiological and pathological changes eventually leading to crystal retention within the kidneys and formation of stone nidi. We have shown that reactive oxygen species (ROS) induced cell injury play significant role in this process [2]. Exposure of renal epithelial cells in culture to high oxalate levels and calcium oxalate (CaOx) crystals leads to the production of ROS, development of oxidative stress and cellular injury [3-7]. CaOx crystals are more injurious in the presence of high oxalate [7]. Since most idiopathic CaOx stone formers are hypercalciuric, crystals form and renal epithelial cells are exposed to them in a high calcium environment. Therefore we decided to investigate renal epithelial cell response to high calcium and crystal cell interaction in the presence of calcium. We determined the release of lactate dehydrogenase (LDH) as marker of cell injury and production of hydrogen peroxide (H2O2) and 8-isoprostane (8-IP) as signs of oxidative stress. Cells were also examined after trypan blue and nuclear DNA staining with 4′,6-diamidino-2-phenylindole (DAPI) to determine their membrane integrity and apoptosis, respectively.
Materials and methods
Cell culture
HK-2 Cells, a proximal tubular epithelial cell line derived from normal human kidney was purchased from American Type Culture Collection, (CRL-2190; Manassas, VA USA). HK-2 cell line has been previously used in our laboratory to investigate the effect of oxalate ions and COM crystals on the cells [2].
Cells were maintained as continuously growing monolayers in 75 cm2 Falcon T-Xask (Fisher, Atlanta,Ga USA) in Dulbecco’s modified essential medium (DMEM/F-12 at 1:1 ratio(Cat#10-092-CN Mediatech, Inc Herndon,VA 20171) supplemented with 10% fetal calf serum (FCS), 2% antibiotic-antimytotic at 37°C in a 5% CO2/air atmosphere incubator. Under these conditions, the cells were grown and the media was changed every 2–3 days. The cells were transferred at the following densities into 24 well plates (5 × 105/well), 96 well plates (105/well) or eight chamber slides. After 95% confluence, the spent media was aspirated and cells were incubated with acclimatization media (DMEM/F-12 with 2% antibiotic–antimytotic solution, 1% insulin/transferring/selenium mix, 0.2% hydrocortisone, 0.68% triiodo-l-thyronine and 0.2% prostaglandin E1 (Atlanta biologicals M232 SA Lawrenceville, GA) for over night to arrest the growth. The cells were then exposed to serum and sodium pyruvate free media supplemented with calcium as CaCl2 at a final concentration of 5 or 10 mmol alone or with CaOx monohydrate (COM) crystal suspension (133 μg/cm2) prepared and used as described in earlier publications. The solutions were prepared just before use. COM suspension was continuously shaken when it was transferred to well. The cells were incubated with additives for 1, 3, 6 and 12 h. Each treatment was done in quadruplicate, unless otherwise stated. After the specified time, the culture media was collected in micro centrifuge tubes and was centrifuged to remove any cells or cell debris. The control was the media collected from the cell wells without additives under same conditions from the same plate. The media was stored at −20°C for further analysis.
LDH assay
Lactate dehydrogenase is a stable cytosolic enzyme that is released when the cell is lysed or there is any injury on the cell membrane. LDH activity from the conditioned media collected from the treated cell wells at specified time intervals was determined colorimetrically with an assay kit Promega Cytotox 96 (Promega Corporation, 2800 Woods Hollow Road, Medison, W1 53711-5399 USA). Aliquots of centrifuged conditioned media (50 μl) in duplicate were transferred to 96-well flat plates. A positive control and blank (control media) were also aliquoted in the designated wells. The substrate, 50 μl was added to all the samples and incubated at room temperature in the dark for half an hour. Following the incubation, stop solution was added and the plate was read at 490 nm on microplate reader (BioRad 3550 microplate reader). The samples were compared with blanks and % yield of LDH was calculated.
Trypan blue assay
After incubation with Ca and Ca and COM for the required length of time, medium was removed and the cells were incubated for 10 min after adding 0.4% Trypan blue solution (#T8145: Sigma Chemical Co.) with Dulbecco’s phosphate buffered saline PBS (Mediatech, Inc Herndon,VA 20171). Cells were viewed under inverted microscope and viability was determined by the number of cells stained blue(dead cells) divided by the total number of cells counted in five fields of each well multiplied by 100 to give the percentage of live and dead cells.
Apoptosis assay
The HK-2 cells were grown in 8-well glass slides and when the cells were confluent they were exposed to different concentrations of calcium and calcium in the presence of COM crystals (133 μg/cm2). The cells in the wells of the glass slide were washed with PBS, fixde with 1% formaldehyde for 10 min at room temperature, stained with 4′,6-diamidino-2-phenylindole (DAPI) for two min and washed with PBS for 5 min. The wells were pulled off and the slides were mounted with crystal mount aqueous/dry mounting medium for enzyme immunocytochemistry cat no. MØ2 (Biomeda corp. Foster city CA) and covered with cover slips. The DAPI is sensitive to light so direct contact of light was avoided. The number of condensed nucleus of HK-2 cells was expressed as condensed nucleus percentage of the cells from three independent experiments.
ROS, hydrogen peroxide (H2O2)
After incubation period, media was removed and placed into micro centrifuge tubes. Hydrogen peroxide quantification was carried out using a colorimetric assay obtained from Pierce (PeroXoquant Quantitative Peroxide Assay Kit: Cat # 23280). An aliquot was taken (amount specific to manufacture’s protocol) and a 1:100 dilution and 1:1 dilution of sample was first measured according to assay protocol to determine optimum sample volume for parameters of standard curved set by kit protocol. Once determination was made for the necessary dilution of sample, the following kit protocol was followed. In a 96-well plate, 10 μl of working reagent (WR: diluted to necessary concentration from stock supplied from kit) was added to 100 μl of sample, standard and reagent blank. The plate was mixed using a plate mixer and incubated at room temperature for up to 20 min (or until a sufficient color of blue was seen in highest concentration of standard). Absorbance was measured at 570 nm (assay wavelength range 560–600 nm) using a Bio-Rad microplate reader (Model 3550). The concentration of hydrogen peroxide in the sample was calculated by reference to its assay absorbance compared to the standard curve.
Lipid peroxidation: 8-isoprostane
Determination of lipid peroxidation was carried out using a kit from Oxford Biomedical Research (Urinary Isoprostane: Cat # EA85). Briefly, after incubation with calcium, COM or calcium and COM, media was collected. Media samples were spun to pellet solid materials and a 100 μl of standard (supplied by kit), samples, and reagent blank (Enhanced dilution buffer supplied by kit) was added to designated wells in a 96-well plate (supplied by kit). Diluted 15-isoprostane (100 μl) F2t HRP conjugate (supplied by kit) was added to all well except reagent blank. Plate was incubated at room temperature for 2 h. After incubation, plate was washed three times with wash buffer (supplied by kit). A total of 200 μl of substrate (supplied by kit) was added to each well and incubated at room temperature for 20–40 min (or until appreciable blue hue was observed in standard blank). Addition of 3 M sulfuric acid was added to each well to stop reaction. Plate was then read using a Bio-Rad 3550 microplate reader at 450 nm. The concentration of isoprostane in the sample was calculated by reference to its assay absorbance compared to the standard curve.
Statistical analysis
All analyses for statistical significance were carried out using GraphPad Prism software. A Student t test and descriptive analysis was carried out to determine significance between various variables. A two-way ANOVA analysis was used to find significance between groups and times. Boniferroni post-test was used for determining significance within specified exposed groups by time periods.
Results
Cell viability and integrity
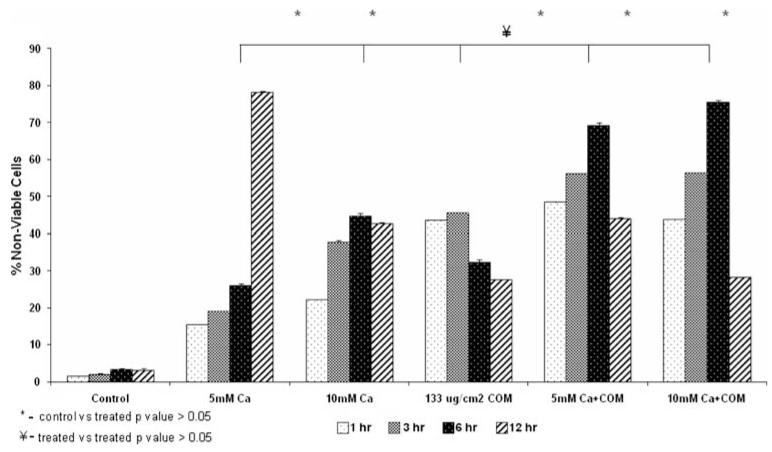
Figure 1 shows result of cell viability assay. All treatments significantly reduced cell viability. Both calcium ions as well as COM crystals were injurious. Dual treatments did not appear any more injurious than individual exposures to calcium or COM crystals. After one hour exposure to 5 mM calcium, 15.43% of cells were non-viable compared with controls at 1.42%. The non-viability of the cells reached to 19% after 3 h exposure. After 6 h, an increase was observed in non-viability that reached to 26% where as the highest percentage of non-viability of cells was observed at 12 h and was 78.28%. At higher concentration of 10 mM Ca2+ the number of nonviable cells after 1 h increased from 1.42 to 22.09%, reached to 37.69, 44.76 and 42.7% after 03, 06 and 12 h exposure, respectively.
Fig. 1.
Percentage of non-viable cells which stained with trypan blue. The cells were exposed to 5 or 10 mM calcium (Ca) or 133 μg/cm2 calcium oxalate monohydrate (COM) crystals separately or calcium and crystals together for 1, 3, 6, or 12 h. All treatments resulted in significant cell death (P > 0.05) compared to normal controls. Exposure to COM crystals was significantly more injurious than exposure to 5 mM Ca. Combined Ca and COM treatments were significantly more injurious than only the treatment with 5 mM Ca
Non-viability of cells exposed to CaOx monohydrate (COM) crystals at a concentration of 133μg/cm2 was 43.58, 45.45, 32.12 and 27.52% at the time intervals of 01, 03, 06 and 12 h, respectively. The cells were also exposed to COM crystals in the presence of 5 and 10 mM Ca2+ ions. At the 5 mM Ca2+ ionic concentration with COM crystals, the number of nonviable cells found was 48.4, 56.69, 69.23 and 43.93% at the exposed time intervals of 01, 03, 06 and 12 h, respectively. During the exposure of cells with COM crystals and 10 mM Ca2+ ions, it was observed that the non-viability of the cells increased from 43.66 for 1 h to 56.26 and 75.4% after 03 and 06 h. Longer treatments to higher concentrations of calcium and crystals resulted in sloughing off the dead cells into the medium and thus an increase in the number of viable cells in the monolayer is an artifact.
Cell exposure to calcium, CaOx crystals, and a combination of two also resulted in a significant increase in the release of LDH (results not shown) in the medium indicating loss of membrane integrity. As expected, LDH release in response to calcium was time and concentration dependent.
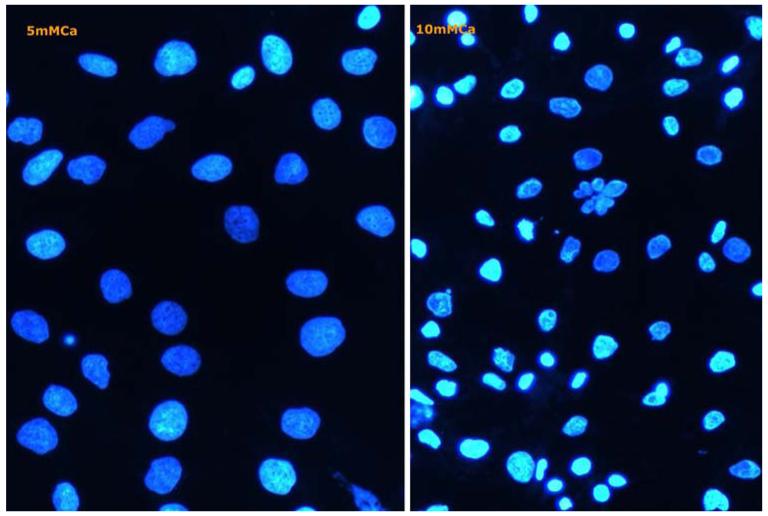
Staining with 4′,6-diamidino-2-phenylindole (DAPI) showed condensed nuclei and apoptotic bodies after an exposure to 10 mmol calcium (Fig. 2) for only 1 h, both by itself or in combination with COM crystals.
Fig. 2.
Cells in culture examined by fluorescence microscopy following staining with 4′-6-diamidino-2-phenylinedole. a After treatment with 5 mM calcium. b Cells treated with 10 mM calcium show highly condensed chromatin and many nuclei fragmented into apoptotic bodies
ROS and lipid peroxidation
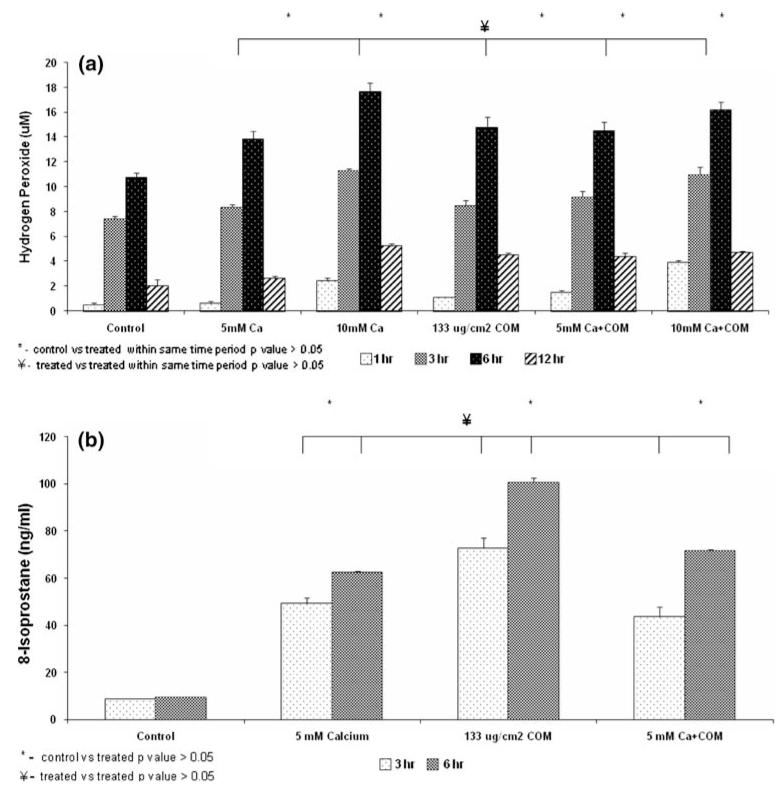
Exposure to calcium with or without COM crystals was associated with the release of H2O2 into the medium in a time and concentration dependent manner (Fig. 3a). Most significant increase was seen after 3 or 6 h of exposures. Overall the exposure to combined calcium and COM crystals did not show any significant increase over exposure to only calcium or COM crystals. Actually the amount of H2O2 release in the medium appears lower after the combined treatments than the separate treatments.
Fig. 3.
Cell treatments as shown in Fig. 1. a Hydrogen peroxide release into the medium. significant (>0.05) amounts were released after treatments for 3 or 6 h to 5 or 10 mM Ca or 133 μg/cm2 COM crystals. There were no significant differences between exposures to crystals and calcium separately or together for same durations. b Production of 8-isoprostane (8-IP) by cells exposed to 5 mM Ca and 133 μg/cm2 COM crystals separately or together for 3 or 6 h. There was a significant (>0.05) increase in 8-IP production by treated cells. There was no significant difference in 8-IP production when cells were exposed to either 5 mM Ca separately or together with COM crystals for 3 or 6 h
There was a significant increase in production of 8-isoprostane (8-IP) by exposures to calcium as well as COM crystals (Fig. 3b). Exposure to the two together also resulted in significantly high amounts of 8-IP compared to the controls but not any more than the individual treatments. Amount of 8-IP was in fact, significantly lower after joint treatment than after individual treatments.
Discussion
There is growing evidence that CaOx nephrolithiasis is associated with renal injury. Data also support the theory that renal injury may be caused by the development of oxidative stress in nephrolithic kidneys. Idiopathic stone formers excrete significant amounts of γ-glutamyl transpeptidase, β-galactosidase, and N-acetyl-β-glucosaminidase in their urine, indicating associated renal epithelial injury [8]. Urinary excretion of lipid peroxides by stone formers is also significantly increased [9]. Similarly experimental induction of CaOx nephrolithiasis in rats leads to urinary excretion of alanine aminopeptidase and N-acetyl-β-glucoseaminidase. Renal and urinary lipid peroxides are also significantly increased [10]. We already mentioned that renal epithelial cells in culture, exposed to high levels of oxalate or CaOx monohydrate crystals, produce ROS and lipid peroxides [2-6]. Treatment with antioxidants leads to significant reduction in production of ROS, lipid peroxides as well as cellular injury. Thus ROS are most likely responsible for the renal injury associated with CaOx nephrolithiasis [2]. Studies presented here show that high levels of calcium are also injurious at least to cells of the renal proximal tubular epithelial origin and ROS are produced during calcium/cell interactions. Amounts of calcium used in our study, 5 or 10 mmol, are higher than physiological levels that normal renal epithelial cells may come in contact with. But under hypercalciuric conditions when urinary excretion of calcium can reach an average of 6.2 mmol (250 mg)/24 h in women and 7.5 mmol (300 mg)/24 h in men, possibility does exist of renal epithelial cells of idiopathic stone patients being exposed, through course of the day, to levels of calcium used in our studies. Calcium at both 5 and 10 mmol concentrations for all time periods tested, appeared insulting with 10 mmol and 12 h exposures being clearly so.
In an earlier study exposure of MDCK (Madin Darby Canine Kidney) cells in culture to 0.5 M calcium was also found to be injurious [11]. Cell injury promotes crystal adhesion through exposure of specific molecules on cell membrane surfaces and is considered significant for crystal retention within the renal tubules [12-15]. Interestingly, COM crystal adhesion to the MDCK cells progressively increased as calcium concentrations were raised from 0 to 50 mM [11]. Further increase in calcium, however, resulted in declining crystal adhesion. Direct measurement of adhesion forces between COM crystals and MDCK cells using atomic force microscopy revealed an increase in adhesion force between MDCK cells and COM crystals as calcium concentrations were raised from 0 to 100 mM [16]. Any further increase in calcium concentration resulted in decreasing adhesion forces.
HK2 cells in culture were injured by exposure to calcium, COM crystals or calcium with COM crystals. Exposure to COM crystals was more injurious than to only calcium or calcium with COM crystals as evidenced by LDH release, trypan blue exclusion, and 8-IP or H2O2 production. These results are quite different from our earlier observations in which cells were exposed to COM crystals with oxalate. When LLC-PK1 and MDCK cells were exposed to oxalate, COM crystals or oxalate with COM crystals, COM crystals with oxalate caused more damage than COM crystals or oxalate alone. Apparently oxalate and COM crystals act synergistically while calcium and COM crystals are not. Calcium and COM crystals may activate same cellular pathways while oxalate and COM crystals may trigger different machinery. Calcium may also affect crystal/cell interfaces by developing stereochemical interactions with the crystal and cell surfaces at the atomic and molecular levels. Excess calcium is readily incorporated into COM crystals.
Hypercalciuria is considered an important risk factor for the development of calcific kidney stones because of calcium’s role in the crystallization of calcium phosphate and CaOx. However calcium is more than a cationic component of the crystals. It is a second messenger and involved in redox signaling, in both the physiological and pathological circumstances [17]. Excess calcium can exert positive feedback on ROS generation resulting in the development of oxidative stress triggering pathological pathways, disease and cell death. Earlier studies have presented evidence for calcium’s role in modulating crystal attachment to the renal epithelial cells and crystal retention within the kidneys. Studies presented here show that high, extracellularly supplied calcium is injurious and that the injury may be caused by the production of ROS and the development of oxidative stress.
Acknowledgments
Research supported by NIH grants # RO1DK065658, Center for the Study of Lithiasis and Pathological calcification. Dr. Khaskheli is recipient of a postdoctoral fellowship of Higher Education Commission of Government of Pakistan.
Contributor Information
Muhammad H. Khaskhali, Department of Chemistry, Shah Abdul Latif University, Khairpur, Sindh, Pakistan; Department of Pathology, Center for the Study of Lithiasis, College of Medicine, University of Florida, Gainesville, FL, USA
Karen J. Byer, Department of Pathology, Center for the Study of Lithiasis, College of Medicine, University of Florida, Gainesville, FL, USA
Saeed R. Khan, Department of Pathology, Center for the Study of Lithiasis, College of Medicine, University of Florida, Gainesville, FL, USA
References
- 1.Worcester E, Coe FL. Nephrolithiasis. Prim Care Clin Off Pract. 2008;35:369–391. doi: 10.1016/j.pop.2008.01.005. doi:10.1016/j.pop.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan SR. Hyperoxaluria-induced oxidative stress and anti-oxidants for renal protection. Urol Res. 2005;33:349–357. doi: 10.1007/s00240-005-0492-4. doi:10.1007/s00240-005-0492-4. [DOI] [PubMed] [Google Scholar]
- 3.Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res. 2003;31:3–9. doi: 10.1007/s00240-002-0286-x. [DOI] [PubMed] [Google Scholar]
- 4.Jonassen JA, Cao LC, Honeyman T, Scheid CR. Mechanisms mediating oxalate-induced alterations in renal cell functions. Crt Rev Eukar Gene Expr. 2003;13:55–72. doi: 10.1615/critreveukaryotgeneexpr.v13.i1.50. doi:10.1615/CritRevEukaryotGeneExpr.v13.i1.50. [DOI] [PubMed] [Google Scholar]
- 5.Khand FD, Gordge MP, Robertson WG, Noronha-Dutra AA, Hothersall JS. Mitochondrial superoxide production during oxalate mediated oxidative stress in renal epithelial cells. Free Radic Biol Med. 2002;32:1339–1350. doi: 10.1016/s0891-5849(02)00846-8. doi:10.1016/S0891-5849(02) 00846-8. [DOI] [PubMed] [Google Scholar]
- 6.Thamilselvan S, Byer KJ, Hackett RL, Khan SR. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol. 2000;164:224–229. doi:10.1016/S0022-5347(05)67499-X. [PubMed] [Google Scholar]
- 7.Hackett RL, Shevock PN. Madin-Darby Canine Kidney Cells are injured by exposure to oxalate and calcium oxalate crystals. Urol Res. 1994;22:197–204. doi: 10.1007/BF00541892. doi:10.1007/BF00541892. [DOI] [PubMed] [Google Scholar]
- 8.Baggio B, Gambaro G, Ossi E, Favaro S, Boesatti A. Increased urinary excretion of renal enzymes in idiopathic calcium oxalate nephrolithiasis. J Urol. 1983;129:1161. doi: 10.1016/s0022-5347(17)52619-1. [DOI] [PubMed] [Google Scholar]
- 9.Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P. Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res. 2005;33:65–69. doi: 10.1007/s00240-004-0444-4. doi:10.1007/s00240-004-0444-4. [DOI] [PubMed] [Google Scholar]
- 10.Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol. 1997;157:1059–1063. doi:10.1016/S0022-5347(01)65141-3. [PubMed] [Google Scholar]
- 11.Lieske JC, Farell, Deganello S. The effect of ions at the surface of calcium oxalate monohydrate crystals on cell-crystal interactions. Urol Res. 2004;32:117–123. doi: 10.1007/s00240-003-0391-5. doi:10.1007/s00240-003-0391-5. [DOI] [PubMed] [Google Scholar]
- 12.Verkoelen CF. Crystal retention in renal stone disease: a crucial role for the glycosaminoglycan hyaluronan? J Am Soc Nephrol. 2006;17:1673–1687. doi: 10.1681/ASN.2006010088. doi:10.1681/ASN.2006010088. [DOI] [PubMed] [Google Scholar]
- 13.Khan SR. CaOx crystal interaction with renal epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res. 1995;23:71–79. doi: 10.1007/BF00307936. doi:10.1007/BF00307936. [DOI] [PubMed] [Google Scholar]
- 14.Khan SR, Byer KJ, Thamilselvan S, Hackett RL, McCormack WT, Benson NA, Vaughn KL, Erdos GW. Crystal–cell interaction and apoptosis in oxalate-associated injury of renal epithelial cells. J Am Soc Nephrol. 1999;10:S457–S463. [PubMed] [Google Scholar]
- 15.Lieska JC, Toback FG. Renal cell–urinary crystal interactions. Curr Opin Nephrol Hypertens. 2000;9:349–355. doi: 10.1097/00041552-200007000-00005. doi:10.1097/00041552-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Rabinovich YI, Daosukho S, Byer K, El-Shall H, Khan SR. Direct measurements of adhesion forces between calcium oxalate monohydrate and kidney epithelial cells in the presence of Ca2+ and Mg2+ ions. J Colloid Interface Sci. 2008;325:594–601. doi: 10.1016/j.jcis.2008.06.024. doi:10.1016/j.jcis.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1275–1312. doi: 10.1089/ars.2007.1886. doi:10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]