Abstract
Anti-vector immunity mitigates immune responses induced by recombinant adenovirus vector vaccines, limiting their prime-boost capabilities. We have developed a novel gene delivery and expression platform (Ad5 [E1-, E2b-]) that induces immune responses despite preexisting and/or developed concomitant Ad5 immunity. In the present study, we evaluated if this new Ad5 platform could overcome the adverse condition of pre-existing Ad5 immunity to induce effective immune responses in prime-boost immunization regimens against two different infectious diseases in the same animal. Ad5 immune rhesus macaques (RM) were immunized multiple times with the Ad5 [E1-, E2b-] platform expressing antigens from simian immunodeficiency virus (SIV). Immunized RM developed cell-mediated immunity against SIV antigens Gag, Pol, Nef and Env as well as antibody against Env. Vaccinated and vector control RMs were challenged intra-rectally with homologous SIVmac239. During a 7-week follow-up, there was perturbation of SIV load in some immunized RM. At 7 weeks post-challenge, eight immunized animals (53%) did not have detectable SIV, compared to two RM controls (13%) (P<0.02; log-rank Mantel-Cox test). There was no correlation of protective MHC contributing to infection control. The RM without detectable circulating SIV, now hyper immune to Ad5, were then vaccinated with the same Ad5 [E1-, E2b-] platform expressing H1N1 influenza hemaglutinin (HA). Thirty days post Ad5 [E1-, E2b-]-HA vaccination, significant levels of influenza neutralizing antibody were induced in all animals that increased after an Ad5 [E1-, E2b-]-HA homologous boost. These data demonstrate the versatility of this new vector platform to immunize against two separate disease targets in the same animal despite the presence of immunity against the delivery platform, permitting homologous repeat immunizations with an Ad5 gene delivery platform.
Keywords: HIV vaccine; Ad5 immunity; Adenovirus vector; SIVmac239; Ad5 [E1-, E2b-]
1. Introduction
Gene delivery platforms have been intensively investigated as vaccine candidates because they can induce potent cell-mediated immunity (CMI) and humoral responses. A major challenge in use of recombinant viral vectors vaccines has been their inherent immunogenicity causing pre-emptive immunologic clearance of the vector and a significant reduction in the desired transgene directed immune responses [1–4]. Adenovirus serotype 5 (Ad5) based vector vaccines have been thwarted because preexisting immunity to Ad5 is widespread in humans due to natural infection that hinders even a primary immunization in clinical studies [3–5]. Ad5 seropositivity is prevalent and studies suggest that cross-reactive Ad-specific T cell responses are essentially universal [6–8].
An improved Ad5 vector platform with additional deletions in the E2b gene region (Ad5 [E1-, E2b-]) has been reported by us to induce immune responses in Ad5 immune animal models [9–15]. Previously, we determined that the Ad5 [E1-, E2b-] platform induced comparable levels of immunity against simian immunodeficiency virus (SIV) targets (Gag, Nef) in Ad5 naïve and Ad5 immune rhesus macaques (RM) in homologous vaccination regimens [14]. Comparable levels of immune responses were subsequently induced in RM to a third SIV antigen (HIV-Pol) vectored by Ad5 [E1-, E2b-], despite hyper-Ad5 immunity (Ad5 NAb titers averaging 1:5700) [14]. We utilized a Chinese-origin RM SIVmac239 challenge model to determine if immune responses induced by a multivalent Ad5 [E1-, E2b-]-SIV vaccine in Ad5 immune RM could result in SIV controlling effects [16]. We report that SIV-specific immune responses induced by vaccinations with Ad5 [E1-, E2b-]-gag/pol/nef/env resulted in control of circulating SIV in a significant number of animals.
To further test our hypothesis that the Ad5 [E1-, E2b-] vector is a platform technology that can be used to induce immune responses to a multitude of diseases within the same host, we vaccinated RM that controlled SIV infection against a second infectious agent, influenza H1N1. The homologous Ad5 [E1-, E2b-] vector backbone expressing H1N1 hemagglutinin (HA) was administered in a second prime-boost protocol in the hyper-Ad5 immune RM, which resulted in the induction of significant levels of influenza neutralizing antibody. This proof-of-concept study confirms the ability to administer multiple homologous immunizations using the Ad5 [E1-, E2b-] vector in the presence of anti-vector immunity and induce effective immune responses against multiple diseases.
2. Materials and methods
2.1. Animals
Thirty SIV and H1N1 naïve Chinese-origin RM were purchased, housed, and handled by BIOQUAL, Inc., Rockville, MD in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), the Animal Welfare Act as amended, the Public Health Service Policy on Humane Care and Use of Laboratory Animals, 2002 and the NIH guidelines for Research Involving Recombinant DNA Molecules. Animals were sedated with 10 mg/kg ketamine and 1 mg/kg acepromazine when required. PBMC and sera from individual animals were collected at BIOQUAL and sent to Etubics Corporation for immune response assessments and to the University of Wisconsin-Madison for MHC class I genotyping. BIOQUAL performed animal body temperature determinations, weights, blood chemistries, and hematology parameters.
2.2. Ad5 vector vaccine construction
The Ad5 [E1-, E2b-] platform has deletions in the E1 gene, E3 gene, polymerase (pol) and preterminal (pTP) protein genes in the early 2 (E2b) gene region [9]. The previously described SIV Gag and SIV nef sequences [14] were employed in this study. The SIV Pol and Env (gp140) inserts were kindly provided by Dr. Daniel Barouch (Harvard Medical School). The previously described HA influenza H1N1 gene insert was also used [15]. Gag, nef, pol, env or HA vector vaccines were constructed and transgene expression verified as described [2,10,11,17]. The ratio of VP to plaque forming units (PFU) was ≥35:1 VP/PFU per lot.
2.3. Immunizations and challenge with SIVmac239
All RM were immunized two times at 2-week intervals (day-33, -14) intradermally with a needle in the hind leg with 1010 VP of Ad5 [E1-]-null (no inserted transgene) (Table 1), which, as reported, induces Ad5 neutralizing antibody (NAb) titers of approximately 1:200 [14]. This level of Ad5 immunity has been considered as “high” Ad5 pre-existing immunity in clinical trials [10–15]. Following Ad5 immunization, RM were randomized into two groups of 15 each based on sex, weight and TRIM5α genotyping (supplementary Table I). Fifteen Ad5-immune RM were immunized subcutaneously in the hind leg with a needle with 1010 VP of a 1:1:1:1 mixture of SIV Ad5 [E1-, E2b-]-gag/pol/nef/env (4 × 1010 VP/injection) on days 0, 14, 28 and 42. Subcutaneous immunization was chosen since this is the intended route of vaccination and is currently being employed in our cancer clinical trial using the Ad5 [E1-, E2b-] vector (ClinicalTrials.gov identifier NCT01147965). To control for non-specific vector-induced immune responses, 15 Ad5-immune RM were immunized with 4 × 1010 VP of Ad5 [E1-, E2b-]-null on the same immunization days. Peripheral blood mononuclear cells (PBMC) and serum were collected from RM immediately before the first Ad5 [E1-, E2b-] vaccination on day 0 (baseline) and 2 weeks after every vaccination. Fourteen days following last vaccination (day 56), all RM were challenged intrarectally with two inoculations of 450 TCID50 SIVmac239 (total of 900 TCID50) over a 24-h period, designed to achieve at least a 90% infection rate in control animals. Similar challenge methods and titers have been previously employed [18,19]
Table 1.
Study protocol.
| Study Day | -33 | -21 | 0 | 14 | 28 | 42 | 56 | 119 | 147 | 161 |
|---|---|---|---|---|---|---|---|---|---|---|
| Ad5 [E1-]-null administration | X | X | ||||||||
| Ad5 [E1-, E2b-]-SIV immunization | X | X | X | X | ||||||
| SIVmac239 challenge | X | |||||||||
| Termination of SIV progressor RM | X | |||||||||
| Ad5 [E1-, E2b-]-HA immunization of SIV controller RM | X | X | ||||||||
| Termination of study | X |
2.4. Immunizations with Ad5 [E1-, E2b-]-H1N1-HA
RM that controlled blood-borne SIV infection to below detectable levels were immunized with 1011 VP of Ad5 [E1-, E2b-]-HA subcutaneously. This vaccine utilizes the same Ad5 [E1-, E2b-] platform and contains the HA gene [16]. Serum samples were collected at initiation of immunization, during, and after immunizations to assess hemagglutination inhibition (HAI) titers.
2.5. Ad5 neutralizing antibody (NAb) and HAI assays
Endpoint Ad5 NAb titers were determined as described employing an MTS tetrazolium bioreduction assay [10,12–14].
HAI assays were performed as described [16] with HAI titers <1:20 considered negative.
2.6. Enzyme-linked immunospot (ELISpot) assay
SIV Gag, Nef, Pol, or Env-specific IFN-γ secretion in RM PBMC was determined employing ELISpot assays previously described [14] using SIVmac239 Gag (15-mer) Peptides-Complete Set, SIV-mac239 Nef (15-mer) Peptides-Complete set, SIVmac239 Pol (15-mer) peptides-Complete Set, and SIVmac239 Env (15-mer) Peptides-Complete Set, respectively (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH).
2.7. Enzyme linked immunosorbent assay (ELISA) for SIV-Gag, Nef, Pol, and Env gp140
Sera from RM were collected and frozen at −20°C until assayed. A quantitative ELISA technique using 50 ng of purified SIV-Gag, Pol, Nef or gp120 Env, respectively, was employed [13,20]. Briefly, ELISA plates were incubated with antigen at room temperature overnight, washed with PBS containing 1% Tween-20 (PBS/Tween) and blocked with PBS/Tween. Sera (200 µL), diluted 1:100 in PBS/Tween, was added to each well, incubated for 1 h at room temperature, then incubated with 200 µL of a 1:5000 dilution of rabbit peroxidase-conjugated anti-monkey IgG (γ-chain specific) (Sigma Chemicals, St. Louis, MO) for 1 h at room temperature. Plates were washed three times with PBS/Tween and 1,2-phenylenediamine substrate solution (200 µL) was added to each well. The reaction was stopped by with 5 N HCl solution (50 µL). Quantitative IgG determinations using an IgG reference standard were calculated as described [12,16].
2.8. Real-time quantitative PCR and MHC class I genotyping
Levels of SIV RNA in plasma were determined by real-time quantitative PCR as described [21,22].
Comprehensive MHC class I genotyping for Mamu-A and -B alleles was performed as described [23,24].
2.9. Statistical analysis
Statistically significant differences in mean immune responses between groups of animals were determined by Student’s t-tests (GraphPad Software, Inc.). Numbers of infected RM over time for each group were compared employing a Kaplan-Meier plot on infected versus non-infected RM and analysis employing the log-rank Mantel-Cox test (GraphPad Software, Inc.). Peak SIV RNA levels in plasma were compared employing the Mann-Whitney test (GraphPad Software, Inc.).
3. Results
3.1. Ad5 [E1-, E2b-]-SIV vaccine induced immune responses
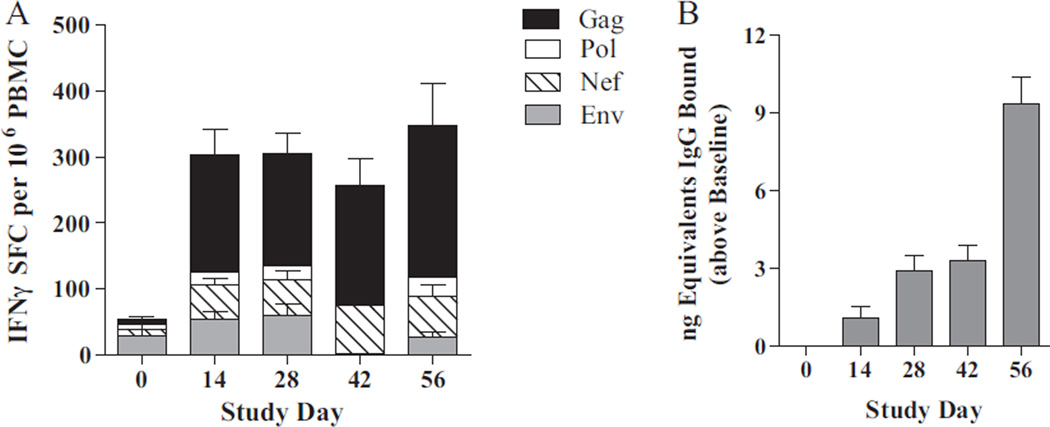
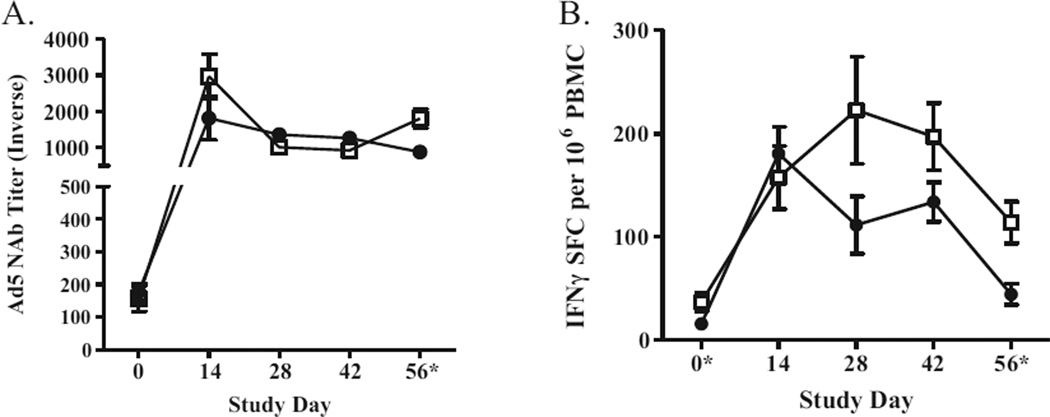
Adverse reactions to immunizations monitored by clinical observations of body weight, temperature, blood chemistries and hematology revealed no significant adverse effects or deviations. Ad5-immunity was induced and confirmed in RM. Three weeks following final Ad5 [E1-]-null injection (day 0), Ad5-directed neutralizing activity was demonstrated by presence of Ad5 NAb (1/176 ± 27 SEM in controls; 1/157 ± 39 SEM in vaccinated; P=0.68). Vaccination with Ad5 [E1-, E2b-]-gag/pol/nef/env induced SIV-specific CMI responses in Ad5-immune RM as determined by ELISpot analysis for IFN-γ secreting PBMC (Fig. 1A). Two weeks following final immunization, vaccinated RM had diverse magnitudes of SIVmac239-specific CMI responses with the highest responses directed against SIV Gag. There were no SIV-directed immune responses in control animals. Ad5-directed CMI responses were observed in both vaccinated and control RM, which peaked during study days 14–28 and decreased following cessation of immunizations (Fig. 2).
Fig. 1.
Vaccine-induced immune responses in RM before SIVmac239 challenge. PBMC and sera were isolated from vaccinated RM at baseline (day 0) and during the course of 4 immunizations. PBMC were assessed for Gag(black), Pol(white), Nef(striped) or Env(grey) induced IFN-γ secretion by ELISpot analysis (A). Note the CMI responses induced during the course of multiple immunizations. No SIV CMI responses were detected in controls immunized with Ad5 [E1-, E2b-]-null. An IgG response (baseline-subtracted) was detected against gp120 env in vaccinated RM employing a quantitative ELISA technique (B). No antibody was detected against SIV-gag, pol, or nef.
Fig. 2.
Ad5 vector-induced immune responses. PBMC and sera were isolated from vaccinated and vector control RM at baseline (day 0) and during the course of 4 immunizations (A). Ad5 NAb endpoint titers peaked 2 weeks (day 14) after the first injection with either Ad5 [E1-, E2b-]-null (black circle) or multivalent Ad5 [E1-, E2b-]-SIV-gag/pol/nef/env (white square). Thereafter, Ad5 NAb levels decreased to newly established but higher baseline levels. Error bars indicate ± SEM. B. Ad5 CMI responses peaked on day 14 in vector control animals (black circle) and day 28 in Ad5 [E1-, E2b-]-SIV immunized RM. These responses were significantly higher in the vaccinated RM on the first day of Ad5 [E1-, E2b-]-gag/pol/nef/env immunization. *Days that there was a significant difference between the vaccinated and vector control RM (P<0.05).
Analysis of serum samples revealed that significant SIV gp120 Env antibody was induced in vaccinated RM (Fig. 1B). No Gag, Pol or Nef antibodies were detected and whether or not lack of antibody production is due to the route of immunization and/or other factors is beyond the scope of this study and is subject to further research. Ad5 NAb titers peaked on day 14 in both groups and decreased thereafter but remained higher than initial baseline values (Fig. 1C).
3.2. SIVmac239 challenge
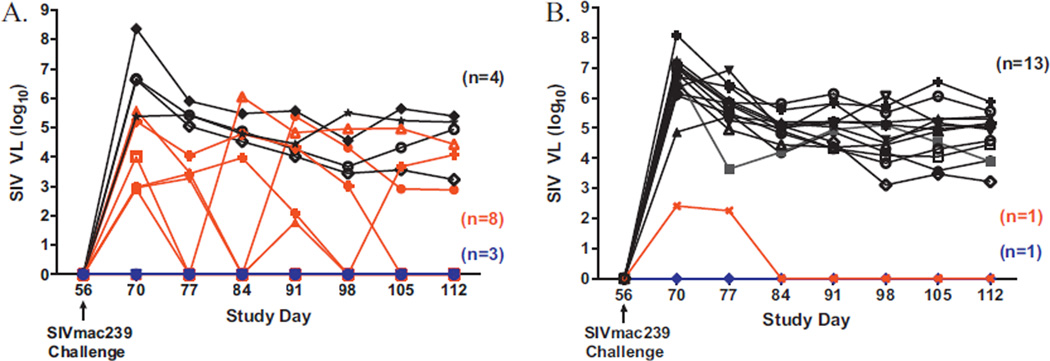
On day 56 post-initiation of immunization, all 30 RM were challenged intrarectally with two inoculations of 450 TCID50 SIVmac239 virus (provided by Dr. Ronald Derosiers, Harvard University) within a 24-h period [25]. Two weeks post-challenge (day 70), initial SIV viral loads were assessed. Five SIV vaccinated RM (33.3%) and one control RM control (6.6%) had below detectable levels of SIV in plasma (<40 RNA copies/mL). Over the next 7 weeks there was evidence of viral control in immunized RM. Three vaccinated RM and one control RM had no detectable SIV in plasma at any time points assessed (Fig. 2). Five vaccinated RM that initially had detectable circulating SIV controlled the virus to below detectable levels in plasma on day 77 (n=2), day 84 (n=2) and day 105 (n = 1) post-SIV challenge, respectively. One control animal that had low levels of SIV post-challenge also controlled SIV to below detectable levels in plasma by day 84 (Fig. 2B). Two vaccinated RM that initially had undetectable virus post-challenge became virus positive. Four immunized RM (26.7%) and 13 controls (86.7%) did not show evidence of SIV control and were terminated. Eight vaccinated RM (53.3%) and two controls (13.3%) had below detectable levels of SIVmac239 at 15 weeks post-challenge. Fig. 3.
Fig. 3.
SIV viral load (RNA copies/mL plasma). SIV viral load was assessed 2 weeks post-SIVmac239 challenge (day 70) and weekly thereafter. Ad5-immune RM immunized with Ad5 [E1-, E2b-]-gag/pol/nef/env had varying degrees of SIV viral control (A). Three vaccinated RM had undetectable levels of SIV in plasma for the duration of the study (A, blue). Eight vaccinated RM exhibited viral control (A, red). Four vaccinated had persistent SIV levels post SIV challenge A (black). Ad5-immune controls generally had no control over SIV had persistent SIV viremia post SIVmac239 challenge (B, black). One control RM had undetectable SIV for the duration of the study (B, blue) and one control RM had a viral blip that resolved by day 84 (B, red). Thirteen controls.
To determine if the RM that controlled SIV to below detectable levels developed de novo CMI responses to SIV, PBMC were assessed for recognition of immunizing Gag, Pol, Nef and Env as well as non-immunizing Tat, Rev and Vif. Vaccinated RM that were SIV viremic but controlled the virus to below detectable levels had anamnestic immune responses to immunizing and non-immunizing SIV antigens (data not shown), indicating that an SIV infection was established in these animals. The three vaccinated RM that remained aviremic did not develop de novo CMI responses to non-immunizing SIV antigens Tat, Vif and Rev post-SIV challenge, indicating that an SIV infection was not established in these animals. The two control RM without progressive SIV infection also did not develop de novo CMI responses to non-immunizing SIV antigens Tat, Vif and Rev post SIV challenge.
Major histocompatibility complex (MHC) classes I and II molecules determine the repertoire of T cell responses that can develop against SIV and/or any other foreign pathogen [26]. Although Chinese RM are not as well defined in MHC loci as Indian RM, a few allelic variations are identified that contribute to natural protection or control of SIV infection including B03 and B17. To determine if any particular MHC allele affected the magnitude of vaccine induced CMI responses or control of SIV viremia following challenge, MHC typing was performed on all of the RM (Supplementary Table 1) [23,24]. There was no observed correlation between SIV viral control and genetic parameters assessed.
3.3. Vaccination against a second infectious disease H1N1 influenza
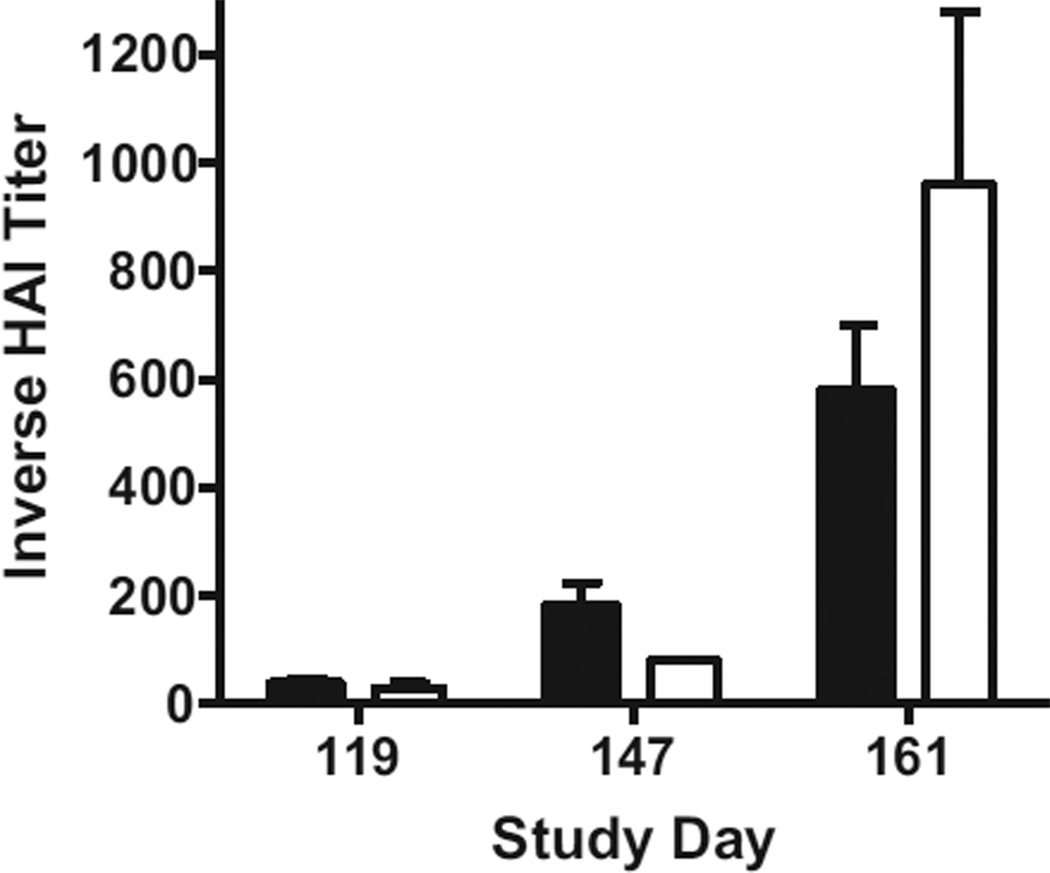
To determine if the Ad5 [E1-, E2b-] platform could be used to induce an immune response to a different infectious disease after immunization and control of SIV, the 8 SIV vaccinated animals that controlled SIV to below detectable levels and were hyper-immune to Ad5 were subsequently immunized with Ad5 [E1-, E2b-]-HA on days 119 and 147. The two control RM without progressing SIV were also immunized using the prime-boost protocol to determine if HA specific immune responses could be induced despite concomitant Ad5-immunity. Ad5 NAb titers averaged 1:406 ± 102 (SEM) in the 8 SIV vaccinated RM and 750 ± 250 (SEM) in control RM on the first day of Ad5 [E1-, E2b-]-HA vaccination. Sera were assessed for H1N1 neutralizing antibody activity 30 days after the first H1N1 vaccination. All 10 vaccinated RM vaccinated sero-converted, exhibiting levels of HAI titers generally considered to provide protection from influenza infection (>1:40) (Fig. 4) [27]. The RM were boosted with Ad5 [E1-, E2b-]-HA on day 147 to determine if anti-influenza responses could be elevated. Ad5 hyper-immunity was present with Ad5 NAb levels averaging 1:2025 ± 690 (SEM) in SIV-immunized RM and 1:5000 ± 0 (SEM) in control RM on day 147. Two weeks after homologous Ad5 [E1-, E2b-]-HA boost (day 161), HAI titers increased over 10-fold from pervious values, averaging 1:580 ± 120 (SEM) in SIV-vaccinated RM and 1:960 ± 320 (SEM) in control RM (Fig. 4).
Fig. 4.
Hemagglutinin inhibition (HAI) titers in RM. Eight SIV-vaccinated RM (black) and two vector control RM (white) that controlled SIV to below detectable levels were subsequently immunized with Ad5 [E1-, E2b-]-HA on days 119 and 147. Sera were assessed for the development of H1N1 neutralizing antibody activity 30 days after the first H1N1 vaccination (day 147). All 10 RM vaccinated with Ad5 [E1-, E2b-]-HA seroconverted. The RM were then boosted with Ad5 [E1-, E2b-]-HA on day 147 and HAI titers were assessed 2 weeks later (day 161). The boost increased HAI in both groups, averaging 1:580 ± 120 (SEM) in SIV vaccinated RM and 1:960 ± 320 in SIV control RM.
4. Discussion
We investigated the anti-SIV effect of immunizations with a multivalent SIV vaccine using a new Ad5 [E1-, E2b-] platform. Importantly, RM were made Ad5-immune to determine if the Ad5 [E1-, E2b-] platform could remain effective in the presence of vector immunity. Employing a multiple homologous immunization protocol, SIV-specific CMI responses were induced after immunizations with Ad5 [E1-, E2b-]-gag/nef/pol/env. This confirms our previous studies in Ad5-immune RM and Ad5-immune mice, where we observed similar results [10,11,14]. Additionally, SIV specific Env antibodies were detected in Ad5-immune RM after multiple vaccinations. We performed a homologous challenge with SIV-mac239 and assessed the short-term viral controlling effects of immunization. We observed that immunizations resulted in control of SIV viremia in a significant number of animals and speculate that both cellular and humoral immune responses contributed to viremia control, although further detailed examinations of the mechanisms are needed. It has been reported that immunologic correlates indicated that Env-specific antibodies may be critical for blocking acquisition of infection and that multiple cellular and humoral immune responses may correlate with virus control [19]. In future studies, it is important to identify immune-based parameters including levels of polyfunctional T-cell compartments from both systemic and mucosal tissues to achieve greater insight into the mechanism of SIV control. It will also be useful to follow SIV challenged animals for longer periods of time and assess the influence of immunologic memory on the control of viral infection.
Another important aspect of the present study is the demonstration that an immune response can be induced against a second infectious disease target in an Ad5 hyper-immune environment. After two administrations of Ad5-null and four immunizations with Ad5 [E1-, E2b-]-SIV-gag/nef/pol/env, RM were immunized against influenza using the same vector backbone. Neutralizing activity to H1N1 influenza was assessed by HAI because this is a standard test used as a correlate of protection in human clinical trials [27]. HAI activity was induced in RM 30 days after a single immunization and boosted with a second administration of Ad5 [E1-, E2b-]-HA despite high titers of Ad5 NAb (Fig. 4). We previously reported that similar levels of HAI titers induced in mice and ferrets by immunizations with Ad5 [E1-, E2b-]-HA resulted in protection from disease as evidenced by lack of mortality and pathology after lethal H1N1 challenge [16]. This demonstrates the extended utility of the new recombinant Ad5 [E1-, E2b-] vector platform to immunize against one disease with the capacity to immunize against a second disease in the same RM.
Ad5 vectors remain ideal candidates for use as vaccine gene delivery platforms with several important attributes including an extensive safety profile, reliable manufacturing, and direct delivery to patients for immunization or immunotherapy [28,29]. We are currently able to produce sufficient quantity of the Ad5 [E1-, E2b-] vaccine platform to deliver up to 5 × 1011 VP/dose in a current cancer clinical trial (ClinicalTrials.gov identifier NCT01147965). The STEP trial tested an earlier generation recombinant Ad5 [E1-] trivalent HIV vaccine and brought forth a safety concern for the use of recombinant Ad5-based HIV vectored vaccines. It has been reported that there was a trend toward increased risk of HIV-1 acquisition in vaccinated subjects who were Ad5 seropositive at baseline [30,31]. It was observed that within 18 months of enrollment or 1 year after vaccination, uncircumcised men with baseline Ad5 NAb had an increased risk of acquiring HIV infection that waned after about 18 months and became equal to that of volunteers who received placebo [32,33]. Recent evidence indicates that risk behaviors in this group could not account for increased risk of acquiring HIV infection noted in the STEP trial [34]. These results are confounded by studies indicating that uncircumcised men are at increased risk of HIV infection without Ad5 vector immunization [32,33]. Moreover, these findings are in sharp contrast to three different HIV-1 vaccine efficacy trials indicating that pre-existing Ad seropositivity is not associated with HIV-1 acquisition [35]. A recent analysis of baseline serum from participants in the STEP trial revealed that subjects infected with HIV-1 during the study were less immunologically responsive prior to the first vaccination [31]. These qualitative differences in their immune systems prior to immunization may have increased risk of HIV-1 acquisition [31]. Further research will be required to delineate if any biologic factors due to Ad5 vaccination contributed to increased susceptibility to HIV-1 acquisition, although no causal association has been found between Ad5 seropositivity and HIV-1 acquisition despite intense investigation [30,31].
This proof-of-concept study confirms the ability to administer multiple homologous immunizations using the Ad5 [E1-, E2b-] vector in the presence of anti-vector immunity and induce effective immune responses against multiple diseases. The new Ad5 [E1-, E2b-] vector advances the field of vaccine technology by providing a platform that may be utilized in applications requiring multiple homologous immunizations.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Daniel Barouch of the Harvard Medical School for contribution of the SIV-pol and SIV-gp140env plasmids, Dr. Andrea Amalfitano for his consultation regarding vector development and Elizabeth Peters and Matt Collins for their assistance with the study. We also wish to thank Ms. Carol Jones for her management of NIH grant activities and ViraQuest, North Liberty, IA, USA for vaccine production. This work was supported in part by NIH-NIAID Grant 2R44A1071733 to Etubics Corporation. The MHC class I genotyping was supported by Etubics Corporation and performed at the Wisconsin National Primate Research Center with support from NIH-NIAID grant P51RR000167. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2012.09.058.
References
- 1.Tatsis N, Lasaro MO, Lin SW, Haut LH, Xiang ZQ, Zhou D, et al. Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens. J Immunol. 2009;182:6587–6599. doi: 10.4049/jimmunol.0900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol. 2007;81:6594–6604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, et al. Phase I safety and immnogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–1649. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 5.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang Z, Li Y, Cun A, Yang W, Ellenberg S, Switzer WM, et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerging Infect Dis. 2006;12:1596–1599. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutnick NA, Carnathan D, Demers K, Makedonas G, Ertl H, Betts MR. Adenovirus-specific human T cells are pervasive, polyfuntional, and cross reactive. Vaccine. 2010;28:1932–1941. doi: 10.1016/j.vaccine.2009.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frahm N, DeCamp AC, Friedrich DP, Carter DK, Defawe OD, Kublin JG, et al. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest. 2012;122:359–367. doi: 10.1172/JCI60202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–933. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabitzsch ES, Xu Y, Yoshida L, Balint J, Gayle RB, Amalfitanto A, et al. A preliminary and comparative evaluation of a novel Ad5 [E1-, E2b-] recombinant based vaccine used to induce cell mediated immune responses. Immunol Lett. 2009;122:44–51. doi: 10.1016/j.imlet.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabitzsch ES, Xu Y, Yoshida LH, Balint J, Amalfitano A, Jones FR. Novel adenovirus type 5 vaccine platform induces cellular immunity against HIV-1 Gag, Pol, Nef despite the presence of Ad5 immunity. Vaccine. 2009;27:6394–6398. doi: 10.1016/j.vaccine.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabitzsch ES, Xu Y, Balint JP, Jr, Hartman ZC, Lyerly HK, Jones FR. Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral [E1-, E2b-]-CEA. Cancer Immunol Immunother. 2010;59:1131–1135. doi: 10.1007/s00262-010-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabitzsch ES, Xu Y, Balcaitis S, Balint JP, Jones FR. An Ad5(E1-, E2b-)-HER2/neu vector induces immune responses and inhibits HER2/neu expressing tumor progression in Ad5 immune mice. Cancer Gene Ther. 2011;18:326–335. doi: 10.1038/cgt.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabitzsch ES, Xu Y, Balcaitis S, Balint JP, Induction Jones FR. Comparison of SIV immunity in Ad5 Naïve and Ad5 Immune Non-human Primates using an Ad5[E1-, E2b-] based vaccine. Vaccine. 2011;29:8101–8107. doi: 10.1016/j.vaccine.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osada T, Yang XY, Hartman ZC, Glass O, Hodges BL, Niedzwiecki D, et al. Optimization of vaccine responses with an E1, E2b, E3 deleted Ad5 vector circumvents pre-existing anti-vector immunity. Cancer Gene Ther. 2009;16:673–682. doi: 10.1038/cgt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones FR, Gabitzsch ES, Xu Y, Balint JP, Borisevich V, Smith J, et al. Prevention of influenza virus shedding and protection from lethal H1 N1 challenge using a consensus 2009 H1 N1 HA and NA adenovirus vector vaccine. Vaccine. 2011;29:7020–7026. doi: 10.1016/j.vaccine.2011.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balint JP, Jr, Jones FR. Detection of elevated levels of antiidiotypic antibody levels in immune thrombocytopenic patients expressing antiplatelet antibody. Blood. 1994;84:664–665. [PubMed] [Google Scholar]
- 18.Lewis MG, Norelli S, Collins M, Barreca ML, Iraci N, Chirullo B, et al. Response of a simian immunodeficiency virus (SIVmac251) to raltegravir: a basis for a new treatment for simian AIDS and an animal model for studying lentiviral persistence during antiretroviral therapy. Retrovirology. 2010;7:21. doi: 10.1186/1742-4690-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagenaur LA, Sanders-Beer BE, Brichacek B, Pal R, Liu X, Liu Y, et al. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol. 2011;6:648–657. doi: 10.1038/mi.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiseman RW, Karl JA, Bimber BN, O'Leary CE, Lank SM, Tuscher JJ, et al. Major histocompatibility complex genotyping with massively parallel pyrosequencing. Nat Med. 2009;15:1322–1326. doi: 10.1038/nm.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol. 2007;81:8827–8832. doi: 10.1128/JVI.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis MG, Bellah S, McKinnon K, Yalley-Ogunro J, Zack PM, Elkins WR, et al. Titration and characterization of two Rhesus-derived SIVmac challenge stocks. AIDS Res Hum Retroviruses. 1994;10:213–220. doi: 10.1089/aid.1994.10.213. [DOI] [PubMed] [Google Scholar]
- 23.Parham P. Putting a face to MHC restriction. J Immunol. 2005;174:3–5. doi: 10.4049/jimmunol.174.1.3. [DOI] [PubMed] [Google Scholar]
- 24.Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines. 2007 May; Accessed at: http://www.fda.gov/downloads/BiologicsBloodVaccines/;.
- 25.Chen S, Chunhui L, Xiaoxiang W, Yaozheng L, Daishu H, Weizhong G, et al. Variability of bio-clinical parameters in Chinese-origin rhesus macaques infected with simian immunodeficiency virus: a nonhuman primate AIDS model. PLoS ONE. 2011;6:e23177. doi: 10.1371/journal.pone.0023177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alpert M, Harvey J, Lauer W, Reeves R, Piatak M, Carville A, et al. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PloS Pathog. 2012;8:e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos SK, Barry MA. Current advances and future challenges in adenoviral vector biology and targeting. Curr Gene Ther. 2007;7:189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatsis N, Ertl HCJ. Adenoviruses as vaccine vectors. Molecular Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the STEP Study): a double-blind, randomized, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng C, Wang L, Gall J, Nason M, Schwartz R, McElrath J, et al. Decreased pre-existing Ad5 capsid and Ad35 neutralizing antibodies increase HIV-1 infection risk in the Step trial independent of vaccination. PLoS ONE. 2012;7(4):e33969. doi: 10.1371/journal.pone.0033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duerr A, Huang Y, Buchbinder S, Coombs RW, Sanchez J, del Rio C, et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J Infect Dis. 2012;206:258–266. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemckerk AA, Sumida SM, Holterman L, Vogels R, Truitt DM, Lynch DM, et al. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity. J Virol. 2005;79:9694–9701. doi: 10.1128/JVI.79.15.9694-9701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koblin BA, Mayer KH, Noonan E, Wang CY, Marmor M, Sanxhez J. Sexual risk behaviors, circumcision status, and preexisting immunity to adenovirus type 5 among men who have sex with men participating in a randomized HIV-1 vaccine efficacy trial: step study. J. Acquir Immune Defic Syndr. 2012;60:405–413. doi: 10.1097/QAI.0b013e31825325aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephenson KA, Hural J, Buchbinder SP, Sinangil F, Barouch DH. Preexisting adenovirus seropositivity is not associated with HIV-1 acquisition in three HIV-1 vaccine efficacy trials. J Infect Dis. 2012;205:1806–1810. doi: 10.1093/infdis/jis285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.