SUMMARY
This review considers recent evidence showing that cells in the reticular activating system (RAS) exhibit 1) electrical coupling mainly in GABAergic cells, and 2) gamma band activity in virtually all of the cells. Specifically, cells in the mesopontine pedunculopontine nucleus (PPN), intralaminar parafascicular nucleus (Pf), and pontine dorsal subcoeruleus nucleus dorsalis (SubCD) 1) show electrical coupling, and 2) all fire in the beta/gamma band range when maximally activated, but no higher. The mechanism behind electrical coupling is important because the stimulant modafinil was shown to increase electrical coupling. We also provide recent findings demonstrating that all cells in the PPN and Pf have high threshold, voltage-dependent P/Q-type calcium channels that are essential to gamma band activity. On the other hand, all SubCD, and some PPN, cells manifested sodium-dependent subthreshold oscillations. A novel mechanism for sleep-wake control based on transmitter interactions, electrical coupling, and gamma band activity is described. We speculate that continuous sensory input will modulate coupling and induce gamma band activity in the RAS that could participate in the processes of preconscious awareness, and provide the essential stream of information for the formulation of many of our actions.
Keywords: Arousal, Calcium channels, Gamma band, Gap junctions, Modafinil, Subthreshold oscillations
Introduction
The reticular activating system (RAS) controls sleep and waking, and fight-vs-flight responses. While this system provides signals that modulate our sleep–wake states, it also serves to help us respond to the world around us. For example, strong stimuli activate ascending RAS projections to the thalamus and then the cortex to cause cortical activation. Such stimuli simultaneously trigger descending projections that influence the spinal cord in the form of postural changes in tone resulting from the startle response, and induce locomotor events in fight-vs-flight responses. The same system is responsible for the relative lack of sensory awareness during slow wave sleep (SWS), as well as the atonia of paradoxical sleep that prevents us from acting out our dreams. This system also modulates the activity of virtually every other in the central nervous system (CNS). We detect changes in sleep–wake states using recordings of electrical activity along the scalp. The electroencephalogram (EEG) measures voltage fluctuations resulting from neuronal activity, and diagnostic applications generally focus on the spectral content of the EEG, that is, the amplitude and frequency of EEG oscillations. What determines the spectral content of the EEG we measure on a daily basis? What is the ionic basis for the rhythms we detect with the EEG during and at the transitions between sleep–wake states? How are oscillations across brain areas generated and controlled? Does this knowledge help us modulate these states pharmacologically and physiologically? How much of this spectral content originates in the RAS? The two major elements that determine the activity of large assemblies of neurons such as that detected in the EEG are coherence and frequency.
Coherence and frequency, two determinants of large scale activity
Coherence is the term for how groups of neurons, firing in coordination, can create a signal that is mirrored instantaneously and precisely by other groups of neurons across the brain.1 These transient episodes of coherence across different parts of the brain may be an electrical signature of thought and actions.1 A recent discovery demonstrated the presence of electrical coupling in three nuclei of the RAS, a mechanism that allows groups of neurons to fire together. Electrical coupling is modulated by the stimulant modafinil, which increases coupling via gap junctions, to drive coherence at higher frequencies to induce arousal.2 Briefly, since most coupled neurons in the RAS are GABAergic, the modafinil-induced coupling decreases input resistance, decreasing activity and gamma amino-butyric acid (GABA) release, thus disinhibiting efferents to all other systems, which leads to overall higher coherence in activity in the RAS,2–4 and thalamocortical (TC) systems.5 Because increased coupling in GABAergic neurons will lead to decreased GABA release, the tendency will be to disinhibit most other transmitter systems, leading to increased excitation, especially during waking. However, if modafinil increases electrical coupling, it should enable better coherence at all frequencies, both during waking and even at night after its effects are waning, during sleeping. We will address the evidence for the presence of electrical coupling in the RAS, and how it is modulated by transmitters and pharmacological agents such as anesthetics.
The other aspect of large scale activity is frequency of firing, especially of ensemble activity, that is also essential to the neural encoding process. The present review is based on another major discovery, the presence of gamma band activity in the same three RAS nuclei.6–9 As we will see below, gamma band activity has been associated with attentional mechanisms, sensory perception and memory. Recent data suggest that many, perhaps all, of the neurons in these RAS regions fire at gamma band frequency when maximally activated, but no higher. These results suggest that brainstem regions not only can generate, but are capped at, such frequencies. By “capped” we mean that the cells cannot fire at rates higher than gamma band. That is, the oscillations generated by the opening and closing of calcium channels simply do not go any faster than gamma band frequencies. Gamma band activity has been described in other subcortical regions like the thalamus, hippocampus, and cerebellum. The goal then becomes one to identify the mechanisms behind gamma band activity generation in the RAS.6–9 We will first address the classical role of gamma band activity, the presence and mechanisms behind gamma band activity in subcortical brain regions, then turn to the mechanisms behind gamma band activity in the RAS, and finally speculate on the potential role of such activity at brainstem levels, in a very old, phylogenetically speaking, region such as the RAS.
Electrical coupling
Electrical coupling in the mammalian brain was first described in the 1970's, with connexin 36 (Cx36) being the only gap junction protein in neurons.10 Connexins oligomerize into hemichannels and are transported to the membrane, congregate in ‘plaques,’ allow the passage of ions and molecules as large as cyclic adenosine monophosphate (cAMP), and have a half-life of several hours. Cx36 gap junctions are the least voltage dependent and are closed by low intracellular pH and low intracellular calcium. Spikelets are stereotypical, usually rhythmic, subthreshold depolarizing potentials thought to reflect firing in the coupled neurons. Electrical coupling and Cx36 labeling are present in the reticular nucleus of the thalamus.11–13 Electrical coupling is also evident in thalamic relay neurons (TRN), but only in the presence of metabotropic glutamate (GLUT) receptor agonists.14 Electrical synapses appear mainly between GABAergic neurons in the thalamic reticular nucleus, where they may promote synchronization of burst firing in the cortex and enhance the synchrony of gamma oscillations.15–17 That is, there are electrical synapses in regions that modulate sleep and waking.
Electrical coupling in the RAS
Gap junctions can be blocked through membrane fluidization such as that induced by the anesthetics halothane and propofol.18,19 Thus, these short-acting anesthetics are also gap junction blockers. Oleamide promotes sleep and blocks gap junctions.20 Anandamide enhances adenosine levels to induce sleep21 and blocks gap junctions.22 One mechanism by which these agents may induce sleep and/or anesthesia is through blockade of electrical coupling in the RAS and other regions. Carbenoxolone, a widely used gap junction blocker, decreases the synchronicity of gamma oscillations, as well as seizure activity.23 Carbenoxolone was used for the treatment of ulcers by blocking gap junctions between acid-secreting cells, but compliance was low because it induced sleepiness. Quinine and its derivative, mefloquine, also block gap junctions, but these anti-malarial agents are soporific as well.25,23 On the other hand, increased electrical coupling can be induced by trimethylamine, a gap junction opener, perhaps by increasing intracellular pH. Therefore, agents that affect gap junctions also affect sleep–wake rhythms.
Modafinil (MOD) is approved for the treatment of excessive sleepiness in narcolepsy, obstructive sleep apnea, and shift work sleep disorder, and is also prescribed in a number of neuropsychiatric conditions. Many publications on this agent acknowledge that the mechanism of action of modafinil is unknown, although there is general agreement that it increases glutamatergic, adrenergic and histaminergic transmission and decreases GABAergic transmission.24 Studies using voltage-sensitive dyes have shown that inhibitory interneurons modulate cortical activation by afferent input. Moreover, cortical interneurons exhibit gamma band (~40 Hz) oscillations that are reduced by pharmacologic blockade of gap junctions.16 The presence of both electrical coupling and chemical synapses between inhibitory interneuron networks are thought to enhance the timing of action potentials (AP).11,15–17 In the cortex, electrical coupling may contribute to action-potential synchronization and network oscillations, to coordination and reinforcement of inhibitory postsynaptic potentials (IPSPs), and to coincidence detection in inhibitory networks.16 There is extensive electrical coupling in the cortex during development, but epileptiform activity is virtually absent. Therefore, electrical coupling may result in a “shunting effect” by decreasing the input resistance of coupled cells, thereby reducing the excitability of cortical interneurons. Such a “shunting effect” has been proposed as the mechanism behind the general absence of epileptiform discharges during early postnatal development of the rat neocortex.25 That same “shunting effect” has been proposed for mode of action of modafinil.
In a landmark study, modafinil was found to increase electrical coupling between cortical interneurons, thalamic reticular neurons, and inferior olive neurons.5 Following pharmacologic blockade of connexin permeability, modafinil restored electrotonic coupling. The effects of modafinil were counteracted by the gap junction blocker mefloquine. These authors proposed that modafinil may be acting in a wide variety of cerebral areas by increasing electrotonic coupling in such a way that the high input resistance typical of GABAergic neurons is reduced. These authors proposed that this “shunting effect” of modafinil may activate the whole thalamocortical system by mildly downregulating inhibitory networks while increasing synchronous activation of both interneurons and noninhibitory neurons.5
We recently described the presence of dye and electrical coupling in the RAS, specifically in the parafascicular nucleus (Pf), pedunculopontine nucleus (PPN) and subcoeruleus nucleus dorsalis (SubCD).2–4 Dye coupling is present when a single cell is injected with fluorescent dye, but multiple cells are found to be labeled, while electrical coupling is determined by simultaneously recording from two nearby cells, blocking all action potential generation and synaptic activity, then intracellularly stimulating one cell and recording an albeit decreased response in the other and vice versa. We also found that modafinil decreased the input resistance of PPN and SubCD neurons, in keeping with results in the cortex, reticular thalamus, and inferior olive. The effects of modafinil were evident in the absence of changes in resting membrane potential or of changes in the amplitude of induced excitatory postsynaptic currents (EPSCs), and were blocked by low concentrations of the gap junction blocker mefloquine, also in the absence of changes in resting membrane potential or in the amplitude of induced EPSCs.4 This suggests that these compounds do not act indirectly by affecting voltage-sensitive channels such as potassium channels, but rather modulate electrical coupling via gap junctions. Mefloquine can block potassium channels, but if that were the case, it would lead to an inward current, change the resting membrane potential, and increase spikelets, not decrease them. These findings in general suggest that increasing electrical coupling may promote states of synchronization of sleep–wake rhythms, thus controlling changes in state. The occurrence of coincident rhythmic IPSPs, especially during cholinergic receptor activation, suggests that a syncytium of inhibitory GABAergic neurons is present in the Pf, PPN and/or SubCD, and helps to generate synchronous oscillations. Thus suggesting a role for electrical coupling in sleep–wake control.
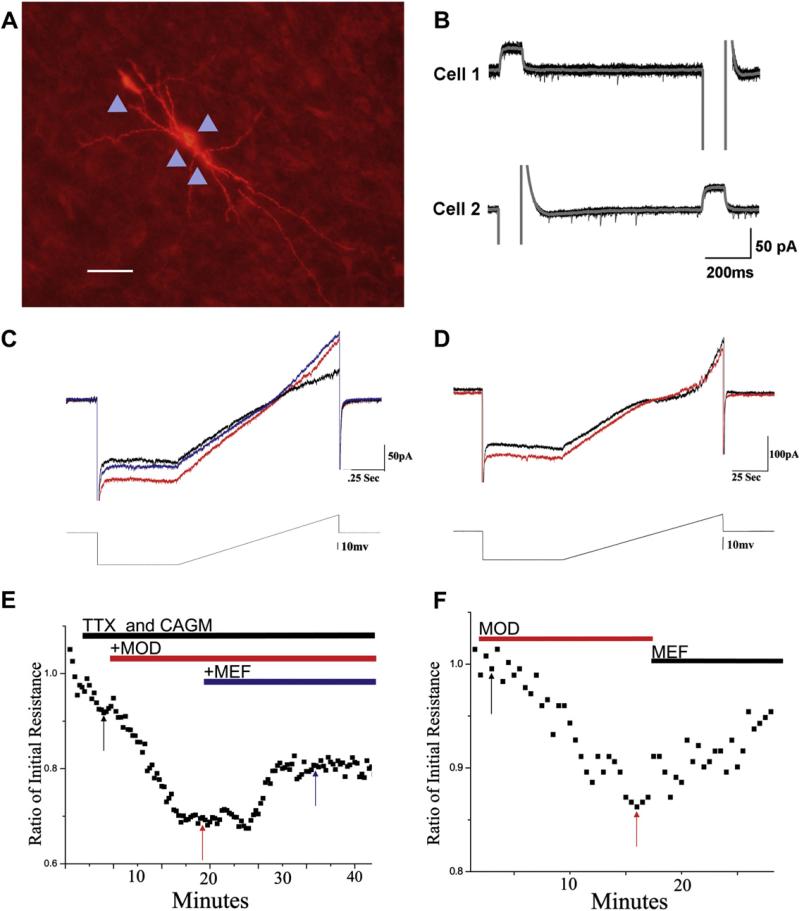
We should emphasize that gap junctions are not necessary for generating subthreshold oscillations, rather they are required for clustering coherent oscillatory activity.26 Oscillatory properties of single neurons endow the system with important reset dynamics, while gap junctions are mainly required for synchronized neural activity.26 For example, oscillations in single inferior olive neurons persisted over a limited range of voltages in the presence of gap junction blockers, leading one lab to conclude that gap junctions allow oscillations to persist over a wide range of membrane potentials making their frequency independent of membrane potential.26,27 This is in keeping with the suggestion that the output from a syncytium of electrically coupled cells may maintain oscillations over a wide range of voltages that would otherwise inactivate rhythmic conductances in single neurons.27 At present, we do not know if the electrically coupled cells in the RAS form a network or exist as clusters of cells. Fig. 1 summarizes the results reported on electrical coupling of RAS regions. Intracellular injection of neurobiotin in many cases led to dye coupling of 2–5 PPN neurons (Fig. 1A, see arrowheads), suggesting that electrically coupled neurons are found in clusters. Simultaneous recordings from two SubCD cells in the presence of tetrodotoxin (TTX – used to block action potential generation) and fast synaptic blockers (used to prevent synaptic input, thus ensuring that the response is only mediated by the cell being recorded) showed that intracellular current injection in one cell led to a current response in the other coupled cell and vice versa, suggesting that they were electrically coupled via gap junctions (Fig. 1B). The ratio of these current responses provides the coupling ratio, which was in the range of 2–3% for RAS neurons.2–4 While this is a small change in membrane potential, it may mean the difference between firing or not firing action potentials, and, in this case, firing them in unison.
Fig. 1.
Electrical coupling in the reticular activating system (RAS). A) Fluorescence labeling for neurobiotin injected into one pedunculopontine nucleus (PPN) cell that manifested spikelets. Multiple (four) other PPN cells were dye coupled by the injection (see arrowheads). B) Protocol for testing electrical coupling and coupling ratio. Under tetrodotoxin (TTX) and fast synaptic blockers, intracellular stimulation of one cell produced a response in the other, suggesting that these subcoeruleus nucleus dorsalis (SubCD) cells were coupled via gap junctions. The ratio of the responses was in the 2–3% range, although not identical in both directions. C) Shows the ramp protocol used to test membrane resistance of coupled neurons. The effects of modafinil (MOD) on a PPN neuron was a decrease in input resistance by the superfusion of MOD (300 μM) in the presence of fast synaptic blockers (CAGM: CNQX, APV, gabazine, and mecamylamine all at 10 μM). The cell was recorded under voltage-clamp mode. A ramp protocol was applied in order to test the change of membrane resistance under TTX and CAGM (black line), such that a higher current was required to compensate for the voltage change in the presence of MOD, indicating a decrease in resistance that could only occur if the neuron was electrically coupled (red line). The decrease in resistance with MOD that was partially blocked by the gap junction blocker mefloquine (MEF, 25 μM) (blue line). D) Shows a ramp protocol conducted on a SubCD neuron revealing a decrease in resistance by MOD (red line), that was partially reversed by MEF (25 μM) application (black line). E) Results from the recordings shown in C, in which testing of input resistance was carried out every 10 s (black squares) in the presence of TTX and CAGM (black arrow denotes black record shown in C). MOD decreased input resistance (red arrow denotes red record shown in C), while MEF partially blocked the effects of MOD and increased input resistance (blue arrow denotes blue record shown in C). F) Results from the recordings shown in D, in which testing of input resistance was carried out every 10 s (black squares) in the presence of TTX and CAGM (black arrow denotes black record shown in D). MOD decreased input resistance (red arrow denotes red record shown in D), while MEF partially blocked the effects of MOD and increased input resistance.
Fig. 1C shows the ramp protocol used to test the membrane resistance of two simultaneously recorded, coupled PPN neurons. In this case, higher current than that applied in the control condition was required in the presence of modafinil, an effect blocked by the gap junction blocker mefloquine. Fig. 1D shows the decrease in membrane resistance induced by modafinil on a subCD neuron was reversed by mefloquine. The graphs in Fig. 1E and F show the time course of changes in input resistance for the effects shown in Fig. 1C and D, respectively. Basically, modafinil led to decrease in input resistance that was mostly reversed by mefloquine.
Connexin 36
The amount of Cx36 protein in the Pf, PPN and SubCD was high early in development (~10 d) and decreased to adult levels by 30 d.2–4 Such a developmental decrease in Cx36 protein levels, with considerable amounts of Cx36 protein still present in the adult, suggest that this gap junction protein may participate in developmental regulation of circuitry and contribute to sleep–wake control in the adult. A study of Cx36 knockout (KO) mice showed that gap junctions in the inferior olive were mostly abolished, but the genetically uncoupled neurons still generated subthreshold oscillations of their membrane potential at a similar amplitude and frequency as those recorded in the wild-type.28 That is, single cell oscillations may occur in such Cx36 KO cells as a result of compensatory ionic mechanisms not encountered in the normal animal.28 Others showed instead that oscillatory properties of uncoupled inferior olive neurons are not caused by long-term compensatory changes but are primarily due to the single-cell properties of normal inferior olive cells.27 Thus gap junctions are required for synchronizing the oscillatory responses of neurons and for clustering of coherent rhythmic activity.
While gross motor activity patterns appeared normal in the Cx36 KO mouse, detailed analysis of motor patterns showed a 10- to 20-ms degradation in coordination, and a delay of more than 20 ms in the optokinetic reflex.29 These differences appear vital to survival. In terms of consciousness and sleep, cortical gamma band oscillations in vitro are impaired in Cx36 KO mice.30 These studies taken together suggest that gap junctions confer an advantage in timing, probably due to their ability to promote coherence in brain rhythms for optimal performance. The unique ability of coupled cells to maintain synchrony across a wide range of membrane potentials probably allows brain rhythms to persist for longer periods without waning.27 Recently, use-dependent plasticity, which has been extensively described for chemical synapses, was described for electrical synapses in the form of long-term depression.31 Therefore, modification of electrical synapses manifested by coupled neurons may be a powerful mechanism for reorganizing of electrically coupled networks. It is not yet known if prolonged use of modafinil leads to a reorganization of electrically coupled networks.
Gamma band activity
The EEG manifests low amplitude, high frequency activity at beta/gamma frequencies (~20–30/30–90 Hz) during waking and paradoxical sleep. Gamma oscillations are thought to participate in sensory perception, problem solving, and memory.32–34 Coherence at these frequencies occurs at cortical or thalamocortical levels.35,36 Gamma band activation in thalamocortical networks and in other neuronal groups (i.e., hippocampal and striatal afferents and efferents) is thought to contribute to the merger, or “binding”, of sensory information originating in separate regions.37 Conversely, deficits in gamma oscillations have been suggested as a pathophysiologic feature of diseases like schizophrenia and Alzheimer's disease.38–41
Gamma oscillations were first proposed to emerge from the dynamic interaction between intrinsic neuronal and synaptic properties of thalamocortical networks.37,38 The networks behind such activity include inhibitory cortical interneurons with intrinsic membrane potential oscillatory activity in the gamma range,35,38,42 many of which are electrically coupled,43 as well as of fast rhythmic bursting pyramidal neurons that are also electrically coupled.44 Thalamocortical excitatory neurons have intrinsic properties (i.e., membrane voltage-gated ion channels) needed to generate subthreshold gamma band membrane potential oscillations.45
While cortical interneurons can generate membrane potential gamma oscillations through the activation of voltage-dependent, persistent sodium channels,35 and metabotropic glutamate receptors,46 the mechanism responsible for gamma band activity in thalamocortical (TC) neurons involves high threshold P/Q- and N-type voltage-gated calcium channels located in the dendrites.45,46 Moreover, the same intrinsic properties mediating gamma band oscillations are present in the thalamus of several vertebrate species, indicating considerable evolutionary conservation.47
P/Q-type channels (also known as Cav2.1 channels) are present widely in the brain.48–50 N-type calcium channels are found in the rat auditory brainstem, are restricted to the early postnatal period, and are replaced by P/Q-type channels later in development.51 Immunocytochemical techniques have demonstrated the presence of N-type channels in brainstem structures.52 Importantly, P/Q-type mutant mice have deficient gamma band activity in the EEG, abnormal sleep–wake states, ataxia, are prone to seizures (low frequency synchrony), and die by 3 weeks of age.50 These channels appear essential to survival.
Gamma band in subcortical regions
Gamma band activity has been reported in regions other than the cortex. Hippocampal oscillatory activity in the gamma range (30–60 Hz) has been functionally associated with entorhinal cortex afferents.53 Entorhinal cortex neurons also oscillate at gamma band frequencies, suggesting a key role for such afferents in maintaining hippocampal gamma oscillations.54 Gamma band activity in the CA1 area has been divided into fast (>65 Hz) and slow (~25–60 Hz) frequency components that differentially couple CA1 and CA3 subfields, respectively.55 Such differences have been proposed to “bind” CA1 fast gamma oscillations with very high frequency activity from entorhinal cortex, which is in charge of providing information about object and place recognition in rodents. On the other hand, CA1 slow gamma oscillations would be locked to the slower frequencies present in the CA3 area in charge of memory storage.55,56
Cerebellar gamma band activity also has been described in the Purkinje cell layer around the apex of the lobule, and to a lesser extent in distal white matter.57,58 GABA-a receptors are critical for gamma oscillation generation in Purkinje cells.57 Cortico-cerebellar coherence at gamma frequencies is evident in monkeys during performance of a manual precision grip task, and cerebellothalamic activity is synchronized with neocortical activity at gamma frequencies. A cross-frequency phase synchronization between gamma band and lower frequencies can occur within the olivocerebellar system itself, in which olivary neurons that operate at the lower frequency bands could provide the underlying timing signal.57 Thus, Purkinje cells would resonate at higher frequencies to the beat of the olivary climbing fiber rhythm. Finally, it has been proposed that both cerebellar and thalamocortical networks might oscillate at the same frequencies to enable information exchange among these brain areas.58
Gamma in the RAS – the PPN
The PPN is most active during waking and paradoxical sleep, is part of the RAS that modulates ascending projections through the thalamus (modulating arousal) and descending projections through the pons and medulla (modulating posture and locomotion). The PPN is composed of different populations of cholinergic, glutamatergic and GABAergic neurons.59 Extracellular recordings of PPN neurons in vivo identified six categories of thalamic projecting PPN cells distinguished by their firing properties relative to pontogeniculo-occipital (PGO) wave generation.60 Some of these neurons had low rates of spontaneous firing (<10 Hz), but most had high rates of tonic firing in the beta/gamma range (20–80 Hz). That is, PPN neurons have been found to fire at gamma band frequency in alert animals. PPN neurons increase firing during rapid eye movement (REM) sleep (“REM-on”), or both waking and REM sleep (“Wake/REM-on”), but decrease during slow-wave sleep (SWS),60–62 suggestive of increased excitation only during activated states. Stimulation of the PPN potentiated the appearance of fast (20–40 Hz) oscillations in the cortical EEG, outlasting stimulation by 10–20 s.63 These findings suggest that PPN neurons are mainly active during states marked by high frequency activity such as waking and REM sleep.
A recent study found that all or most PPN cells fired maximally at gamma band frequency when depolarized using current steps.7 A later study showed that, in the presence of tetrodotoxin (TTX, to block action potential generation by blockade of sodium channels) and fast synaptic blockers (APV to block n-methyl-d-aspartic acid (NMDA) receptors, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to block AMPA/ kainic acid(KA) receptors, gabazine to block GABA receptors, and strychnine to block glycine receptors), the remaining oscillatory activity observed in PPN neurons during current clamp square pulse depolarization was due to activation of intrinsic membrane properties in the form of high threshold voltage-dependent calcium channels.8 Gamma band oscillations were visible between –25 mV and –5 mV somatic membrane voltage range, and were absent at membrane potentials below –30 mV orabove 0 mV, i.e., at the voltage levels of high threshold calcium channels.8 These results suggest that gamma band activity in PPN neurons may be due to channels that are activated at high membrane potentials such as high threshold, voltage-dependent calcium channels.
Using voltage clamp configuration, no clear oscillatory currents were observed at holding potentials below –30 mV. However, clear beta/gamma band oscillations were observed in the power spectra at both 20 –mV and –10 mV holding potentials. The highest gamma band power amplitudes were observed at a –10 mV holding potential.9 That is, the window for opening of these channels was in the –20 mV to –10 mV range. The amplitudes of the peaks in the power spectrum were reduced after series resistance compensation (40–60%; i.e., to reduce any space clamp problems during recordings), suggesting that PPN neuronal oscillatory activity in voltage clamp was generated in neuronal compartments distant from the soma, as previously shown for thalamic neurons.45 The distant nature of the channels explains why the membrane potential at the cell body has to be driven by the recording electrode to such high levels. Basically, the depolarization induced by the recording electrode at the cell body must reach high levels in order to depolarize the distant dendrites, which themselves may be close to physiological levels, such as –45 mV rather than –20 mV as recorded at the cell body. However, the identification of the exact kind of calcium channels mediating this effect in the PPN still remained to be established.
In PPN neurons, the specific N-type blocker ω-conotoxin-GVIA (ω-CgTX) only reduced gamma band oscillation amplitude and power spectrum, while the specific P/Q-type blocker ω-agatoxin-IVA (ω-Aga) totally abolished them.8 These and other results showed that both voltage-dependent N- and P/Q-type calcium channels mediated the depolarizing phase of gamma band oscillations in the PPN nucleus. However, only P/Q-type channels appeared to be essential for gamma oscillation generation. Moreover, voltage clamp results suggested that calcium channels might be located distally to the somata, mediating their effects in distant dendrites of PPN neurons,8 similar to thalamic neurons.45
The average oscillation frequency of PPN neurons (age 8–13 d) was in the beta range at 23 ± 1 Hz when perfused with synaptic blockers and TTX only. However, when the nonspecific cholinergic agonist carbachol (CAR) was added, the average frequency of gamma oscillations was significantly higher and in the gamma range (47 ± 2 Hz). These oscillations were blocked by application of ω-Aga. Interestingly, analysis of the power spectrum of oscillatory activity of all cells at different frequency ranges (from theta to gamma) showed that CAR significantly reduced the power amplitude at lower frequencies (i.e., theta, alpha, and beta bands), while there was no difference in average amplitude of oscillations at gamma band frequency.8 These data suggest that synaptic activation by cholinergic input can drive oscillations in PPN neurons to gamma band range while decreasing oscillations at lower frequencies.
These results a) confirmed the presence of gamma band activity previously reported in PPN neurons,7 and b) identified mechanisms underlying the generation of gamma band oscillations in the PPN.8 There is now little doubt that, despite the fact that there are three different types of PPN cells,59 all PPN neurons have the ability to manifest gamma band activity that is enhanced by CAR, such as that reported for CAR in cortical, thalamic, hippocampal, and cerebellar cells. However, the maximal frequency of firing of PPN cells, even under the influence of CAR, is in the gamma band range, but no higher.
Gamma in the RAS – the Pf
The Pf is a component of the intralaminar thalamus (ILT), which is traditionally considered a part of the “non-specific” TC system. Pf cells differ from typical “specific” TC neurons in morphology, electrophysiological properties and some synaptic connections. Pf neurons have long, sparsely branching processes in their proximal dendrites instead of compact bushy primary dendrites like TC relay cells.64 TC relay neurons are present throughout the “specific” and some “non-specific” thalamic nuclei, and are bushy, multidendritic cells with stereotypical intrinsic properties, i.e., bistable states of tonic vs bursting patterns of activity due to the ubiquitous incidence of low threshold spikes (LTS) mediated by T-currents.38 However, our previous electrophysiological studies demonstrated that “nonspecific” Pf cells exhibited reduced incidence of calcium-mediated LTS currents,64 compared to “specific” TC neurons.65 These findings suggest the possibility that Pf neurons may play a different role in the modulation of thalamocortical activity compared to “specific” TC neurons. In vivo, ILT neurons show high frequency spike bursts recurring rhythmically at ~40 Hz during wakefulness and REM sleep.66 These authors stated, “One of the most remarkable characteristics of these cells was the firing of high frequency bursts during natural states of wakefulness and REM sleep. At this time, this is the only known thalamic cell class exhibiting such behavior during brain-activated states”.66 These findings suggest that Pf cells also fire at high frequencies during waking and REM sleep in vivo.
As one of the targets of the cholinergic arm of the RAS, the ILT receives dense projections with symmetrical and asymmetrical terminals from the PPN and laterodorsal tegmental (LDT) nuclei,67 which participate in the modulation of cortical arousal, sleep–wake cycles and sensory awareness.37,63 In addition, Pf neurons are thought to be involved in maintaining the state of consciousness and selective attention in humans,68 and they also receive vagal input and participate in motor control, as well as pain modulation. That is, this region is strongly influenced by PPN output, and is critical to a number of higher processes.
Another recent study used steps of increasing current amplitudes in current clamp mode and showed that Pf firing frequency increased with increasing current steps but plateaued at gamma band frequencies, as in the PPN.9 Gamma band oscillations were generated by current ramps between –25 mV and –5 mV somatic membrane voltage range, and were absent at membrane potentials below –30 mV or above 0 mV. Power spectrum analysis of the membrane oscillations induced during ramp recordings revealed that 60% of the cells exhibited the highest peak of oscillatory activity at the gamma range, while some 38% of cells had the highest peak at beta frequency. However, those cells showing frequencies below gamma band were typically at earlier ages.9
These authors also showed that CAR could increase the firing frequency of younger cells to the gamma range, and their frequency would eventually plateau within the gamma range, but no higher. In the Pf, the P/Q-type channel blocker ω-Aga totally abolished gamma band oscillations. Interestingly, the N-type blocker ω-CgTX only reduced gamma band oscillation amplitude. These results showed that both voltage-dependent P/Q- and N-type calcium channels may mediate the depolarizing phase of gamma band oscillations in the Pf nucleus. However, only P/Q-type channels appeared to be essential, while N-type channels were only permissive, for gamma band oscillation generation. The specific calcium channel blockers had the same type of blocking effect on Pf neuron oscillatory activity regardless of age.9 In conclusion, the Pf is similar to the PPN in the mechanisms responsible for its gamma band activity, including the fact that all cells exhibit the same properties and are capped at gamma band frequencies.
Comparison of Pf and PPN with other regions
In such structures as the striatum and subthalamic nucleus, depolarizing current steps linearly increase firing frequency to >500 Hz and >250 Hz, respectively,69,70 but do not plateau. In monkey and rat prefrontal cortex, basket cells also do not plateau, almost linearly increasing firing frequency.71 On the other hand, pyramidal cells in mouse neocortex plateau below gamma band frequency, even after application of very high amplitude current steps (600–1000 pA).72 These results suggest that the capping of firing frequency of Pf and PPN cells is unusual compared to other brain regions. The implication is that the Pf and PPN are exquisitely suited to driving higher structures at gamma band frequencies, presumably during waking and REM sleep. But is the RAS the only region modulating waking and REM sleep? While much attention has been paid to hypothalamic neurons in the control of waking, De Lecea's lab has optogenetically engineered animals that have rhodopsin cation channels in orexin and in noradrenergic locus coeruleus (LC) neurons.73 Their findings showed that light activation of orexin neurons requires several seconds of stimulation with a latency ~20 s to induce waking, implying that its output must travel elsewhere before it awakens. When they light-activated LC cells, the animals awoke immediately, but if LC was inactivated, orexin neuron stimulation failed to awaken the animals. These results suggest that orexin neurons must first affect their descending RAS target, the LC, in order to manifest a waking effect in vivo. That is, the lateral hypothalamic system may act through the RAS to elicit arousal. Another region that has garnered considerable attention in the control of waking is the basal forebrain. However, early studies showed that precollicular transections in which the basal forebrain is anterior to the transection, eliminate fast activity related to waking and REM sleep,74 i.e., the basal forebrain by itself cannot drive the cortex to maintain gamma band activity in vivo. This was confirmed by studies in which transection at the level of the posterior edge of the inferior colliculus to the anterior hypothalamus, thus disconnecting the basal forebrain and cortex from the brainstem, induced profound coma.75 That is, the RAS appears to be the final common pathway for the high EEG frequency states of waking and REM sleep.
The results described above show that when Pf or PPN neurons are depolarized with increasing current steps, they may fire initially at higher frequency, but the firing frequency then plateaus at gamma frequency (30–60 Hz). This plateau in firing rate was observed in all Pf and PPN cells. The main difference between Pf and PPN was that PPN neurons did not show the high frequency instantaneous rate at the beginning of the current steps. That is, PPN neurons fired at ~50 Hz initially and then at ~30–40 Hz during the rest of the step, while Pf cells fired at ~80 Hz initially, then at ~50–60 Hz for the remainder of the step. Thus, Pf neurons may provide slightly differing initial signaling than PPN neurons, but both ensure the maintenance of gamma band activity when maximally activated. In addition, electrical and pharmacological activation of the Pf or PPN induced gamma band population responses. These results suggest that the Pf and PPN may generate gamma band activity (but no higher) when maximally activated, and perhaps impart gamma band activity on its targets. This newly discovered intrinsic membrane property of RAS neurons represents a novel mechanism for the induction of activated states such as waking and REM sleep.
Gamma in the RAS – the SubCD
REM sleep is distinguished from other states by low amplitude, high frequency EEG activity, muscle atonia, and PGO waves in cats (P waves in the rat). Nuclei located in the pons, including the SubCD, are critical for generation of this state.76–79 The SubCD is most active during REM sleep, and injection of the nonspecific cholinergic agonist CAR or the glutamate receptor agonist KA into this area induced a REM sleep like state with muscle atonia and PGO waves.80–82 Lesion of this area produced REM sleep without muscle atonia or P waves,83,84 or diminished REM sleep.76 The SubCD receives afferent input from several nuclei, including cholinergic, and perhaps glutamatergic, afferents from the PPN and LDT.85 The SubCD projects to other areas, including the thalamus, hippocampus, pons, and medulla.86
A recent study showed that some neurons in the SubCD fired at frequencies above gamma band (>100 Hz) at the beginning of a stimulus, but all neurons fired maximally (plateaued) at beta/gamma band following the initial portion of the current step.6 Voltage- and sodium channel-dependent subthreshold oscillations appear to be involved in generating this activity. Subthreshold oscillations were isolated using APV, CNQX, GBZ, and STR to block fast inhibitory and excitatory spontaneous synaptic activity. At membrane potentials below AP threshold (–48 mV), subthreshold oscillations were observed and persisted at membrane potentials above AP threshold, where they were evident between APs (–43 mV). Subthreshold oscillations were also observed following inactivation of sodium channels underlying APs (–40 mV), suggesting the existence of two populations of voltage-gated sodium channels, one related to AP generation and the other related to subthreshold oscillations.6
A sodium-dependent mechanism was revealed using TTX, an extracellular sodium channel blocker, and QX-314, an intracellular sodium channel blocker. Low concentrations of TTX (0.01 μM) completely blocked AP generation and reduced the power of gamma band oscillations but did not abolish subthreshold oscillations, while high concentrations of TTX (10 μM) completely blocked the remaining subthreshold gamma oscillations. QX-314 in the intracellular recording solution blocked both APs and subthreshold gamma oscillations. These results suggest that beta/gamma frequency, sodium-dependent subthreshold oscillations may underlie the gamma frequency AP firing of SubCD neurons.6
Previous studies in the SubCD reported the presence of gamma firing frequency in vivo and in vitro following depolarizing pulses.87,88 Extracellular recordings from cells in the PGO-wave generating site in cats recorded the presence of “PGO-on” cells, which increased their firing rates before the first PGO wave until the end of REM sleep, but had low firing rates during waking and slow wave sleep without PGO waves.87 These cells discharged high frequency spike bursts (>500 Hz) during PGO-related states and also fired tonically at 25–100 Hz.
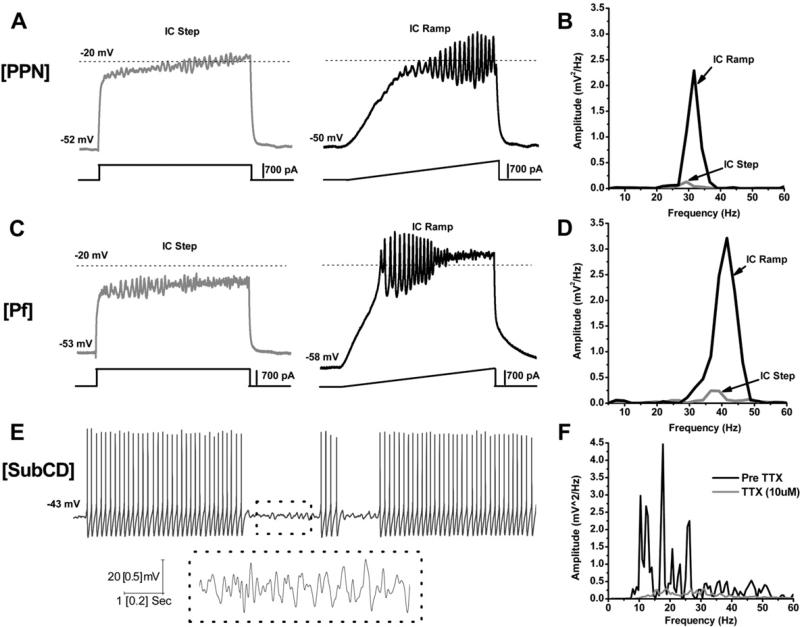
Fig. 2 summarizes the results reported for beta/gamma band activity generation in these three RAS nuclei. These results suggest that SubCD neurons do not significantly change the frequency of the sodium-dependent subthreshold oscillations early in development or after exposure to CAR. In fact, these frequencies are in the beta range and remain at these rates following CAR.6 Similarly, subthreshold oscillations in PPN neurons also appear to remain in the beta range. However, both PPN and Pf neurons exhibit high threshold calcium channel dependent oscillations that are P/Q- and N-type channel-dependent.8,9 The firing rates of PPN and Pf cells are in the beta range early (9–12 d, before major changes in REM sleep) and middle ages (13–16 d, at the start of the decrease in REM sleep) in development, but PPN and Pf cells increase in firing to the gamma range during the greatest developmental decrease in REM sleep, and are driven at similar rates by CAR, but still remain within the gamma range after exposure to CAR.
Fig. 2.
Gamma band activity in the reticular activating system (RAS). A) Representative membrane potential responses of the same pedunculopontine nucleus (PPN) neuron to depolarizing 1 s square step (gray record, left), and to 1 s long ramp (black record, right) obtained in the presence of fast synaptic blockers and tetrodotoxin (TTX). B) Overlapping curves comparing power spectrum amplitudes for oscillations obtained in A, pulses vs ramps. Note the higher amplitude of the oscillations obtained in the same neuron using ramps vs square steps. C) Representative membrane potential responses of the same parafascicular nucleus (Pf) neuron to depolarizing 1 s square step (gray record, left), and to 1 s long ramp (black record, right) obtained in the presence of fast synaptic blockers and TTX. D) Overlapping curves comparing power spectrum amplitudes for oscillations obtained in C, pulses vs ramps. Again, note the higher amplitude of the oscillations obtained using ramps vs square steps. E) Representative subthreshold oscillations at membrane potentials above action potential (AP) threshold (–43 mV). The dotted boxes include 1 s of recordings (upper records) that are also shown at higher resolution (lower records), revealing gamma frequency oscillations at –43 mV holding potential. F) Power spectrum confirming that the subthreshold oscillations were at beta/gamma frequencies, but no higher.
In summary, these results suggest that subthreshold oscillations in the RAS tend to cycle in the beta range, whether they are in PPN or SubCD neurons. This suggests that, since both nuclei are involved in the modulation of REM sleep, beta band will be the “preferred” frequency during that state. On the other hand, high threshold calcium channel-dependent oscillations in the RAS tend to cycle in the gamma range, whether they are in PPN or Pf neurons. This suggests that, since both nuclei are involved in the modulation of arousal and waking, gamma band will be the “preferred” frequency of that state, especially later in development and under the influence of cholinergic input.
Functional considerations
There is now little doubt that the RAS can generate beta/gamma band activity, and neurons appear “capped” to fire maximally at those frequencies. Because gamma waves can occur during slow wave states and anesthesia, a close relation to consciousness has been questioned.89 It was suggested that consciousness is associated with continuous gamma band activity, as opposed to an interrupted pattern of activity.90 The original description of the RAS specifically suggested that it participates in tonic or continuous arousal, and lesions of this region were found to eliminate tonic arousal.91 This raises the question of how a circuit can maintain such rapid, recurrent activation for long periods. Expecting a circuit of five or 10 synapses to reliably relay 20–60 Hz cycling without failing is unrealistic. Without the intrinsic properties afforded by rapidly oscillating channels, such as those described recently for PPN, Pf, and subthreshold oscillations in SubCD, as well as the presence of electrically coupled neurons that help maintain firing across different membrane potentials, beta/gamma band activity could not be maintained. The combination of channels capable of fast oscillations, and of circuitry that involves activating these channels, probably are both required for the maintenance of gamma band activity.6–9,35,38,45,47,50 The group of RAS nuclei as a whole, in which every cell in every nucleus exhibits beta/gamma band activity, and in which a subgroup of cells exhibit electrical coupling, then becomes a gamma-making machine. We speculate that it is the activation of the RAS during waking and REM sleep that induces coherent activity (through electrically coupled cells) and high frequency oscillations (through P/Q-type calcium channel and subthreshold oscillation activity), and lead to the maintenance of the background of gamma activity necessary to support a state capable of reliably assessing the world around us on a continuous basis. That is, these mechanisms may underlie preconscious awareness.
Preconscious awareness
People often act in order to meet desired goals, and feel that conscious will is the cause of their behavior. Scientific research suggests otherwise. Under some conditions, actions are initiated even though we are unconscious of the goal. Libet et al. were the first to show that when people consciously set a goal to engage in a behavior, their conscious will to act begins unconsciously.92 Libet et al. studied the readiness potential (RP), a negative DC shift present long before the execution of a voluntary movement. Their subjects were asked to move voluntarily, and were also asked to subjectively time the will to move as well as the onset of movement. The initial and later phases of the RP preceded the consciously determined will to move by hundreds of milliseconds. The authors concluded that cerebral initiation of spontaneous, freely voluntary acts can begin unconsciously, before there is any subjective awareness that a decision to act was initiated cerebrally. Even simple movements appear to be generated subconsciously, and the conscious sense of volition comes later.93 This review described the details of studies showing that voluntary movements can be triggered with stimuli that are not perceived, that movement may well occur prior to the apparent planning of the movement, and that not only the sense of willing the movement, but also the sense of the movement having occurred, happens before the actual movement.92 Even the pursuit of complex goals operates outside of conscious awareness, actions are initiated even though we are unconscious of complex goals to be attained or their motivating effects on behavior. This is even more significant considering the results of studies on patients who appeared to be minimally conscious and were asked to perform two imagery tasks.94 Different patterns of hemodynamic responses using fMRI were detected with each task, and some of the patients were able to elicit “yes” or “no” responses by generating either imagery signal. These studies suggest that at least some minimally conscious individuals who are unable to elicit an overt behavioral response can still use mental imagery to communicate complex ideas.94
So how does preconscious awareness arise? An attractive model of conscious perception is based on the presence of gamma band activity in thalamocortical regions. During activated states (waking and paradoxical sleep), EEG responses are characterized by low amplitude, high frequency oscillatory activity in the gamma band range (~30–90 Hz). Gamma frequency oscillations have been proposed to participate in sensory perception, problem solving, and memory, and it has been suggested that such coherent events occur at cortical and/or thalamocortical levels. The mechanisms behind such activity, as described above, include the presence of inhibitory GABAergic cortical interneurons that exhibit intrinsic oscillatory activity in the gamma band frequency, many of which are electrically coupled, as well as of fast rhythmic bursting pyramidal neurons that are also electrically coupled. That is, the rhythm is generated initially and maintained, not by the circuit alone, but by the combination of a specific circuit, electrical coupling-induced coherence, and the high frequency intrinsic membrane properties of cortical neurons.
Cognition has been proposed to arise from “specific” thalamocortical projections that carry the content of conscious experience, interacting with “non-specific” thalamocortical projections that carry the context of conscious events.95 The cortical sites peaking at gamma band frequency via “specific” thalamocortical projections are thought to reverberate and summate with coincident “non-specific” gamma band activity.37,96 This summation, along with the coherence provided by electrical coupling, is proposed to provide global binding. Binding is the mechanism whereby the different aspects of a sensation, say, color, motion, shape, all different aspects of sensation, are combined into a unified perceptual experience. Disturbance in this mechanism results in “thalamocortical dysrhythmia (TCD)”, and is thought to be involved in a number of neurological and psychiatric disorders.95
Is there a mechanism, perhaps based on intrinsic oscillatory activity, that generates a similar process but at subcortical levels? As we saw above, gamma band activity has been reported in the hippocampus and cerebellum. Now, three nuclei of the RAS exhibit electrical coupling, providing a novel mechanism for sleep–wake control based on coherence driven by electrical coupling.2 Moreover, virtually every neuron in these nuclei, regardless of cell or transmitter type, exhibited gamma band activity generated by intrinsic membrane properties. Regardless of depolarizing level or input, these cells are “capped” to fire at gamma band frequency (30–60 Hz).6–9 This is a very unique property. Taken together, these results suggest that a similar mechanism to that in the cortex for achieving temporal coherence at high frequencies is present in the PPN, and perhaps its subcortical targets such as the Pf and SubCD nuclei. We suggested that gamma band activity and electrical coupling generated in the PPN may help stabilize coherence related to arousal, providing a stable activation state during waking and paradoxical sleep.7–9 Our overall hypothesis here is that sensory input will induce gamma band activity in the RAS that could participate in preconscious awareness. The RAS seems the ideal site for preconscious awareness since it is phylogenetically conserved, modulates sleep/wake cycles, the startle response, and fight-vs-flight responses that include changes in muscle tone and locomotion.
Concluding remarks
William James proposed that the “stream of consciousness” is “a river flowing forever through a man's conscious waking hours”.97 Wilder Penfield developed the idea of a “centrencephalic integrating system” that fulfills the role of sensory-motor integration essential for consciousness.98 He arrived at a subcortical mechanism for consciousness based on the fact that “there is no place in the cerebral cortex where electrical stimulation will cause a patient to believe or to decide”. He also emphasized that, while localized cortical seizures elicit sensory or motor effects but maintain consciousness, petit mal seizures in mesothalamic regions always eliminate consciousness.98 But, what happens when we awaken?
It has been shown that increases in blood flow in the thalamus and brainstem begin within 5 min of waking, but as much as 15 min elapse before significant cortical changes in blood flow are observed.99 That is, upon waking, significant increases in the brainstem and thalamus precede restoration of blood flow to the frontal lobes.99 The important role of subcortical structures in the determination of states of awareness is gaining attention. In his recent book, Damasio proposed that the brainstem is critical to the formulation of the self, which is critical to the formulation of feelings.100 The fact that we awaken as ourselves, despite low levels of frontal cortical blood flow, supports the view that subcortical structures are essential to this process.
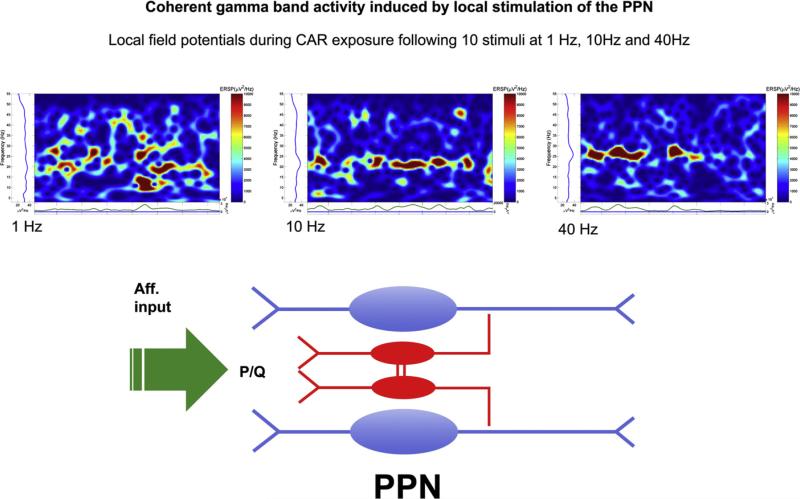
Fig. 3 summarizes local field potential recordings in the PPN in the presence of carbachol (CAR, 50 μM) to activate the slice. Stimulation at 1 Hz vs 10 Hz vs 40 Hz induced increasingly coherent activation until peaks of activation were separated by gaps in activity. Such a pattern would be induced by a system in which output neurons are activated to produce gamma band activity (blue cells) that includes inhibitory interneurons (red coupled cells) that provide coherent recurrent inhibition, i.e., gaps. A similar effect was shown to occur using voltage-sensitive dye recordings in the cortex, in which stimulation at 40 Hz led to more narrowly focused activation than lower frequency stimulation.101 Much additional investigation will be needed to substantiate this speculation, which is proposed only as a starting point for discussion of the nature of this potential process. From a pathological point of view, a similar process to TCD could occur at subcortical levels, in which the timing of brainstem–thalamus oscillations would be disturbed. The deficits produced by such dysregulation could be severe, perhaps resulting in the release of automatic behaviors such as fixed action patterns and tics, in addition to arousal and sensory gating deficits. That is, the deficits could arise from a lack of cortical regulation of these automatisms, resulting in involuntary movements, unregulated arousal, and exaggerated fight-vs-flight responses. We are only beginning to explore the subcortical involvement of preconscious awareness in a number of disorders.
Fig. 3.
Coherent activation of the pedunculopontine nucleus (PPN). Upper panel. Local stimulation of the PPN was applied as 10 pulses at 1 Hz, 10 Hz or 40 Hz, in the presence of carbachol (CAR, 50 μM). The poststimulus event related spectral perturbation (ERSP) of the local field potential recorded in the PPN was calculated. Note that increasing frequency of stimulation induced greater and greater coherence in the response. An ERSP represents a measure of event related brain dynamics induced in the EEG or local field potential spectrum by a stimulus or event. It basically measures average dynamic changes in amplitude of the broad band frequency spectrum as a function of time relative to an experimental event. These analyses use MatLab to generate power spectra for continuous points in time, e.g., during and after stimulation or application of an agent or washout. These graphs plot frequency of activity over time, and the amplitude of the frequency shown is color-coded such that background (control) appears blue, and higher amplitudes appear progressively more yellow, then red. A convenient way of reading these graphs is as a running power spectrum over time.6,7 Lower panel. Hypothesized organization of response to afferent input (green arrow) impinging on PPN dendrites bearing P/Q-type high threshold calcium channels. GABAergic neurons are shown in red and as electrically coupled. Cholinergic and glutamatergic output neurons are shown in blue. Higher frequency stimulation induced coherent peaks of activation that were separated by recurrent gaps. This pattern would be expected following activation of a system which activates output neurons (blue) as well as local inhibitory, electrically coupled neurons (red).
Practice points.
The RAS includes cells that manifest:
Electrical coupling via gap junctions, allowing cell groups to fire with increased coherence;
Electrical coupling that is increased by the stimulant modafinil, leading to decreased GABA release and disinhibition of other transmitter systems;
Electrical coupling that allows cells to maintain synchrony across a wide range of membrane potentials, allowing brain rhythms to persist for longer periods;
Gamma band activity via P/Q-type high threshold voltage-dependent calcium channels or sodium-dependent subthreshold oscillations;
All of the mechanisms for generating and maintaining its own gamma band activity;
Gamma band activity that is proposed to participate in the establishment of preconscious awareness.
Research agenda.
In the future we need to explore:
The design of novel stimulants and anesthetics based on the modulation of gap junctions;
Novel stimulants and anesthetics based on the modulation of gamma band activity in the RAS;
The process of preconscious awareness experimentally; and
Determine the role of this process in disorders of arousal and perception, including schizophrenia, anxiety disorder and depression.
Acknowledgments
This work was supported by R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by P20 GM104325. In addition, Dr. Urbano is a fellow of the John Simon Guggenheim Memorial Foundation (http://www.gf.org/fellows/17153-francisco-urbano) and was supported by FONCyT, Agencia Nacional de Promoción Científica y Tecnológica (http://www.ifibyne.fcen.uba.ar/new/): BID 1728 OC.AR. PICT 2007-1009, PICT 2008-2019 and PIDRI-PRH 2007.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AHP
afterhyperpolarization
- AMPA
2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid
- AP
action potential
- APV
2R-amino-5-phosphonovaleric acid
- APV-DL
2-amino-5-phosphonopentanoic acid
- CA1
cornu ammonis 1
- CA3
cornu ammonis 3
- CAGM
CNQX, APV, gabazine, and mecamylamine
- cAMP
cyclic adenosine monophosphate
- CAR
carbachol
- CL
centrolateral nucleus
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CNS
central nervous system
- Cx
36 connexin 36
- EEG
electroencephalogram
- EPSCs
excitatory postsynaptic currents
- fMRI
functional magnetic resonance imaging
- GABA
gamma amino-butyric acid
- GBZ
gabazine
- GLUT
glutamate
- IA
hyperpolarization activated current
- Ik
delayed rectifier-like potassium current
- ILT
intralaminar thalamus
- IPSPs
inhibitory postsynaptic potentials
- KA
kainic acid
- KO
knockout
- LC
locus coeruleus
- LDT
laterodorsal tegmental nucleus
- LTS
low threshold spikes
- MEF
mefloquine
- MOD
modafinil
- NMDA
n-methyl-d-aspartic acid
- PD
Parkinson's disease
- Pf
parafascicular nucleus
- PGO
ponto-geniculo-occipital
- PPN
pedunculopontine nucleus
- RAS
reticular activating system
- REM
rapid eye movement
- STR
strychnine
- SubCD
subcoeruleus nucleus dorsalis
- SWS
slow-wave sleep
- TC
thalamocortical
- TCD
thalamocortical dysrhythmia
- TRN
thalamic relay neurons
- TTX
tetrodotoxin
- ω-Aga
ω-agatoxin-IVA
- ω-CgTX
ω-conotoxin-GVIA
Footnotes
Conflict of interest
None of the authors have a conflict of interest.
References
* The most important references are denoted by an asterisk.
- 1.Thiagarajan TC, Lebedev MA, Nicolelis MA, Plenz D. Coherence potentials: loss-less, all-or-none network events in the cortex. PLoS Biol. 2010;8:e1000278. doi: 10.1371/journal.pbio.1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–14. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008;31:673–90. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heister DS, Hayar A, Charlesworth A, Yates C, Zhou Y, Garcia-Rill E. Evidence for electrical coupling in the subcoeruleus (SubC) nucleus. J Neurophysiol. 2007;97:3142–314. doi: 10.1152/jn.01316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Urbano FJ, Leznik E, Llinas R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci U S A. 2007;104:12554–9. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Simon C, Kezunovic N, Williams DK, Urbano FJ, Garcia-Rill E. Cholinergic and glutamatergic agonists induce gamma frequency activity in dorsal subcoeruleus nucleus neurons. Am J Physiol Cell Physiol. 2011;301:C327–35. doi: 10.1152/ajpcell.00093.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Simon C, Kezunovic N, Ye M, Hyde J, et al. Gamma band unit and population responses in the pedunculopontine nucleus. J Neurophysiol. 2010;104:463–74. doi: 10.1152/jn.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Kezunovic N, Urbano FJ, Simon C, Hyde J, Hayar A, Williams DK, et al. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN). Eur J Neurosci. 2011;34:404–15. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Kezunovic N, Hyde JR, Simon C, Urbano FJ, Garcia-Rill E. Gamma band activity in the developing parafascicular nucleus (Pf). J Neurophysiol. 2011;107:772–84. doi: 10.1152/jn.00677.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 11.Long MA, Landisman CE, Connors BW. Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J Neurosci. 2004;24:341–9. doi: 10.1523/JNEUROSCI.3358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuentealba P, Steriade M. The reticular nucleus revisited: Intrinsic network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–41. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Liu XB, Jones EG. Fine structural localization of connexin-36 immunore-activity in mouse cerebral cortex and thalamus. J Comp Neurol. 2003;466:457–67. doi: 10.1002/cne.10901. [DOI] [PubMed] [Google Scholar]
- 14.Hughes SW, Blethyn KL, Cope DW, Crunelli V. Properties and origin of spikelets in thalamocortical neurons in vitro. Neuroscience. 2002;3:395–401. doi: 10.1016/s0306-4522(01)00577-2. [DOI] [PubMed] [Google Scholar]
- 15.Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 2005;28:304–9. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FE, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci. 2001;21:9476–86. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–10. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- 18.Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap junctional intercellular communication. Biochem Soc Trans. 2001;29:606–12. doi: 10.1042/bst0290606. [DOI] [PubMed] [Google Scholar]
- 19.He DS, Burt JM. Mechanism and selectivity of the effects of halothane on gap junction channel function. Circ Res. 2000;86:1–10. doi: 10.1161/01.res.86.11.e104. [DOI] [PubMed] [Google Scholar]
- 20.Boer DL, Henriksen SJ, Cravatt BF. Oleamide: an endogenous sleep-inducing lipid and prototypical member of a new class of biological signaling molecules. Curr Pharm Des. 1998;4:303–14. [PubMed] [Google Scholar]
- 21.Murillo-Rodriguez E, Blanco-Centurion C, Sanchez C, Piomelli D, Shiromani PJ. Anandamide enhances extracellular levels of adenosine and induces sleep: an in vivo microdialysis study. Sleep. 2003;26:943–7. doi: 10.1093/sleep/26.8.943. [DOI] [PubMed] [Google Scholar]
- 22.Gigout S, Louvel J, Kawasaki H, D'Antuono M, Armand V, Kurcewicz I, et al. Effects of gap junction blockers on human neocortical synchronization. Neurobiol Dis. 2006;22:496–508. doi: 10.1016/j.nbd.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Rozental R, Srinivas M, Spray DC. How to close a gap junction channel. Methods Mol Biol. 2001;154:447–77. doi: 10.1385/1-59259-043-8:447. [DOI] [PubMed] [Google Scholar]
- 24.Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–66. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 25.Sutor B, Hablitz JJ, Rucker F, ten Bruggencate G. Spread of epileptiform activity in the immature rat neocortex studied with voltage-sensitive dyes and laser scanning microscopy. J Neurophysiol. 1994;72:1756–68. doi: 10.1152/jn.1994.72.4.1756. [DOI] [PubMed] [Google Scholar]
- 26.Leznik E, Llinas R. Role of gap junctions in synchronized oscillations in the inferior olive. J Neurophysiol. 2005;94:2447–56. doi: 10.1152/jn.00353.2005. [DOI] [PubMed] [Google Scholar]
- 27.Llinas R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurons and their pharmacological modulation: an in vitro study. J Physiol. 1986;376:163–82. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Zeeuw CI, Chorev E, Devor A, Van Der Giessen RS, De Jeu MT, Hoongenraad CC. Deformation of network connectivity in the inferior olive of connexin 36-deficient mice is compensated by morphological and electrophysiological changes at the single neuron level. J Neurosci. 2003;23:4700–11. doi: 10.1523/JNEUROSCI.23-11-04700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Placatonakis DG, Bukovsky AA, Zeng XH, Kiem HP, Welsh JP. Fundamental role of inferior olive connexin 36 in muscle coherence during tremor. Proc Natl Acad Sci U S A. 2004;101:7164–9. doi: 10.1073/pnas.0400322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin 36. Neuron. 2001;31:477–85. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 31.Haas JS, Zavala B, Landisman CR. Activity-dependent long-term depression of electrical synapses. Science. 2011;21:389–93. doi: 10.1126/science.1207502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, et al. Coherent oscillations: a mechanism of feature linking in the visual system? Biol Cybern. 1988;60:121–30. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 33.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Nat Acad Sci U S A. 1989;86:1698–702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philips S, Takeda Y. Greater frontal–parietal synchrony at low gamma-band frequencies for inefficient then efficient visual search in human EEG. Int J Psychophysiol. 2009;73:350–4. doi: 10.1016/j.ijpsycho.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Llinas RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Nat Acad Sci U S A. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–74. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- 37.Llinás RR, Paré D. Of dreaming and wakefulness. Neuroscience. 1991;44:521–35. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 38.Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 39.Ribary U, Ioannides AA, Singh KD, Hasson R, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci U S A. 1991;88:11037–41. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stam CJ, van Cappellen A, van Walsum AM, Pijnenburg YA, Berendse HW, de Munck JC, et al. Generalized synchronization of MEG recordings in Alzheimer's disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19:562–74. doi: 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;8:173–89. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steriade M. Handbook of behavioral state control. Cellular and molecular mechanisms. CRC Press; Florida: 1999. Cellular substrates of oscillations in corticothalamic systems during states of vigilance. pp. 327–347. [Google Scholar]
- 43.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–9. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 44.Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FE, Vogt A, et al. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc Nat Acad Sci U S A. 2004;101:7152–7. doi: 10.1073/pnas.0402060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Pedroarena C, Llinás RR. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc Natl Acad Sci U S A. 1997;94:724–8. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in inter-neuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–5. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 47.Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 48.Jones EG. Calcium channels in higher-level brain function. Proc Nat Acad Sci U S A. 2007;14:17903–4. doi: 10.1073/pnas.0709509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hillman D, Chen S, Aung TT, Cherksey B, Sugimori M, Llinas RR. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci U S A. 1991;88:7076–80. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llinas RR, Choi S, Urbano FJ, Shin H-S. Gamma band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Natl Acad Sci U S A. 2007;104:17819–24. doi: 10.1073/pnas.0707945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Caterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992;9:1099–115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- 52.Shen W, Hernandez-Lopes S, Tkatch T, Held E, Surmeier DJ. Kv1.2-contaiing K+ channels regulate subthreshold excitability of striatal medium spiny neurons. J Neurophysiol. 2004;91:1337–49. doi: 10.1152/jn.00414.2003. [DOI] [PubMed] [Google Scholar]
- 53.Charpak S, Paré D, Llinás RR. The entorhinal cortex entrains fast CA1 hippocampal oscillations in the anaesthetized guinea-pig: role of the monosynaptic component of the perforant path. Eur J Neurosci. 1995;7:1548–57. doi: 10.1111/j.1460-9568.1995.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 54.Chrobak JJ, Buzsáki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18:388–98. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colgin L, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–7. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 56.Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology. 2010;25:319–29. doi: 10.1152/physiol.00021.2010. [DOI] [PubMed] [Google Scholar]
- 57.Lang EJ, Sugihara I, Llinás RR. Olivocerebellar modulation of motor cortex ability to generate vibrissal movements in rat. J Physiol (Lond) 2006;571:101–20. doi: 10.1113/jphysiol.2005.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Middleton SJ, Racca C, Cunningham MO, Traub RD, Monyer H, Knopfel T. High-frequency network oscillations in cerebellar cortex. Neuron. 2008;58:763–74. doi: 10.1016/j.neuron.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–58. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steriade M, Paré D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560–79. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakai K, El Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–23. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- 62.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:79–82. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 63.Steriade M, Curro Dossi R, Paré D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Nat Acad Sci U S A. 1991;88:4396–400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phelan KD, Mahler HR, Deere T, Cross CB, Good C, Garcia-Rill E. Postnatal maturational properties of rat parafascicular thalamic neurons recorded in vitro. Thalamus Rel Syst. 2005;1:1–25. doi: 10.1017/S1472928805000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–8. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- 66.Steriade M, Curro-Dossi R, Contreras D. Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (~40 Hz) spike-bursts at ~1000 Hz during waking and rapid eye movement sleep. Neuroscience. 1993;56:1–9. doi: 10.1016/0306-4522(93)90556-u. [DOI] [PubMed] [Google Scholar]
- 67.Erro E, Lanciego JL, Gimenez-Amaya JM. Relationships between thalamostriatal neurons and pedunculopontine projections to the thalamus: a neuroanatomical tract-tracing study in the rat. Exp Brain Res. 1999;127:162–70. doi: 10.1007/s002210050786. [DOI] [PubMed] [Google Scholar]
- 68.Raeva SN. The role of the parafascicular complex (CM-Pf) of the human thalamus in the neuronal mechanisms of selective attention. Neurosci Behav Physiol. 2006;36:287–95. doi: 10.1007/s11055-006-0015-y. [DOI] [PubMed] [Google Scholar]
- 69.Azouz R, Gray CM, Nowak LG, McCormick DA. Physiological properties of inhibitory interneurons in cat striate cortex. Cereb Cortex. 1997;7:534–45. doi: 10.1093/cercor/7.6.534. [DOI] [PubMed] [Google Scholar]
- 70.Barraza D, Kita H, Wilson CJ. Slow spike frequency adaptation in neurons of the rat subthalamic nucleus. J Neurophysiol. 2009;102:3689–97. doi: 10.1152/jn.00759.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Povysheva NV, Zaitsev AV, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS. Parvalbumin-positive basket interneurons in monkey and rat prefrontal cortex. J Neurophysiol. 2008;100:2348–60. doi: 10.1152/jn.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou L, Gall D, Qu Y, Prigogine C, Cheron G, Tissir F, et al. Maturation of “neocortex isole” in vivo in mice. J Neurosci. 2010;30:7928–39. doi: 10.1523/JNEUROSCI.6005-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moruzzi G, Magoun HW. Brainstem reticular formation and activation. Electroenceph Clin Neurophysiol. 1949;1:455–73. [PubMed] [Google Scholar]
- 75.Steriade M, Constantinescu E, Apostol V. Correlation between alterations of the cortical transaminase activity and EEG patterns of sleep and wakefulness induced by brainstem transections. Brain Res. 1969;13:177–80. doi: 10.1016/0006-8993(69)90152-8. [DOI] [PubMed] [Google Scholar]
- 76.Mouret J, Delorme F, Jouvet M. Lesions of the pontine tegmentum and sleep in rats. CR Seances Soc Biol Fil. 1967;161:1603–6. [PubMed] [Google Scholar]
- 77.Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA. A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: spontaneous and drug-induced neuronal activity. Neuroscience. 1990;39:295–304. doi: 10.1016/0306-4522(90)90268-9. [DOI] [PubMed] [Google Scholar]
- 78.Marks GA, Farber J, Roffwarg HP. Metencephalic localization of pontogeniculo occipital waves in the albino rat. Exp Neurol. 1980;69:667–77. doi: 10.1016/0014-4886(80)90059-x. [DOI] [PubMed] [Google Scholar]
- 79.Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306:39–52. doi: 10.1016/0006-8993(84)90354-8. [DOI] [PubMed] [Google Scholar]
- 80.Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30:409–23. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 81.Mitler MM, Dement WC. Cataplectic-like behavior in cats after microinjections of carbachol in pontine reticular formation. Brain Res. 1974;68:335–43. doi: 10.1016/0006-8993(74)90402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat pontomedullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–73. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 83.Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch Ital Biol. 1989;127:133–64. [PubMed] [Google Scholar]
- 84.Sanford LD, Morrison AR, Mann GL, Harris JS, Yoo L, Ross RJ. Sleep patterning and behaviour in cats with pontine lesions creating REM without atonia. J Sleep Res. 1994;3:233–40. doi: 10.1111/j.1365-2869.1994.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 85.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 86.Shiromani PJ, Armstrong DM, Gillin JC. Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltransferase immunohistochemical study. Neurosci Lett. 1998;95:19–23. doi: 10.1016/0304-3940(88)90625-8. [DOI] [PubMed] [Google Scholar]
- 87.Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2004;24:1416–27. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Datta S, Hobson JA. Neuronal activity in the caudolateral peribrachial pons: relationship to PGO waves and rapid eye movements. J Neurophysiol. 1994;71:95–109. doi: 10.1152/jn.1994.71.1.95. [DOI] [PubMed] [Google Scholar]
- 89.Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electro-physiological characterization of neurons in the dorsolateral pontine rapid eye-movement sleep induction zone of the rat: intrinsic membrane properties and responses to carbachol and orexins. Neuroscience. 2006;143:739–55. doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vanderwolf CH. What is the significance of gamma wave activity in the pyriform cortex? Brain Res. 2000;877:125–33. doi: 10.1016/s0006-8993(00)02568-3. [DOI] [PubMed] [Google Scholar]
- 91.Moruzzi G, Magoun HW. Brainstem reticular formation and activation of the EEG. Electroenceph Clin Neurophysiol. 1949;1:455–73. [PubMed] [Google Scholar]
- 92*.Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106:623–42. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- 93*.Hallett M. Volitional control of movement: the physiology of free will. Clin Neurophysiol. 2007;118:1179–92. doi: 10.1016/j.clinph.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cruse D, Chennu S, Chatelle C, Beckinstein TA, Fernandez-Espejo D, Pickard JD. Bedside detection of awareness in the vegetative state: a cohort study. Lancet. 2011;378:2088–94. doi: 10.1016/S0140-6736(11)61224-5. [DOI] [PubMed] [Google Scholar]
- 95*.Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Nat Acad Sci U S A. 1999;96:15222–7. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci U S A. 2002;99:449–54. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.James W. The principles of psychology. Originally published in 1890. Cosimo Classics; New York: 2007. [Google Scholar]
- 98.Penfield W. The mystery of the mind. University Press; Princeton, New Jersey: 1975. [Google Scholar]
- 99.Balkin TJ, Braun AR, Wesensten NJ, Jeffries K, Varga M, Baldwin P, et al. The process of awakening: a PET study of regional brain activity patterns mediating the re-establishment of alertness and consciousness. Brain. 2002;125:2308–19. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- 100.Damasio A. Self comes to mind: constructing the conscious brain. Pantheon Books; New York: 2010. [Google Scholar]
- 101.Contreras D, Llinas R. Voltage-sensitive dye imaging of neocortical spatiotemporal dynamics to afferent activation frequency. J Neurosci. 2001;21:9403–13. doi: 10.1523/JNEUROSCI.21-23-09403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]