Thrombospondins (TSPs) belong to a family of five, secreted, modular glycoproteins encoded by separate genes (Adams and Lawler, 2004, 2011). Group A TSPs (TSP1 and TSP2) are homotrimers, whereas group B TSPs (TSP3, -4, and -5/COMP) are homopentamers (Adams, 2001), and all are secreted as disulfide-bonded complexes. Although the five members of the TSP family differ with respect to structure, cell type, and temporal expression, all TSPs bind to components of the extracellular matrix as well as to a number of cell surface receptors, enabling TSPs to modulate cellular behaviors in a wide array of tissue contexts. TSPs are the prototype of the matricellular protein (Bornstein, 1995) and have been the subject of intense study for four decades.
First identified in 1971 as protein released from thrombin-stimulated platelets, TSP1 (thrombin sensitive protein) was initially studied for its role in platelet aggregation and related hemostatic functions (Baenziger et al., 1971; Gartner and Dockter, 1984; Lahav et al., 1982; Lawler et al., 1978; Phillips et al., 1980). The eponym thrombospondin was proposed by Lawler and co-workers (Lawler et al., 1978) who isolated intact thrombospondin complexes from thrombin-treated platelets in physiological saline. Thrombospondin is a filamentous glycoprotein ~70 nm long and binds to heparin-affinity columns, and thus, to heparan sulfate proteoglycans in vivo (Fig. 1). Over the next two decades, it was discovered that TSP1 is synthesized and secreted by endothelial cells as well as other cell types in culture (Asch et al., 1986; Jaffe et al., 1982; Jaffe et al., 1985; Jaffe et al., 1983; McPherson et al., 1981; Mosher et al., 1982; Raugi and Lovett, 1987). The expression of TSP1 by diverse cell types, identification of interacting proteins, and the subsequent recognition that TSP1 binds to specific cell surface molecules in a saturable manner (Asch et al., 1987; McKeown-Longo et al., 1984; Murphy-Ullrich and Mosher, 1987; Roberts et al., 1985; Silverstein et al., 1984) provided the first evidence that TSPs might regulate cell behavior through direct interactions with cell surface receptors to activate intracellular signaling pathways: these first known receptors turned out to be CD36 (GP88) and heparan sulfate proteolgycans (syndecans) and established precedents for identification of other TSP receptors (Asch et al., 1987; Asch et al., 1992; Chung et al., 1999; Godyna et al., 1995; Goicoechea et al., 2000; Lawler and Hynes, 1989; Lawler et al., 1988; Murphy-Ullrich et al., 1988; Sun et al., 1989; Tuszynski et al., 1993; Wang and Frazier, 1998). Subsequent work showed that TSP1 expression is regulated by growth factors (PDGF, TGF-β), that TSP1 can enhance cellular responses to growth factors such as EGF, antagonize VEGF signaling, or variably regulate FGF family signaling (Gupta et al., 1999; Iruela-Arispe et al., 1999; Majack et al., 1985, 1986; Orr et al., 2003; Penttinen et al., 1988; Taraboletti et al., 1992). Elegant biochemistry, protein structural studies, and cloning and sequencing of the TSP1 gene further elucidated relationships between discrete modular domains of TSPs and specific cellular functions as well as regulators of gene expression (Bornstein et al., 1990; Dixit et al., 1986; Frazier et al., 1987; Lawler, 1986; Slane et al., 1988; Sun and Mosher, 1991). Together these early findings stimulated many diverse and fruitful avenues of investigation which have yielded surprising insights into the diverse functions of this remarkable family of proteins.
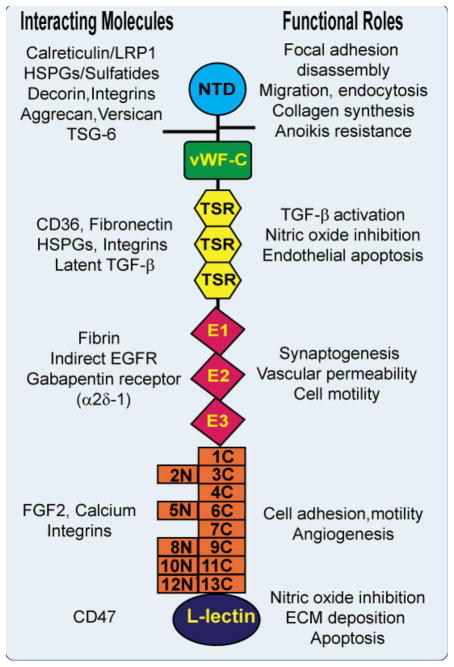
Fig. 1.
Model of TSP1 monomer showing the N-terminal laminin G-like domain, the oligomerization region of interchain disulfide bonding, the procolllagen-like, von Willebrand factor_C module, the properdin-like type 1 (TSR) repeats, the EGF-like repeats, and the TSP type 3 repeats which form the calcium-sensitive wire structure around the C-terminal L lectin type globular domain. The TSP receptors and interacting molecules discussed in this review series are shown to the left of the monomer and the functions induced by TSP interactions with these molecules are shown to the right of the monomer. For the sake of simplicity, only TSP1 is shown, although many of these interactions occur in other TSP family members. Some of the interacting molecules, such as integrins, heparan sulfate proteoglycans (HSPGs) and CD36 that will not be specifically discussed at length in this thematic minireview series, are also depicted. In addition, TSPs interact with other molecules (PDGF, cathepsin G and neutrophil elastase) whose binding site(s) on TSP have not been clearly elucidated and thus are not depicted. It is likely that future studies will identify additional binding molecules.
This thematic series of interrelated mini-reviews will focus on recent additions to the armamentarium of TSP functions.
The review by Mosher and Adams (2012, this issue) employs both evolutionary and structural perspectives to discern the functions of thrombospondins as well as other matricellular proteins, especially in the context of the complex network of intermolecular interactions occurring in the extracellular matrix and at the cell surface.
The review by Roberts and co-workers (2012, this issue) highlights the role of TSP1 as a regulator of local and systemic physiology through its ability to attenuate nitric oxide signaling. The identification of TSP1 as a regulator of nitric oxide signaling has significantly broadened our understanding of the role of TSP1, especially in vascular physiology and pathology.
The review by Risher and Eroglu (2012, this issue) discusses exciting, novel findings documenting a role for TSP in synaptogenesis through interactions between astrocyte-secreted TSPs and their neuronal receptor, calcium channel subunit α2δ-1, also a receptor for gabapentin. Collectively, these new findings have implications for neuronal development and responses to CNS injury.
Finally, Sweetwyne and Murphy-Ullrich (2012, this issue) focus on distinct roles for TSP1 mediated by different domains which impact basic cellular processes key for wound healing, tissue repair, and fibrosis. These entail stimulation of collagen expression through the N-terminal domain calreticulin-binding sequence, transactivation of EGF receptor by the EGF-like domains, and regulation of latent TGF-β activation by the TSR type 1 repeats.
Many of these findings were initially presented at the 2010 FASEB Summer Research Conference on Thrombospondins and Other Matricellular Proteins in Tissue Organization and Homeostasis. Clearly, despite several decades of research, the thrombospondins continue to surprise us with their broad and clinically-significant roles in physiology and disease. The field of matrix biology has been enriched by the often unexpected roles of thrombospondins in normal development and physiology and in the pathophysiology of diverse disease processes.
Acknowledgments
We would like to dedicate this thematic mini-review series to Dr. Paul Bornstein, Emeritus Professor at the University of Washington in Seattle WA, for his pioneering work on various aspects of thrombospondins. We also thank James Smithies for help with the illustration. We would also like to acknowledge the following support for the 2010 FASEB meeting: NIH R13 DK08753 with support from NIDDK, NIAMS, and NIHCD; the NIH Office of Rare Diseases, NIH, NCI Center for Cancer Research, Genentech, Genzyme, Pfizer, and the Company of Biologists, Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol. 2001;17:25–51. doi: 10.1146/annurev.cellbio.17.1.25. [DOI] [PubMed] [Google Scholar]
- Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch AS, Barnwell J, Silverstein RL, Nachman RL. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch AS, Leung LL, Shapiro J, Nachman RL. Human brain glial cells synthesize thrombospondin. Proc Natl Acad Sci U S A. 1986;83:2904–2908. doi: 10.1073/pnas.83.9.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch AS, Silbiger S, Heimer E, Nachman RL. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem Biophys Res Commun. 1992;182:1208–1217. doi: 10.1016/0006-291x(92)91860-s. [DOI] [PubMed] [Google Scholar]
- Baenziger NL, Brodie GN, Majerus PW. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci U S A. 1971;68:240–243. doi: 10.1073/pnas.68.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Alfi D, Devarayalu S, Framson P, Li P. Characterization of the mouse thrombospondin gene and evaluation of the role of the first intron in human gene expression. J Biol Chem. 1990;265:16691–16698. [PubMed] [Google Scholar]
- Chung J, Wang XQ, Lindberg FP, Frazier WA. Thrombospondin-1 acts via IAP/CD47 to synergize with collagen in α2β1-mediated platelet activation. Blood. 1999;94:642–648. [PubMed] [Google Scholar]
- Dixit VM, Hennessy SW, Grant GA, Rotwein P, Frazier WA. Characterization of a cDNA encoding the heparin and collagen binding domains of human thrombospondin. Proc Natl Acad Sci U S A. 1986;83:5449–5453. doi: 10.1073/pnas.83.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier WA, Dixit VM, Galvin NJ, Rotwein PR. Structure of human thrombospondin: complete amino acid sequence derived from cDNA. Semin Thromb Hemost. 1987;13:255–260. doi: 10.1055/s-2007-1003500. [DOI] [PubMed] [Google Scholar]
- Gartner TK, Dockter ME. Secreted platelet thrombospondin binds monovalently to platelets and erythrocytes in the absence of free Ca2+ Thromb Res. 1984;33:19–30. doi: 10.1016/0049-3848(84)90151-8. [DOI] [PubMed] [Google Scholar]
- Godyna S, Liau G, Popa I, Stefansson S, Argraves WS. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J Cell Biol. 1995;129:1403–1410. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea S, Orr AW, Pallero MA, Eggleton P, Murphy-Ullrich JE. Thrombospondin mediates focal adhesion disassembly through interactions with cell surface calreticulin. J Biol Chem. 2000;275:36358–36368. doi: 10.1074/jbc.M005951200. [DOI] [PubMed] [Google Scholar]
- Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis. 1999;3:147–158. doi: 10.1023/a:1009018702832. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Leung LL, Nachman RL, Levin RI, Mosher DF. Thrombospondin is the endogenous lectin of human platelets. Nature. 1982;295:246–248. doi: 10.1038/295246a0. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Ruggiero JT, Falcone DJ. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. 1985;65:79–84. [PubMed] [Google Scholar]
- Jaffe EA, Ruggiero JT, Leung LK, Doyle MJ, McKeown-Longo PJ, Mosher DF. Cultured human fibroblasts synthesize and secrete thrombospondin and incorporate it into extracellular matrix. Proc Natl Acad Sci U S A. 1983;80:998–1002. doi: 10.1073/pnas.80.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav J, Schwartz MA, Hynes RO. Analysis of platelet adhesion with a radioactive chemical crosslinking reagent: interaction of thrombospondin with fibronectin and collagen. Cell. 1982;31:253–262. doi: 10.1016/0092-8674(82)90425-1. [DOI] [PubMed] [Google Scholar]
- Lawler J. The structural and functional properties of thrombospondin. Blood. 1986;67:1197–1209. [PubMed] [Google Scholar]
- Lawler J, Hynes RO. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood. 1989;74:2022–2027. [PubMed] [Google Scholar]
- Lawler J, Weinstein R, Hynes RO. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988;107:2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler JW, Slayter HS, Coligan JE. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978;253:8609–8616. [PubMed] [Google Scholar]
- Majack RA, Cook SC, Bornstein P. Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombospondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol. 1985;101:1059–1070. doi: 10.1083/jcb.101.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack RA, Cook SC, Bornstein P. Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc Natl Acad Sci USA. 1986;83:9050–9054. doi: 10.1073/pnas.83.23.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Hanning R, Mosher DF. Binding and degradation of platelet thrombospondin by cultured fibroblasts. J Cell Biol. 1984;98:22–28. doi: 10.1083/jcb.98.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J, Sage H, Bornstein P. Isolation and characterization of a glycoprotein secreted by aortic endothelial cells in culture. Apparent identity with platelet thrombospondin. J Biol Chem. 1981;256:11330–11336. [PubMed] [Google Scholar]
- Mosher DF, Doyle MJ, Jaffe EA. Synthesis and secretion of thrombospondin by cultured human endothelial cells. J Cell Biol. 1982;93:343–348. doi: 10.1083/jcb.93.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Mosher DF. Interactions of thrombospondin with endothelial cells: receptor-mediated binding and degradation. J Cell Biol. 1987;105:1603–1611. doi: 10.1083/jcb.105.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Westrick LG, Esko JD, Mosher DF. Altered metabolism of thrombospondin by Chinese hamster ovary cells defective in glycosaminoglycan synthesis. J Biol Chem. 1988;263:6400–6406. [PubMed] [Google Scholar]
- Orr AW, Elzie CA, Kucik DF, Murphy-Ullrich JE. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J Cell Sci. 2003;116:2917–2927. doi: 10.1242/jcs.00600. [DOI] [PubMed] [Google Scholar]
- Penttinen RP, Kobayashi S, Bornstein P. Transforming growth factor β increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988;85:1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DR, Jennings LK, Prasanna HR. Ca2+-mediated association of glycoprotein G (thrombin sensitive protein, thrombospondin) with human platelets. J Biol Chem. 1980;255:11629–11632. [PubMed] [Google Scholar]
- Raugi GJ, Lovett DH. Thrombospondin secretion by cultured human glomerular mesangial cells. Am J Pathol. 1987;129:364–372. [PMC free article] [PubMed] [Google Scholar]
- Roberts DD, Haverstick DM, Dixit VM, Frazier WA, Santoro SA, Ginsburg V. The platelet glycoprotein thrombospondin binds specifically to sulfated glycolipids. J Biol Chem. 1985;260:9405–9411. [PubMed] [Google Scholar]
- Silverstein RL, Leung LL, Harpel PC, Nachman RL. Complex formation of platelet thrombospondin with plasminogen. Modulation of activation by tissue activator. J Clin Invest. 1984;74:1625–1633. doi: 10.1172/JCI111578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slane JM, Mosher DF, Lai CS. Conformational change in thrombospondin induced by removal of bound Ca2+. A spin label approach. FEBS Lett. 1988;229:363–366. doi: 10.1016/0014-5793(88)81157-8. [DOI] [PubMed] [Google Scholar]
- Sun X, Mosher DF. Ca2(+)-sensitive binding of thrombospondin to U937 cells is due to the formation of calcium precipitate in the binding medium. J Clin Invest. 1991;87:171–176. doi: 10.1172/JCI114967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Mosher DF, Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J Biol Chem. 1989;264:2885–2889. [PubMed] [Google Scholar]
- Taraboletti G, Belotti D, Giavazzi R. Thrombospondin modulates basic fibroblast growth factor activities on endothelial cells. Exs. 1992;61:210–213. doi: 10.1007/978-3-0348-7001-6_32. [DOI] [PubMed] [Google Scholar]
- Tuszynski GP, Rothman VL, Papale M, Hamilton BK, Eyal J. Identification and characterization of a tumor cell receptor for CSVTCG, a thrombospondin adhesive domain. J Cell Biol. 1993;120:513–521. doi: 10.1083/jcb.120.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Frazier WA. The thrombospondin receptor CD47 (IAP) modulates and associates with α2β1 integrin in vascular smooth muscle cells. Mol Biol Cell. 1998;9:865–874. doi: 10.1091/mbc.9.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]