Abstract
Stem cell therapy for adult stroke has reached limited clinical trials. Here, we provide translational research guidance on stem cell therapy for neonatal hypoxic-ischemic brain injury requiring a careful consideration of clinically relevant animal models, feasible stem cell sources, and validated safety and efficacy endpoint assays, as well as a general understanding of modes of action of this cellular therapy. To this end, we refer to existing translational guidelines, in particular the recommendations outlined in the consortium of academicians, industry partners and regulators called Stem cell Therapeutics as an Emerging Paradigm for Stroke or STEPS. Although the STEPS guidelines are directed at enhancing the successful outcome of cell therapy in adult stroke, we highlight overlapping pathologies between adult stroke and neonatal hypoxic-ischemic brain injury. We are, however, cognizant that the neonatal hypoxic-ischemic brain injury displays disease symptoms distinct from adult stroke in need of an innovative translational approach that facilitates the entry of cell therapy in the clinic. Finally, insights into combination therapy are provided with the vision that stem cell therapy may benefit from available treatments, such as hypothermia, already being tested in children diagnosed with hypoxic-ischemic brain injury.
Keywords: cerebral palsy, stem cells, hypothermia, neurorestoration, translational, consortium, combination therapy
Neonatal Hypoxic-Ischemic Brain Injury: A Disease Target for Cell Therapy
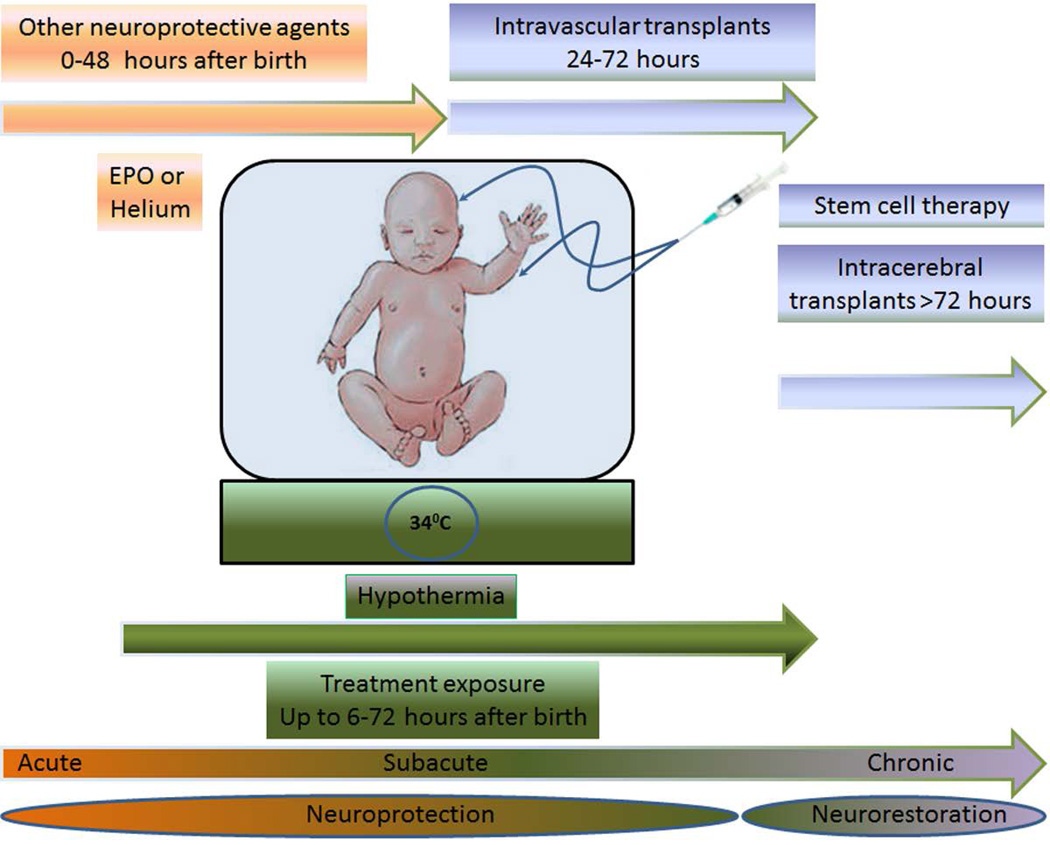
Neonatal hypoxic-ischemic brain injury is the major cause of hypoxic-ischemic encephalopathy (HIE), cerebral palsy (CP), and periventricular leukomalacia (PVL). Children diagnosed with hypoxic-ischemic brain injury present with neurodevelopmental deficits such as learning disabilities, mental retardation, and hearing and visual impairments. HIE is the brain manifestation of systemic asphyxia [1], afflicting 1.5 of 1,000 full-term live birth infants [2–4]. In this paper, the term HIE and the alternative term of neonatal encephalopathy (NE) [5, 6] are used interchangeably. A discussion on these two terminologies has been a topic for debate [7, 8]. Despite a concerted effort among researchers and clinicians to employ sensitive diagnostic tools, encephalopathy has not been diagnosed in premature infants compared to full term infants [9–11]. Mortality in newborns with HIE is as high as 50% [12], and 25% of those survivors display CP symptoms permanently [13, 14]. Ischemic perinatal stroke accounts for 30% of children with CP [15]. PVL, a cerebral white matter injury, is seen in 50% of neonates with extremely low birth weights with 90% of survivors exhibiting CP symptoms [16]; however, ultrasonography studies report lower than 50% incidence of PVL [17–19]. Because of overlapping pathophysiological symptoms between neonatal hypoxic-ischemic brain injury and adult stroke, novel treatments such as cell-based therapies, which are being tested in stroke, may prove effective in neonatal hypoxic-ischemic brain injury. Understanding the neurochemical cascade of events is critical for initiating treatment intervention in neonates [20]. In particular, therapeutic benefits may be achieved by abrogating the “secondary energy failure” or “excito-oxidative cascade” [20, 21], which is characterized by increased excitation of NMDA receptors coupled with aberrant oxidative stress due to mitochondrial dysfunction, altogether depleting energy from the brain seen in babies with hypoxic-ischemic injury [20]. The present treatment for HIE is hypothermia [22–24], which is largely effective in newborns with a gestational age of ≥ 36 weeks [24, 25] diagnosed with moderate to severe HIE [23, 24], but neurodevelopmental deficits persist in 40–50% of patients even after hypothermia [24]. Combination therapy of cell transplantation and hypothermia may benefit neonates with moderate to severe HIE (Figure 1).
Figure 1.
Envisioned combination therapy of cell transplantation and neuroprotective therapies, such as hypothermia, erythropoietin (EPO) and helium for treatment of neonates with HIE or NE. These different treatment interventions as stand alone therapies can be subdivided into acute (0–48 hours after birth), subacute (6–72 hours after birth) or chronic (>72 hours after birth). Treatments targeting acute and subacute stages of neonatal brain injury correspond to neuroprotection, whereas those targeting the chronic stage represent neurorestoration. EPO or helium treatment at the acute stage, and hypothermia and stem cell therapy at the subacute stage via intravascular routes (e.g., intravenous or intra-arterial) harness neuroprotective processes, while stem cell therapy during the chronic stage via intracerebral route promotes neurorestorative mechanisms. The combination of two or more of these therapies will allow abrogation of multiple cell death pathways during the cascade of brain injury thereby arresting not one but many of the cell death signals altogether improving clinical outcome of neonates with HIE or NE.
Translating Stem Cells from Bench to Bedside
Stem cell Therapeutics as an Emerging Paradigm for Stroke (STEPS) is a consortium of academicians, industry partners and regulators, including the National Institutes of Health (NIH) and the U.S. Food and Drug Administration (FDA) have regularly convened to enhance the successful outcome of cell therapy in stroke patients [26–33]. The establishment of a Baby STEPS consortium should allow a safe and effective translation of cell therapy in neonatal hypoxic-ischemic brain injury. We enumerate below critical gating criteria in designing translational studies in order to aid in the formulation of Baby STEPS guidelines.
Experimental Models of Neonatal Hypoxic-Ischemic Brain Injury
Vannucci’s rodent model of neonatal hypoxic-ischemic brain injury resembles many human neonate HIE pathological events [34]. Using 7-day old postnatal rats, which underwent ligation of unilateral carotid artery combined with systemic hypoxia generated cell death to cerebral cortex, subcortical and periventricular white matter, striatum, and hippocampus ipsilateral to the ligated artery [35]. Mice have also been used to create the Vannucci model [36] with varying pathological outcomes depending on the mouse strain [37–40]. Animal species and strain should be considered in HIE modeling.
Equally an important variable to control in experimental HIE is the age of the animals. Young animals tend to be resistant against hypoxia; indeed, 1–2 day old postnatal rats need to be exposed to more severe hypoxia than 7-day old postnatal rats to achieve successful HIE symptoms. However, the younger animals display worse white matter injury than the older rats [36]. Age as a key factor in HIE modeling is further evidenced by a focal subcortical cell loss accompanied by a surge in proliferating oligodendrocyte progenitor cells following HIE in young neonates, but rather modest in older animals [41–45]. Such age-related neurodegeneration and neuroregeneration after HIE requires standardization of animals models in order to better assess the therapeutic effects of experimental treatments.
Another factor to consider in animal models of HIE is gender. Neonatal female rats exhibit much more reduction in infarct volume and better improvement in sensorimotor task after perinatal hypoxic-ischemic brain injury and treatment with erythropoietin compared to male rats exposed to the same treatment conditions [46]. Other laboratory studies have observed gender influence in similar injury models [47, 48], altogether suggesting the need to monitor the gender of animals in experimental HIE.
The approximation of the clinical pathology of HIE is an imperative goal in standardizing the animal models. A species mimicking the human condition should allow better evaluation of experimental treatments for HIE. Among the species employed as closely resembling humans include fetal and neonatal nonhuman primate, sheep, lamb, puppy, piglet, and rabbit [34, 49–54]. The expensive cost of using large animals, however, has hindered access to these clinically relevant experimental models. The piglet model resembles closely the weight and the size of a newborn infant, thus a good platform for translational research of treatment interventions for neonates [55, 56]. Using this piglet model, phosphorylated metabolites were shown to be temperature-sensitive and that the more severe the energy depletion the worse the secondary energy failure, exacerbating neuronal death [55, 56]. These findings implicate the need for therapeutic strategies that can regulate temperature and maintain brain energy.
Assessment of pathological improvements after therapy in experimental models needs to consider developing tests for short and long-term functional outcomes that are species specific that closely approximate the human condition. However, while experimental models of brain injury allow the investigators to control the nature, severity, and timing of injury in relation to origin and progression of pathology, and treatment intervention, characterizing the phenotype of encephalopathy in neonates has been a major challenge. Indeed, this disconnect between the experimental models and the clinical setting has remained a key barrier in implementing “timely” interventions in babies with neonatal encephalopathy, which is in stark contrast to situations in adults with traumatic encephalopathy, or strokes. Recognizing this translational research gap is critical when contemplating with designing therapeutic intervention studies for clinical applications in neonates.
Characterizing Transplantable Stem Cells
A well-defined phenotypic characterization of stem cells is necessary to gain a better understanding of basic stem cell biology and translational potential [57–60]. The identity of the stem cells for transplantation in HIE is critical to validate the cell population that is safe and effective, but also to allow insights into the mechanism of action mediating the functional recovery after transplantation (discussed in detail below). Whereas the original concept of cell transplantation in brain injury is to replace damaged neurons, the recognition of neurodegeneration in multiple cell types has reinvented brain repair not as a single neuronal cell replacement therapy, but as a multi-pronged restorative mechanism. These reparative events involve both exogenous and endogenous neurons, glial cells, endothelial cells, among many other cell types, synergistically acting in tandem with the grafted stem cells’ by-stander effects, such as: trophic, neurogenic, vasculogenic, angiogenic, and synaptogenic properties [61]. Defining the stem cell source is also important for validation and replication of laboratory studies. As we envision the clinical product, the availability of thaw-and-inject transplantable stem cells has a practical application in the clinical setting. A readily available cryopreservable stem cell product, which can be shipped frozen and thawed at the clinic for transplantation, will be preferred for neonatal diseases. Additionally, the use of autologous stem cells, including those harvested from craniofacial neural crest or from fibroblasts for generating induced pluripotent stem cells, is attractive because they circumvent graft rejection and its adverse side effects. A small pilot study shows that intravenous injection of autologous cord blood is safe in CP children [62]. Another study suggests that placental tissue obtained during prenatal chorionic villous sampling or at delivery can be a good source of autologous stem cells which can be grafted during the last month of gestation or the first few months after delivery if neurodegeneration is detected in the baby [63].
Safety and Efficacy Endpoints of Cell Therapy
Routine functional assays of safety and efficacy of experimental treatments in HIE include behavioral tests that can assess motor and cognitive improvements and histological assays, such as markers of decreased cell loss/apoptosis, reduced inflammation, elevated neurogenesis, and suppressed oxidative stress to reveal brain remodeling processes. Approximation of the HIE symptoms is key component in appreciating the clinical relevance of the functional deficits after injury and the recovery following transplantation [64–67]. A need for both short- and long-term characterization of safety and efficacy of stem cells will also be more clinically relevant to assess cell therapy’s immediate and prolonged effects [68]. This characterization can become a challenging issue for neonatal HIE because of endogenous spontaneous recovery in both developmental and maturation periods of the neonatal animal [69], and also seen in pediatric patients [70]. For histological evaluation, the status of the grafted cells and the host HIE needs to be assessed, using phenotypic markers of cell fate for the following: trophic factor effect, immunomodulatory response, neurogenesis, vasculogenesis, angiogenesis and synaptogenesis, as well as inflammation, tumorigenesis or ectopic tissue formation [71]. These histological assays provide insights into the modes of action of the transplanted cells, but also serve as safety measures of any adverse side effects.
Translational Study Protocols
A general rule in designing translational studies is to optimize the dose, delivery route, and timing of stem cell transplantation within clinically relevant parameters. Treating the laboratory as the clinical setting for cell therapy in HIE will enhance the translational potential of the stem cell product. Finding the minimum therapeutic cell dose may be critical and beneficial in order to avoid any potential microembolism at a high dose. Focusing on minimally invasive procedures for cell delivery will circumvent adding more trauma to the already injured brain. For timing of cell delivery, consideration should be given to the neuroprotective phase (<1 day of injury), and the neurorestorative phase (>1 day after injury) [72, 73].
Cellular and Molecular Therapeutic Pathways Underlying Stem Cell Transplantation
Cell replacement and bystander effects are the two major modes of action implicated in stem cell-mediated functional recovery in ischemic brain injury. Cellular and molecular pathways such as neurogenesis, angiogenesis, synaptogensis, immunomodulation, and trophic factor secretion mediate neurorestorative mechanisms [32, 33, 74]. The recent use of real-time visualization techniques (i.e., magnetic resonance imaging) allows tracking of the transplants and imaging of the host neurorestorative mechanisms [75–81] originally performed in stroke and extended to HIE models [82–84].
Are We There Yet? Remaining Preclinical Issues Prior to Embarking in Cell Therapy for Neonatal Hypoxic-Ischemic Injury
Extreme caution in determining the safety and efficacy of stem cell therapy should accompany the clinical trials in neonatal hypoxic-ischemic injury. Two limited clinical trials in the US (Medical College of Georgia and Duke University) are evaluating the safety and efficacy of umbilical cord blood transplants in CP pediatric patients. Although intravenous transplantation of autologous cord blood in CP children has been found safe, long-term efficacy readouts remain to be addressed [62, 85]. Autologous bone marrow-derived MSCs have also been transplanted and found safe, but only in a single case report of CP patient ([86]). Notwithstanding, the preferred stem cells for transplantation are those derived from autologous sources. Lineage committed (neurons, glia, astrocytes) or brain region specific (cortex, hippocampus) cells have also been proposed for stem cell sources, but this cell replacement strategy has been challenged, in that convincing evidence supports the stem cell by-stander mechanism (neurotrophic, anti-inflammatory, anti-oxidative) as the primary mode of action of cell therapy, indicating non-lineage committed or non-brain region differentiated cells as equally efficacious cells for transplantation therapy. As noted above, the translation of cell therapy in neonatal ischemic-injury patients should be guided by clinically relevant animal models, utilizing a well-defined set of stem cells, tested rigorously in the laboratory and passed the safety and efficacy parameters, and at least a general understanding of the mode of action underlying the stem cells’ therapeutic benefits. In declaring that stem cell therapy has reached its prime time for clinical application, an objective measure of predictive neurologic outcomes of this novel treatment in neonatal hypoxic-ischemic injury remains elusive. Current anecdotal reports of clinical improvement following cell therapy in children with CP or HIE should not compromise the Baby STEPS’ footing on the need for solid preclinical studies to support the clinical trials. The discussion above on the Baby STEPS guidelines may be applicable to other experimental therapies for neonatal hypoxic-ischemic injury [87–90] and should be used in concert with existing pediatric stroke recommendations for research and treatment interventions [91–94].
Combination Therapy of Cell Transplantation and Hypothermia
All of the current brain oriented therapies, such as magnesium, calcium channel blockers and NMDA receptor antagonists, seek to interrupt the cascade triggered by HIE and thereby limits the extent of injury. To date, all therapies in human neonates who have suffered from HIE have had disappointing results in preventing the continued neuronal loss (reviewed in [95, 96]). Hypothermia, in experimental animal models of HIE, decreases glutamate release [97], attenuates secondary energy failure [23, 55, 97–99], normalizes protein synthesis [100] and attenuates free radical-induced injury [96]. Several small safety trials of hypothermia performed in human neonates [98, 99] gave promising results, while three large randomized trials of hypothermia demonstrated improvement in neurodevelopmental outcomes in neonates with mild to moderate HIE, but no improvement in neonates with severe HIE [22–24]. Recently, hypothermia has been shown to be neuroprotective by reducing the risk of neurodevelopmental disability at 18 months of age in newborns with either moderate or severe HIE [101]. Hence, while neuroprotective approaches may play a role in reducing the ongoing or escalating damage, repairing already damaged regions will still require a cellular replacement approach that may be applicable for neonates with moderate to severe HIE.
Hypothermia has been shown to afford strong neuroprotective effects against HIE pathological events, including aberrant stages of region-specific brain maturation [102], blood brain barrier (BBB) impairment [103], and mitochondrial dysfunction-induced apoptosis [104]. As highlighted above, efficacy of hypothermia is best reproduced within the first 6 hours of life for the infant with moderate to severe HIE [105–107], suggesting that hypothermia treatment protocol may benefit from a combination of therapeutic strategies [108] [109]. Several new interventions that are in clinical trial stages, including erythropoietin and helium [110–112], should also be considered for this combination therapy. More importantly, the treatment regimen for hypothermia plus these adjunctive therapies is likely to be based on the evolving pathophysiology of neonatal brain injury, as elegantly reviewed by Ferriero and colleagues [113, 114]. Combination, instead of stand-alone, therapies may be more beneficial to combat the multiple pathophysiological cell death cascades; early detection of at-risk newborns may also facilitate the prevention or the reduction in the incidence of lifelong disabilities associated with neonatal brain injury [113, 114].
As discussed above, accumulating experimental data have indicated the mobilization of bone marrow-derived stem cells, such as mesenchymal stem cells (MSCs), in brain plasticity and therapy of HIE to the affected area [115]. In the clinic, MSCs can be obtained from umbilical cord blood, adipose tissue, amniotic fluid/tissue or menstrual blood [116]. As alluded earlier, autologous MSCs may be the preferred stem cells to avoid adverse effects associated with graft rejection, but allogeneic MSCs may also be equally safe and effective due to their immature immune system, as well as their capacity to secrete anti-inflammatory factors [116]. MSCs are capable of differentiation into variety of phenotype cells [117] [110], and have been demonstrated to exert a therapeutic benefit against brain injury [105]. However, little is known regarding MSC treatment for HIE, especially in combination with hypothermia.
The observation that seizure onset beyond the first 12 hours of life is not only common in newborns with HIE [118], but also is associated with severe brain injury [50], advances the notion of a critical relationship between the onset of neonatal seizure and initiation of the therapy. Accordingly, any treatment regimen, including hypothermia, is likely to exert benefit if initiated within 6 hours after hypoxic-ischemic injury and continuing over the next 12 hours or even beyond (i.e., for 72 hours) [118]. The mechanism underlying hypothermia remains elusive, but may include its capacity to reduce oxidative stress, energy deficit, and inflammation [119]. Because of the dismal prognosis of infants with HIE, clinical enthusiasm for a novel treatment is understandable [120].
The use of delta opioid agonists may resemble certain physiological correlates of hibernation, including hypothermia [121], which may involve direct opioid receptor activation, as well as non-opioid mechanisms [122–124]. Interestingly, delta opioids may regulate neural stem and progenitor cell proliferation and differentiation [125], and may even enhance cell-based therapeutics in in vitro and in vivo disease models [126]. Our recent study [127] revealed that moderate hypothermia is efficacious in an in vitro model of hypoxic-ischemic injury, which was enhanced by MSC treatment. We also showed that the delta opioid system, along with other non-opioid neuroprotective processes, primarily contributes to the observed neuroprotection in HIE. Stem cell therapy using MSCs significantly improved the therapeutic outcome of moderate hypothermia. Primary rat neurons were exposed to oxygen-glucose deprivation (OGD) condition, a model of hypoxic-ischemic injury, then incubated at 25°C (severe hypothermia), 34°C (moderate hypothermia), and 37°C (normothermia) with or without subsequent co-culture with mesenchymal stem cells (MSCs). Combination treatment of moderate hypothermia and MSCs proved to be the optimal condition for preserving cell survival and mitochondrial activity after OGD exposure. Pharmacologic induction of hypothermia in human embryonic kidney cells (HEK293) via treatment with delta opioid peptide (DADLE) resembled moderate hypothermia’s attenuation of OGD-mediated cell alterations, which were much more pronounced in HEK293 cells overexpressing the delta opioid receptor. Further, the addition of DADLE to 34°C hypothermia and stem cell treatment in primary rat neurons showed synergistic neuroprotective effects against OGD which were significantly more robust than the dual combination of moderate hypothermia and MSCs, and were significantly reduced, but not completely abolished, by the opioid receptor antagonist naltrexone altogether implicating a ligand-receptor mechanism of neuroprotection. Investigations into other therapeutic signaling pathways revealed growth factor upregulation (i.e., GDNF) and anti-apoptotic function accompanying the observed therapeutic benefits. These results support combination therapy of hypothermia and stem cells for hypoxic-ischemic injury, which may have direct impact on current clinical trials using stand-alone hypothermia or stem cells for treating neonatal hypoxic-ischemic brain injury.
Conclusions
Stem cell therapy has emerged as an experimental treatment for neonatal hypoxic-ischemic brain injury. There is an urgent demand to introduce this therapy in the clinic for children with neonatal hypoxic-ischemic brain injury. Unfortunately, additional translational research studies are warranted in order to advance this cellular therapy from the laboratory to the clinic. The clinical entry of cell transplantation in neonatal hypoxic-ischemic brain injury will benefit from published laboratory studies and ongoing clinical trials of stem cell therapy in adult stroke. However, while neonatal hypoxic-ischemic injury shares overlapping pathologies with adult stroke, the former displays unique disease symptoms that will require a modified translational approach prior to clinical application. Consideration of combination therapy involving hypothermia and stem cell transplantation may improve the outcome of cell therapy in neonatal hypoxic-ischemic injury. The primary outcomes of this combination therapy can be measured by functional parameters such as behavioral tests and histological assays of brain status. Moreover, the use of biomarkers via neuroimaging may serve as surrogate markers of brain remodeling which allows close monitoring of the transplant recipient at different time points post-intervention over several months or even years, thereby facilitating the long-term demonstration of stable functional effects by the combination therapy.
Future perspective
Stem cell therapy for neonatal hypoxic-ischemic brain injury remains experimental. Limited clinical trials of transplantation of autologous umbilical cord blood cells for CP children are underway, but extending this therapy to other neonatal diseases will require solid preclinical safety and efficacy data for each indication. Standardized experimental models with quantitative functional endpoints and predictive clinical outcomes are an urgent need for translational research. Intermediate goals over the next five years will be optimizing the route of delivery, cell dose and timing of transplantation after diagnosis of neonatal brain injury. Target patient population for the initial clinical trials will be full term infants because of the difficulty in detecting encephalopathy in premature babies. It is envisioned that a decade from now, once more sensitive diagnostic tools (neuroimaging) and laboratory data are available, cell therapy will be tested in preterm infants with encephalopathy. The recognition that HIE or NE is associated with multiple cell death pathways will also attract research investigations on combination therapies over the next ten years. In particular, hypothermia and other neuroprotective strategies currently in clinical stage will be tested as adjunctive therapies to stem cell transplantation. Rigorous experimental testing of stem cells and combination therapies can be leveraged by adhering to relevant translational research guidelines [e.g., 128] to enhance the safe and effective clinical outcome of these interventions for treating neonatal hypoxic-ischemic brain injury.
Executive Summary/Executive summary headings.
Neonatal Hypoxic-Ischemic Brain Injury: A Disease Target for Cell Therapy
Neonatal hypoxia-ischemia brain injury leads to HIE, CP and PVL.
Cell-based therapy may prove safe and effective for HIE.
Translating Stem Cells from Bench to Bedside
-
The importance of implementing a baby STEPS program facilitates the translation of stem cell therapy, as stand alone or in combination with neuroprotective therapies, from the laboratory to the clinic.
Experimental Models of Neonatal Hypoxic-Ischemic Brain Injury.-
○Appropriate animal models that allow predictive clinical outcomes are necessary in assessment of novel therapies for HIE.
-
○Unfortunately, current animal models do not faithfully mimic many of HIE pathology and symptoms.
-
○A standardized animal model for HIE that provides quantitative safety and efficacy endpoints is required for translational research.
Characterizing Transplantable Stem Cells.-
○Creating a well defined cell line population that is not only safe but also effective with long-term and stable effects.
-
○The ideal transplantable stem cell is envisioned as a cell product that is readily available on clinical site, deliverable via non-invasive procedure, and well tolerated by the transplant recipient.
Safety and Efficacy Endpoints of Cell Therapy.-
○Quantifiable outcome measures need be core experimental design for demonstrating safety and efficacy in both histological and behavioral parameters with good predictive clinical values.
-
○Because of the brain plasticity inherent in the neonates, delineating spontaneous from treatment-mediated effects should be considered.
-
○Endpoints should reveal acute as well as prolonged safety and efficacy of the stem cell product.
Translational Study Protocols.-
○Preclinical investigations are needed to explore delivery methods of stem cells that are safe and effective via less invasive route, with clinically relevant cell dose and therapeutic window appropriate for neonates.
Cellular and Molecular Therapeutic Pathways Underlying Stem Cell Transplantation.-
○Providing insights on the molecular and cellular pathways that mediate therapeutic benefits of stem cell therapy will help to optimize the treatment regimen.
-
○Revealing the physiological status of the transplanted cells, as well as the host brain tissue will provide additional guidance on the graft-host interaction during disease progression and treatment intervention.
-
○
Are We There Yet? Remaining Preclinical Issues Prior to Embarking in Cell Therapy for Neonatal Hypoxic-Ischemic Injury
Consideration is given to gating items that still need to be addressed in the laboratory prior to initiating clinical studies.
Identifying optimal stem cell product should evaluate advantages and limitations of autologous and allogeneic tissue sources.
Combination Therapy of Cell Transplantation and Hypothermia
Promising neuroprotective therapies for HIE, such as EPO and helium, with emphasis on hypothermia are discussed.
Rationale is provided for investigating the therapeutic potential of combining stem cells and hypothermia for HIE.
Conclusion
Stem cell therapy remains an experimental treatment for HIE.
Rigorous translational research designed to assess safety and efficacy of stem cell therapy will pave the way for its entry to the clinic for applications in neonates.
Combination therapy of stem cells and hypothermia for HIE may lead to improved clinical outcome.
Acknowledgement
CVB is supported by NIH NINDS 1R01NS071956-01, James and Esther King Foundation for Biomedical Research Program 1KG01-33966, SanBio Inc., Celgene Cellular Therapeutics, KMPHC and NeuralStem Inc.
Abbreviations
- HI
Hypoxic-ischemia
- HIE
Hypoxic-ischemic encephalopathy
- NE
Neonatal encephalopathy
- CP
Cerebral palsy
- PVL
Periventricular Leukomalacia
- STEPS
Stem cell Therapeutics as an Emerging Paradigm for Stroke
- MSCs
mesenchymal stem cells
References
* References of interest
** considerable interest
- 1.Vannucci R. Hypoxia Ischemia: Pathogenesis and Neuropathology. (Sixth) St. Louis, MO: Mosby; 1997. [Google Scholar]
- 2.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Low JA, Lindsay BG, Derrick EJ. Threshold of metabolic acidosis associated with newborn complications. Am J Obstet Gynecol. 1997;177(6):1391–1394. doi: 10.1016/s0002-9378(97)70080-2. [DOI] [PubMed] [Google Scholar]
- 4.Mulligan JC, Painter MJ, O'donoghue PA, Macdonald HM, Allan AC, Taylor PM. Neonatal asphyxia. II. Neonatal mortality and long-term sequelae. J Pediatr. 1980;96(5):903–907. doi: 10.1016/s0022-3476(80)80575-0. [DOI] [PubMed] [Google Scholar]
- 5.Nelson KB. Neonatal encephalopathy: etiology and outcome. Dev Med Child Neurol. 2005;47(5):292. doi: 10.1017/s0012162205000563. [DOI] [PubMed] [Google Scholar]
- 6. Nelson KB, Leviton A. Hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2006;354(15):1643–1645. author reply 1643–1645. This paper provides the rationale for hypothermia therapy in neonates with hypoxic-ischemic encephalopathy
- 7.Dammann O, Ferriero D, Gressens P. Neonatal encephalopathy or hypoxic-ischemic encephalopathy? Appropriate terminology matters. Pediatr Res. 2011;70(1):1–2. doi: 10.1203/PDR.0b013e318223f38d. [DOI] [PubMed] [Google Scholar]
- 8.Leviton A. Why the term neonatal encephalopathy should be preferred over neonatal hypoxic-ischemic encephalopathy. Am J Obstet Gynecol. 2012 doi: 10.1016/j.ajog.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Blume HK, Loch CM, Li CI. Neonatal encephalopathy and socioeconomic status: population-based case-control study. Arch Pediatr Adolesc Med. 2007;161(7):663–668. doi: 10.1001/archpedi.161.7.663. [DOI] [PubMed] [Google Scholar]
- 10.De Vries LS, Van Haastert IC, Benders MJ, Groenendaal F. Myth: cerebral palsy cannot be predicted by neonatal brain imaging. Semin Fetal Neonatal Med. 2011;16(5):279–287. doi: 10.1016/j.siny.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11. Volpe JJ. The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 2009;16(4):167–178. doi: 10.1016/j.spen.2009.09.005. This paper presents the challenges in detecting encephalopathy in preterm infants.
- 12.Macdonald HM, Mulligan JC, Allen AC, Taylor PM. Neonatal asphyxia. I. Relationship of obstetric and neonatal complications to neonatal mortality in 38,405 consecutive deliveries. J Pediatr. 1980;96(5):898–902. doi: 10.1016/s0022-3476(80)80574-9. [DOI] [PubMed] [Google Scholar]
- 13.Finer NN, Robertson CM, Richards RT, Pinnell LE, Peters KL. Hypoxic-ischemic encephalopathy in term neonates: perinatal factors and outcome. J Pediatr. 1981;98(1):112–117. doi: 10.1016/s0022-3476(81)80555-0. [DOI] [PubMed] [Google Scholar]
- 14.Robertson CM, Finer NN, Grace MG. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr. 1989;114(5):753–760. doi: 10.1016/s0022-3476(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 15.Raju TN. Ischemic perinatal stroke: challenge and opportunities. Int J Stroke. 2008;3(3):169–172. doi: 10.1111/j.1747-4949.2008.00205.x. [DOI] [PubMed] [Google Scholar]
- 16.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HJ, Wei KL, Yao YJ. [Multicenter investigation for incidence of periventricular leukomalacia in premature infants in China] Zhongguo Dang Dai Er Ke Za Zhi. 2008;10(6):686–692. [PubMed] [Google Scholar]
- 18.Baud O, D'allest AM, Lacaze-Masmonteil T, et al. The early diagnosis of periventricular leukomalacia in premature infants with positive rolandic sharp waves on serial electroencephalography. J Pediatr. 1998;132(5):813–817. doi: 10.1016/s0022-3476(98)70309-9. [DOI] [PubMed] [Google Scholar]
- 19.Olsen P, Paakko E, Vainionpaa L, Pyhtinen J, Jarvelin MR. Magnetic resonance imaging of periventricular leukomalacia and its clinical correlation in children. Ann Neurol. 1997;41(6):754–761. doi: 10.1002/ana.410410611. [DOI] [PubMed] [Google Scholar]
- 20. Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10(4):372–382. doi: 10.1016/S1474-4422(11)70016-3. This paper reviews neuroprotective strategies for neonatal disorders.
- 21.Hope PL, Reynolds EO. Investigation of cerebral energy metabolism in newborn infants by phosphorus nuclear magnetic resonance spectroscopy. Clin Perinatol. 1985;12(1):261–275. [PubMed] [Google Scholar]
- 22.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 23. Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. This paper reports the clinical results of hypothermia therapy for NE.
- 24. Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. This paper reports the clinical results of hypothermia therapy for HIE.
- 25.Rutherford MA, Azzopardi D, Whitelaw A, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116(4):1001–1006. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 26.Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 27.Recommendations for clinical trial evaluation of acute stroke therapies. Stroke. 2001;32(7):1598–1606. doi: 10.1161/01.str.32.7.1598. [DOI] [PubMed] [Google Scholar]
- 28.Feuerstein GZ, Zaleska MM, Krams M, et al. Missing steps in the STAIR case: a Translational Medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J Cereb Blood Flow Metab. 2008;28(1):217–219. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- 29.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopp M, Steinberg GK, Kondziolka D, et al. Who's in favor of translational cell therapy for stroke: STEPS forward please? Cell Transplant. 2009;18(7):691–693. doi: 10.3727/096368909X470883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borlongan CV, Chopp M, Steinberg GK, et al. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regen Med. 2008;3(3):249–250. doi: 10.2217/17460751.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borlongan CV. Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke. 2009;40(3 Suppl):S146–S148. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40(2):510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 34. Northington FJ. Brief update on animal models of hypoxic-ischemic encephalopathy and neonatal stroke. ILAR J. 2006;47(1):32–38. doi: 10.1093/ilar.47.1.32. This paper reviews experimental models of HIE.
- 35.Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci. 2005;27(2–4):81–86. doi: 10.1159/000085978. [DOI] [PubMed] [Google Scholar]
- 36.Ditelberg JS, Sheldon RA, Epstein CJ, Ferriero DM. Brain injury after perinatal hypoxia-ischemia is exacerbated in copper/zinc superoxide dismutase transgenic mice. Pediatr Res. 1996;39(2):204–208. doi: 10.1203/00006450-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810(1–2):114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 38.Fullerton HJ, Ditelberg JS, Chen SF, et al. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann Neurol. 1998;44(3):357–364. doi: 10.1002/ana.410440311. [DOI] [PubMed] [Google Scholar]
- 39.Graham EM, Sheldon RA, Flock DL, et al. Neonatal mice lacking functional Fas death receptors are resistant to hypoxic-ischemic brain injury. Neurobiol Dis. 2004;17(1):89–98. doi: 10.1016/j.nbd.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Hagberg H, Wilson MA, Matsushita H, et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90(5):1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 41.Mcquillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23(8):3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S, Wu EX, Cai K, Lau HF, Cheung PT, Khong PL. Mild hypoxic-ischemic injury in the neonatal rat brain: longitudinal evaluation of white matter using diffusion tensor MR imaging. AJNR Am J Neuroradiol. 2009;30(10):1907–1913. doi: 10.3174/ajnr.A1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Z, Liu J, Cheung PY, Chen C. Long-term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic-ischemic brain injury. Brain Res. 2009;1301:100–109. doi: 10.1016/j.brainres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Wu EX, Tam CN, Lau HF, Cheung PT, Khong PL. Characterization of white matter injury in a hypoxic-ischemic neonatal rat model by diffusion tensor MRI. Stroke. 2008;39(8):2348–2353. doi: 10.1161/STROKEAHA.107.509927. [DOI] [PubMed] [Google Scholar]
- 45.Chang YC, Huang CC, Hung PL, Huang HM. Rolipram, a phosphodiesterase type IV inhibitor, exacerbates periventricular white matter lesions in rat pups. Pediatr Res. 2008;64(3):234–239. doi: 10.1203/PDR.0b013e31817cfc87. [DOI] [PubMed] [Google Scholar]
- 46.Wen TC, Rogido M, Peng H, Genetta T, Moore J, Sola A. Gender differences in long-term beneficial effects of erythropoietin given after neonatal stroke in postnatal day-7 rats. Neuroscience. 2006;139(3):803–811. doi: 10.1016/j.neuroscience.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 47.Guo TL, Germolec DR, Musgrove DL, et al. Myelotoxicity in genistein-, nonylphenol-, methoxychlor-, vinclozolin- or ethinyl estradiol-exposed F1 generations of Sprague-Dawley rats following developmental and adult exposures. Toxicology. 2005;211(3):207–219. doi: 10.1016/j.tox.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Pequignot JM, Spielvogel H, Caceres E, et al. Influence of gender and endogenous sex steroids on catecholaminergic structures involved in physiological adaptation to hypoxia. Pflugers Arch. 1997;433(5):580–586. doi: 10.1007/s004240050317. [DOI] [PubMed] [Google Scholar]
- 49.Raju TN. Some animal models for the study of perinatal asphyxia. Biol Neonate. 1992;62(4):202–214. doi: 10.1159/000243873. [DOI] [PubMed] [Google Scholar]
- 50.Bjorkman ST, Miller SM, Rose SE, Burke C, Colditz PB. Seizures are associated with brain injury severity in a neonatal model of hypoxia-ischemia. Neuroscience. 2010;166(1):157–167. doi: 10.1016/j.neuroscience.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 51.Zhang D, Hathi M, Yang ZJ, Ding H, Koehler R, Thakor N. Hypoxic-ischemic brain injury in neonatal piglets with different histological outcomes: An amplitude-integrated EEG study. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:1127–1130. doi: 10.1109/IEMBS.2009.5333439. [DOI] [PubMed] [Google Scholar]
- 52.Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009;40(3):156–167. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 53.Tai WC, Burke KA, Dominguez JF, Gundamraj L, Turman JE., Jr Growth deficits in a postnatal day 3 rat model of hypoxic-ischemic brain injury. Behav Brain Res. 2009;202(1):40–49. doi: 10.1016/j.bbr.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 54.Derrick M, Luo NL, Bregman JC, et al. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci. 2004;24(1):24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laptook AR, Corbett RJ, Burns D, Sterett R. Neonatal ischemic neuroprotection by modest hypothermia is associated with attenuated brain acidosis. Stroke. 1995;26(7):1240–1246. doi: 10.1161/01.str.26.7.1240. [DOI] [PubMed] [Google Scholar]
- 56.Iwata O, Iwata S, Thornton JS, et al. "Therapeutic time window" duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. 2007;1154:173–180. doi: 10.1016/j.brainres.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 57.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149(2):310–321. doi: 10.1006/exnr.1997.6730. [DOI] [PubMed] [Google Scholar]
- 58.Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55(4):565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- 59.Nelson PT, Kondziolka D, Wechsler L, et al. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160(4):1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hara K, Yasuhara T, Maki M, et al. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog Neurobiol. 2008;85(3):318–334. doi: 10.1016/j.pneurobio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 61.Yu G, Fournier C, Hess DC, Borlongan CV. Transplantation of carotid body cells in the treatment of neurological disorders. Neurosci Biobehav Rev. 2005;28(8):803–810. doi: 10.1016/j.neubiorev.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 62. Sun J, Allison J, Mclaughlin C, et al. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion. 2010;50(9):1980–1987. doi: 10.1111/j.1537-2995.2010.02720.x. Landmark pilot study report on autologous transplantation of umbilical cord blood in children.
- 63.Portmann-Lanz CB, Schoeberlein A, Huber A, et al. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194(3):664–673. doi: 10.1016/j.ajog.2006.01.101. [DOI] [PubMed] [Google Scholar]
- 64.Robertson CM, Finer NN. Long-term follow-up of term neonates with perinatal asphyxia. Clin Perinatol. 1993;20(2):483–500. [PubMed] [Google Scholar]
- 65.Lauterbach MD, Raz S, Sander CJ. Neonatal hypoxic risk in preterm birth infants: the influence of sex and severity of respiratory distress on cognitive recovery. Neuropsychology. 2001;15(3):411–420. [PubMed] [Google Scholar]
- 66.Espy KA, Senn TE, Charak DA, Tyler J, Wiebe SA. Perinatal pH and neuropsychological outcomes at age 3 years in children born preterm: an exploratory study. Dev Neuropsychol. 2007;32(2):669–682. doi: 10.1080/87565640701376003. [DOI] [PubMed] [Google Scholar]
- 67.Kaandorp JJ, Benders MJ, Rademaker CM, et al. Antenatal allopurinol for reduction of birth asphyxia induced brain damage (ALLO-Trial); a randomized double blind placebo controlled multicenter study. BMC Pregnancy Childbirth. 2010;10:8. doi: 10.1186/1471-2393-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke. 2008;39(4):1307–1313. doi: 10.1161/STROKEAHA.107.499822. [DOI] [PubMed] [Google Scholar]
- 69.Carroll JE, Borlongan CV. Adult stem cell therapy for acute brain injury in children. CNS Neurol Disord Drug Targets. 2008;7(4):361–369. doi: 10.2174/187152708786441812. [DOI] [PubMed] [Google Scholar]
- 70.Kim CT, Han J, Kim H. Pediatric stroke recovery: a descriptive analysis. Arch Phys Med Rehabil. 2009;90(4):657–662. doi: 10.1016/j.apmr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 71.Yasuhara T, Hara K, Maki M, et al. Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats. J Cereb Blood Flow Metab. 2008;28(11):1804–1810. doi: 10.1038/jcbfm.2008.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif. 2008;41(Suppl 1):94–114. doi: 10.1111/j.1365-2184.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. 2008;8(8):1193–1201. doi: 10.1586/14737175.8.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parolini O, Alviano F, Bergwerf I, et al. Toward cell therapy using placenta-derived cells: disease mechanisms, cell biology, preclinical studies, and regulatory aspects at the round table. Stem Cells Dev. 2010;19(2):143–154. doi: 10.1089/scd.2009.0404. [DOI] [PubMed] [Google Scholar]
- 75.Hoehn M, Wiedermann D, Justicia C, et al. Cell tracking using magnetic resonance imaging. J Physiol. 2007;584(Pt 1):25–30. doi: 10.1113/jphysiol.2007.139451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Modo M, Mellodew K, Cash D, et al. Mapping transplanted stem cell migration after a stroke: a serial, in vivo magnetic resonance imaging study. Neuroimage. 2004;21(1):311–317. doi: 10.1016/j.neuroimage.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 77.Jendelova P, Herynek V, Urdzikova L, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76(2):232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 78.Shyu WC, Chen CP, Lin SZ, Lee YJ, Li H. Efficient tracking of non-iron-labeled mesenchymal stem cells with serial MRI in chronic stroke rats. Stroke. 2007;38(2):367–374. doi: 10.1161/01.STR.0000254463.24655.14. [DOI] [PubMed] [Google Scholar]
- 79.Song M, Kim Y, Ryu S, Song I, Kim SU, Yoon BW. MRI tracking of intravenously transplanted human neural stem cells in rat focal ischemia model. Neurosci Res. 2009;64(2):235–239. doi: 10.1016/j.neures.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 80.Daadi MM, Li Z, Arac A, et al. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17(7):1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee ES, Chan J, Shuter B, et al. Microgel iron oxide nanoparticles for tracking human fetal mesenchymal stem cells through magnetic resonance imaging. Stem Cells. 2009;27(8):1921–1931. doi: 10.1002/stem.112. [DOI] [PubMed] [Google Scholar]
- 82.Ashwal S, Obenaus A, Snyder EY. Neuroimaging as a basis for rational stem cell therapy. Pediatr Neurol. 2009;40(3):227–236. doi: 10.1016/j.pediatrneurol.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 83.Chau V, Poskitt KJ, Miller SP. Advanced neuroimaging techniques for the term newborn with encephalopathy. Pediatr Neurol. 2009;40(3):181–188. doi: 10.1016/j.pediatrneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 84.Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40(11):3415–3421. doi: 10.1161/STROKEAHA.109.564633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liao Y, Cotten M, Tan S, Kurtzberg J, Cairo MS. Rescuing the neonatal brain from hypoxic injury with autologous cord blood. Bone Marrow Transplant. 2012 Sep 10; doi: 10.1038/bmt.2012.169. [Epub ahead of print] PubMed PMID: 22964590. An update report on the status of on-going clinical study of autologously transpalnted cord blood cells in CP children.
- 86.Li M, Yu A, Zhang F, Dai G, Cheng H, Wang X, An Y. Treatment of one case of cerebral palsy combined with posterior visual pathway injury using autologousbone marrow mesenchymal stem cells. J Transl Med. 2012 May 18;10(1) doi: 10.1186/1479-5876-10-100. [Epubahead of print] PubMed PMID: 22607263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jordan LC, Rafay MF, Smith SE, et al. Antithrombotic treatment in neonatal cerebral sinovenous thrombosis: results of the International Pediatric Stroke Study. J Pediatr. 2010;156(5):704–710. 710, e701–e710, e702. doi: 10.1016/j.jpeds.2009.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grunwald IQ, Walter S, Shamdeen MG, et al. New mechanical recanalization devices - the future in pediatric stroke treatment? J Invasive Cardiol. 2010;22(2):63–66. [PubMed] [Google Scholar]
- 89.Normann S, De Veber G, Fobker M, et al. Role of endogenous testosterone concentration in pediatric stroke. Ann Neurol. 2009;66(6):754–758. doi: 10.1002/ana.21840. [DOI] [PubMed] [Google Scholar]
- 90.Kenet G, Lutkhoff LK, Albisetti M, et al. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta-analysis of observational studies. Circulation. 2010;121(16):1838–1847. doi: 10.1161/CIRCULATIONAHA.109.913673. [DOI] [PubMed] [Google Scholar]
- 91. Ganesan V. Pediatric stroke guidelines: where will these take future research and treatment options for childhood stroke? Expert Rev Neurother. 2009;9(5):639–648. doi: 10.1586/ern.09.14. This paper reports translational research guidelines for pediatric stroke.
- 92.Amlie-Lefond C, Bernard TJ, Sebire G, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. 2009;119(10):1417–1423. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berman DR, Liu Y, Barks J, Mozurkewich E. Treatment with docosahexaenoic acid after hypoxia-ischemia improves forepaw placing in a rat model of perinatal hypoxia-ischemia. Am J Obstet Gynecol. 2010;203(4):385, e381–e385. doi: 10.1016/j.ajog.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou Y, Fathali N, Lekic T, et al. Remote limb ischemic postconditioning protects against neonatal hypoxic-ischemic brain injury in rat pups by the opioid receptor/Akt pathway. Stroke. 2011;42(2):439–444. doi: 10.1161/STROKEAHA.110.592162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shankaran S, Laptook A, Wright LL, et al. Whole-body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics. 2002;110(2 Pt 1):377–385. doi: 10.1542/peds.110.2.377. [DOI] [PubMed] [Google Scholar]
- 96.Lei B, Tan X, Cai H, Xu Q, Guo Q. Effect of moderate hypothermia on lipid peroxidation in canine brain tissue after cardiac arrest and resuscitation. Stroke. 1994;25(1):147–152. doi: 10.1161/01.str.25.1.147. [DOI] [PubMed] [Google Scholar]
- 97.Thoresen M, Penrice J, Lorek A, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995;37(5):667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- 98.Battin MR, Dezoete JA, Gunn TR, Gluckman PD, Gunn AJ. Neurodevelopmental outcome of infants treated with head cooling and mild hypothermia after perinatal asphyxia. Pediatrics. 2001;107(3):480–484. doi: 10.1542/peds.107.3.480. [DOI] [PubMed] [Google Scholar]
- 99.Battin MR, Penrice J, Gunn TR, Gunn AJ. Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics. 2003;111(2):244–251. doi: 10.1542/peds.111.2.244. [DOI] [PubMed] [Google Scholar]
- 100.Bergstedt K, Hu BR, Wieloch T. Postischaemic changes in protein synthesis in the rat brain: effects of hypothermia. Exp Brain Res. 1993;95(1):91–99. doi: 10.1007/BF00229658. [DOI] [PubMed] [Google Scholar]
- 101.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558–566. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 102.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yenari M, Kitagawa K, Lyden P, Perez-Pinzon M. Metabolic downregulation: a key to successful neuroprotection? Stroke. 2008;39(10):2910–2917. doi: 10.1161/STROKEAHA.108.514471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robertson NJ, Nakakeeto M, Hagmann C, et al. Therapeutic hypothermia for birth asphyxia in low-resource settings: a pilot randomised controlled trial. Lancet. 2008;372(9641):801–803. doi: 10.1016/S0140-6736(08)61329-X. [DOI] [PubMed] [Google Scholar]
- 106.Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159(5):731–735. e731. doi: 10.1016/j.jpeds.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Higgins RD, Raju T, Edwards AD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159(5):851–858. e851. doi: 10.1016/j.jpeds.2011.08.004. This paper provides rationale for combination therapy for NE.
- 108.Jatana M, Singh I, Singh AK, Jenkins D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2006;59(5):684–689. doi: 10.1203/01.pdr.0000215045.91122.44. [DOI] [PubMed] [Google Scholar]
- 109.Jenkins DD, Chang E, Singh I. Neuroprotective interventions: is it too late? J Child Neurol. 2009;24(9):1212–1219. doi: 10.1177/0883073809338412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for Neuroprotection in Neonatal Encephalopathy: Safety and Pharmacokinetics. Pediatrics. 2012 doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiong T, Qu Y, Mu D, Ferriero D. Erythropoietin for neonatal brain injury: opportunity and challenge. Int J Dev Neurosci. 2011;29(6):583–591. doi: 10.1016/j.ijdevneu.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 112.Dickinson R, Franks NP. Bench-to-bedside review: Molecular pharmacology and clinical use of inert gases in anesthesia and neuroprotection. Crit Care. 2010;14(4):229. doi: 10.1186/cc9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351(19):1985–1995. doi: 10.1056/NEJMra041996. This paper highlights the need for undertanding disease pathophysiology to better enhance treatment intervention in neonatal brain injury.
- 114.Bonifacio SL, Glass HC, Peloquin S, Ferriero DM. A new neurological focus in neonatal intensive care. Nat Rev Neurol. 2011;7(9):485–494. doi: 10.1038/nrneurol.2011.119. [DOI] [PubMed] [Google Scholar]
- 115.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95(2):213–228. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borlongan CV, Kaneko Y, Maki M, et al. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19(4):439–452. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ozawa K, Sato K, Oh I, et al. Cell and gene therapy using mesenchymal stem cells (MSCs) J Autoimmun. 2008;30(3):121–127. doi: 10.1016/j.jaut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 118.Gunn AJ, Gunn TR. The 'pharmacology' of neuronal rescue with cerebral hypothermia. Early Hum Dev. 1998;53(1):19–35. doi: 10.1016/s0378-3782(98)00033-4. [DOI] [PubMed] [Google Scholar]
- 119.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14(11):1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Borlongan CV, Lind JG, Dillon-Carter O, et al. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004;1010(1–2):108–116. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 122.Borlongan CV, Lind JG, Dillon-Carter O, et al. Intracerebral xenografts of mouse bone marrow cells in adult rats facilitate restoration of cerebral blood flow and blood-brain barrier. Brain Res. 2004;1009(1–2):26–33. doi: 10.1016/j.brainres.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 123.Boutin H, Dauphin F, Mackenzie ET, Jauzac P. Differential time-course decreases in nonselective, mu-, delta-, and kappa-opioid receptors after focal cerebral ischemia in mice. Stroke. 1999;30(6):1271–1277. doi: 10.1161/01.str.30.6.1271. discussion 1278. [DOI] [PubMed] [Google Scholar]
- 124.Kevelaitis E, Peynet J, Mouas C, Launay JM, Menasche P. Opening of potassium channels: the common cardioprotective link between preconditioning and natural hibernation? Circulation. 1999;99(23):3079–3085. doi: 10.1161/01.cir.99.23.3079. [DOI] [PubMed] [Google Scholar]
- 125.Tsai SY, Lee CT, Hayashi T, Freed WJ, Su TP. Delta opioid peptide DADLE and naltrexone cause cell cycle arrest and differentiation in a CNS neural progenitor cell line. Synapse. 2010;64(4):267–273. doi: 10.1002/syn.20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borlongan CV, Su TP, Wang Y. Treatment with delta opioid peptide enhances in vitro and in vivo survival of rat dopaminergic neurons. Neuroreport. 2000;11(5):923–926. doi: 10.1097/00001756-200004070-00005. [DOI] [PubMed] [Google Scholar]
- 127.Kaneko Y, Tajiri N, Su TP, Wang Y, Borlongan CV. Combination treatment of hypothermia and mesenchymal stromal cells amplifies neuroprotection in primary rat neurons exposed to hypoxic-ischemic-like injury in vitro: role of the opioid system. PLoS ONE. 2012 doi: 10.1371/journal.pone.0047583. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Borlongan CV, Weiss MD. Baby STEPS: a giant leap for cell therapy in neonatal brain injury. Pediatr Res. 2011 Jul;70(1):3–9. doi: 10.1203/PDR.0b013e31821d0d00. Review. PubMed PMID:21659957;PubMed Central PMCID: PMC3117246. [DOI] [PMC free article] [PubMed] [Google Scholar]