Abstract
Changes in mitochondrial bioenergetics have been proposed to be critical for triggering and effecting anesthetic-induced preconditioning (APC) against cardiac ischemia and reperfusion injury. The objective of this study was to analyze changes in mitochondrial protein levels and link those changes to potential functional changes. A 18O-labeling method was applied for relative comparison of cardiac mitochondrial samples from control and isoflurane exposed rats before and after ischemia and reperfusion. Wistar rats were exposed to isoflurane for 30 min (APC) or did not receive the anesthetic (control). Rats were subjected to 30 min coronary occlusion and 15 min reperfusion without (ischemia) or after APC (ischemia + APC). The following comparisons were made: control vs. APC, control vs. ischemia, and APC vs. ischemia + APC. Proteins were analyzed by liquid chromatography-mass spectrometry. A total of 98 proteins currently annotated as mitochondrial proteins in the UniProt database were positively identified from three replicate experiments. Most of the changes during APC and ischemia occur in complexes of the electron transport chain. Overall, fewer changes in ETC complexes were found when comparing APC with APC + ischemia than when comparing control and ischemia. This corresponds to the preservation of bioenergetics due to APC after ischemia and reperfusion as indicated by preserved ATP level and generation. APC itself induced changes in complex I, but those changes were not correlated with activity changes in mitochondria after APC. Thus, a proteomic mass spectral approach does not only assess quantitative changes without prior knowledge of proteins, but also allows insight into the mechanisms of ischemia and reperfusion injury and APC.
Keywords: anesthetic preconditioning, 18O labeling, mitochondrial proteome, electron transport chain
similar to the powerful endogenous cardioprotective mechanism of ischemic preconditioning (22), pharmacological preconditioning with volatile anesthetics [anesthetic preconditioning (APC)] has emerged as a considerably less risk-bearing but equally effective cardioprotective intervention (27). Brief exposure to volatile anesthetics such as isoflurane, sevoflurane, enflurane, or desflurane protects the myocardium from ischemia and reperfusion injury, when applied prior to prolonged ischemic insult.
Extensive research has advanced the understanding of the mechanisms of APC. It is now recognized that the cellular response to APC involves several prosurvival signaling pathways, including activation of proteins such as protein kinase C (PKC), mitogen-activated protein kinases (MAPK), protein kinase B (Akt), glycogen synthase kinase (GSK)3β, as well as ion channels in the sarcolemmal and mitochondrial membrane (23). Mitochondria have emerged as major target of these prosurvival signaling pathways but act also as a trigger for the protective effect of volatile anesthetics. Although it is known that mitochondria play a vital role in the cardioprotection conferred by volatile anesthetics, the exact target proteins have not been identified. It has long been established that drugs with anesthetic properties have a depressant effect on mitochondrial function (9, 11). The slowing of respiration rates at various complexes in the respiratory chain may cause an electron leak and subsequently lead to augmented generation of ROS. Indeed, similar to ischemic preconditioning, there is compelling evidence for a role of ROS in the phenomenon of APC (25). It is important to note that in contrast to the requirement that minute amounts of ROS must be generated to initiate APC, the protection by APC also involves a decrease in detrimental large bursts of ROS that occur during ischemia and on reperfusion (16). Elevated ROS production on reperfusion, together with increased mitochondrial Ca2+, may cause an opening of the mitochondrial permeability transition pore, leading to both apoptotic and necrotic cell death. However, the molecular basis for isoflurane-induced changes in mitochondrial function is not understood. Direct interaction of anesthetics with the mitochondrial membranes and proteins and/or protein kinase-induced phosphorylation of mitochondrial proteins may very well alter the mitochondrial proteome. Previous studies on protein changes in untreated cardiomyocytes and cardiomyocytes after treatment with diazoxide, adenosine, glycogen synthase 3β-inhibitor, or ischemic preconditioning found that mitochondrial energetic proteins comprised the majority of identified changes (1, 28). Similarly, mitochondrial and stress response proteins were changed in the rat heart after application of volatile anesthetics in vivo (15). On the basis of these initial findings, we aimed in this study to quantitatively analyze changes in protein levels specific to cardiac mitochondria not only after exposure to the preconditioning agent but after exposure to ischemia and reperfusion as well. Quantitative proteomic approaches using isotopic labeling and mass spectrometry allowed for the relative comparison of protein abundances in cardiac mitochondrial samples from control and isoflurane exposed rats before and after ischemia (12, 20).
EXPERIMENTAL PROCEDURES
Animal protocol.
We used male adult Wistar rats (200–250 g) that were housed in accordance with the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (Milwaukee, WI); experiments were conducted in accordance with U.S. National Institutes of Health standards, and all protocols were reviewed and approved by the Institutional Animal Care and Use Commitee. Myocardial infarction was initiated on rats as previously described (18). Animals were anesthetized with thiobutabarbital sodium (100–150 mg/kg), tracheotomy was performed, and trachea was cannulated. Rats then underwent mechanical ventilation with a rodent ventilator (Harvard Apparatus 683, South Natick, MA), with positive end-expiratory pressure using an air-oxygen mixture. After a left thoracotomy was performed in the fifth intercostal space, the pericardium was opened. Overall, there were four experimental groups: Control, APC, ischemia + reperfusion (“ischemia”), and APC + ischemia. The APC groups received isoflurane (Baxter, Deerfield, IL) at 1.0 minimum alveolar concentration [MAC] for 30 min using a vaporizer (Ohio Medical Products 100F, Madison, WI), followed by a 15 min memory period. The control group remained without isoflurane treatment. Both control and APC groups underwent tracheal intubation and thoracotomy with no coronary artery occlusion. In the other two experimental groups, myocardial ischemia (30 min) was induced by occluding left descending coronary artery, and reperfusion (15 min) was initiated by loosening the suture. The short reperfusion protocol was chosen to study the acute changes in mitochondrial proteome following ischemia, or APC + ischemia. After 15 min of reoxygenation, the left ventricular area at risk (left ventricle below the left coronary artery occlusion) was excised and mitochondria were isolated as described in the following section.
Isolation of an enriched rat cardiac mitochondrial fraction.
The freshly isolated cardiac tissue was minced in isolation buffer [in mM: 50 sucrose, 200 mannitol, 5 KH2PO4, 1 ethylene glycol-bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 5 3-(N-morpholino)propanesulfonic acid and 0.1% bovine serum albumin, pH 7.3]. Albumin was added to stabilize mitochondria during homogenization. The tissue was homogenized twice for 5 s with a T 25 disperser (IKA-Werke, Staufen, Germany), and mitochondria isolated by differential centrifugation. The homogenate was first centrifuged at 8,000 g for 10 min; the pellet was removed and resuspended in isolation buffer with a Potter-Elvehjem homogenizer. The pellet was than centrifuged at 750 g for 10 min, and supernatant was collected. The supernatant was centrifuged at 8,000 g for 10 min to pellet the enriched mitochondria. The final mitochondrial pellet was resuspended in isolation buffer without bovine serum albumin. Protein concentration was determined by a modified Lowry assay kit (Bio-Rad, Hercules, CA). For the remainder of this manuscript the enriched mitochondrial fraction obtained by this isolation technique will be referred to as mitochondria.
Mitochondrial protein extraction and digestion.
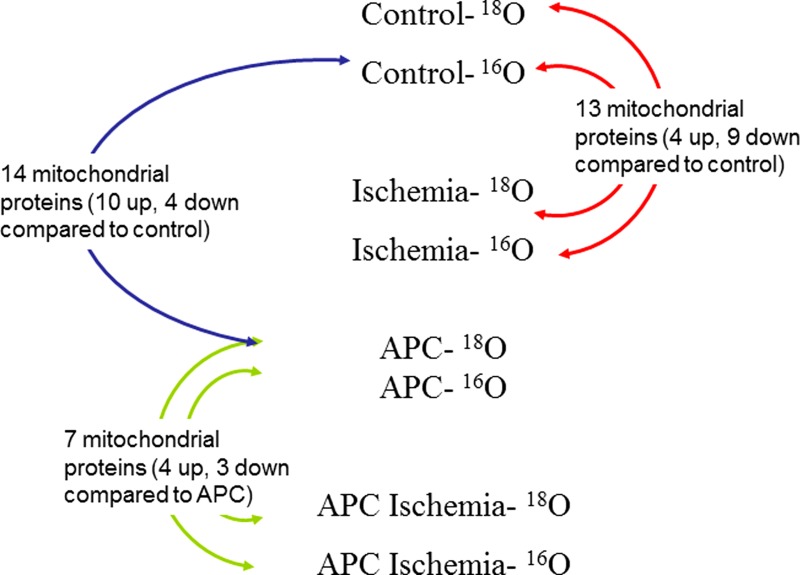
Myocyte mitochondria were lysed using M-PER Mammalian Protein Extraction Reagent (Pierce, Rockford, IL). For each of the four treatments (control, APC, ischemia, and APC + ischemia), 300 μg of soluble mitochondrial protein was reduced with 10 mM dithiothreitol at 37 °C for 30 min. Samples where then incubated without light in 55 mM iodoacetamide at 37°C for 45 min. After reduction and alkylation, samples were precipitated with 4 (vol/vol) ice-cold acetone and incubated on ice for 2 h. Proteins were then labeled with either H216O or H218O (Cambridge Isotope Laboratories, Andover, MA) according to previously published protocols (12, 20). Briefly, precipitated proteins for each treatment were divided equally and resuspended in 250 mM ammonium bicarbonate made with either H216O or H218O. Samples were digested with 2 ng trypsin resuspended in either H216O or ≥95% H218O (Promega, Madison, WI), overnight at 37°C. After the first 12 h of digestion, 20% (vol/vol) acetonitrile plus 2 ng trypsin was added to each sample. The digests were then incubated at 37°C for another 12 h. Digestions were stopped with the addition of 1 μl 1% (vol/vol) formic acid. Alternatively, samples were digested on trypsin spin columns (20). Prior to being desalted on modified C18 Zip-Tips (Millipore, Billerica, MA), samples were mixed 1:1 according to the diagram illustrated in Fig. 1.
Fig. 1.
Isotopic 18O labeling scheme for group comparison of mitochondrial protein isolated from male Wistar rats for liquid chromatography-tandem mass spectrometry. Proteins were labeled during tryptic digestion in ± H218O containing 250 mM ammonium bicarbonate. APC, anesthetic preconditioning.
Mass spectrometry and quantitative analysis.
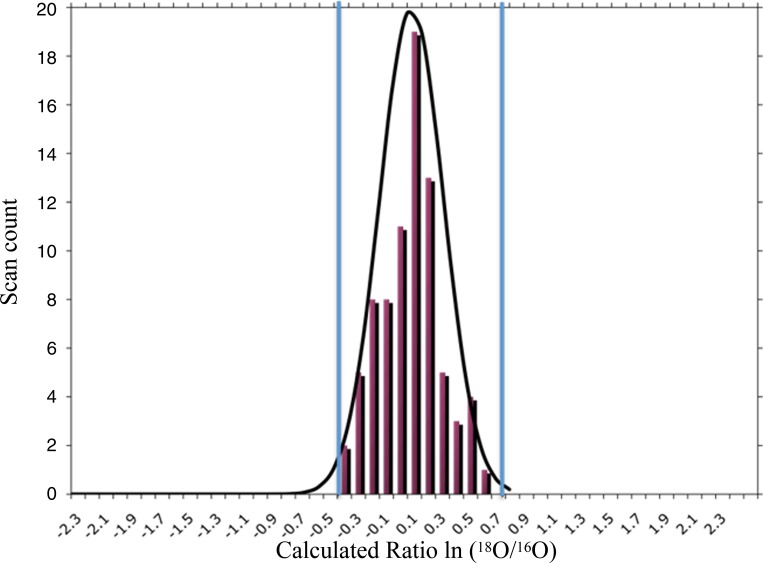
Mass spectral analysis was carried out on a ThermoFinnigan LCQ Deca XP plus ion trap mass spectrometer interfaced with a Surveyor LC system through an RP microcapillary column (100 mm inner diameter) packed with 10 cm of 5 μm C18 RP particles (Phenomenex, Cheshire, UK). Solvent A was 5% (vol/vol) acetonitrile in 0.1% (vol/vol) formic acid and solvent B was 95% (vol/vol) acetonitrile in 0.1% (vol/vol) formic acid. The protein digest (150 μg of each of the four samples outlined in the labeling scheme of Fig. 1) was injected onto the microcapillary column and resolved at the rate of 1 μl/min, by the following gradient conditions: 0–25 min 0–5% B, 25–180 min 5–35% B, 180–220 min 35–65% B, 220–240 min 65–100% B. B at 100% at was held for 10 min, then switched to 100% A, and held for another 40 min. To quantify 16O -and 18O-labeled peptides, the instrument was run initially with a full scan (m/z 400–2,000), followed by a zoom scan of the most intense ion, followed by a data dependent MS/MS scan to sequence the ion. The MS data obtained were interpreted using SEQUEST against uniprot_rodent.fasta database for protein identification (6). Peptides were filtered using the following xcorr values vs. charge state: charge state +1 xcorr 1.8, charge state +2 xcorr 2.0, charge state+3 xcorr 2.2. The described xcorr value vs. charge state optimizes the number of zoom scans available for analysis in ZoomQuant (10). A protein was considered to be positively identified if had a false discovery rate of <5%. A 5% false discovery rate corresponds to a protein being identified with at least two scans and a protein probability score of >0.85 (21). A protein was considered to have a change in regulation if its ratio was at least two standard deviations away from the mean calculated from trials mixing labeled and unlabeled control rat mitochondrial proteins (independent isolations) one to one. Figure 2 illustrates the histogram of results from the control experiments and the estimation of the standard deviation used to determine the significance of protein changes during treatment.
Fig. 2.
Histogram of peptide scan 18O ratios from 2 separately isolated rat heart mitochondria (control) mixed 1 to 1 with a theoretical Gaussian distribution on top. Ratios were calculated by the software program ZoomQuant. The blue lines indicated a level of 2 SDs. A protein was considered to have a change in regulation between 2 groups if its ratio was at least 2 SDs away from the mean calculated from trials mixing labeled and unlabeled control rat mitochondrial proteins (independent isolations) 1 to 1.
Western blotting.
Equal amount (50 μg) of cardiac mitochondria from control, APC, ischemia, and APC + ischemia groups were dissolved in loading buffer and heated at 95°C for 3 min. Samples were separated on a 4–15% polyacrylamide gel (Bio-Rad) and electrophoretically transferred to a nitrocellulose membrane (Bio-Rad). After being blocked with 5% milk in Tris-buffered saline containing 0.1% Tween 20, nitrocellulose membranes were incubated overnight at 4°C in 0.1% Tween 20 containing 5% milk and a 1:1,000 dilution of goat polyclonal antibody against the 75 kDa subunit (NDUFS1) of the NADH oxidoreductase (complex I) (Santa Cruz, Santa Cruz, CA). Membranes were washed three times with 0.1% Tween 20 for 5 min and incubated with a 1:10,000 dilution of horseradish peroxidase-labeled anti-goat immunoglobulin G (Santa Cruz) in 0.1% Tween 20 containing 5% milk. Bound antibody was detected by enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ) on radiographic film. Ponceau 0.1% (Sigma-Aldrich) staining of nitrocellulose membranes was used to verify equal protein loading. Quantitative analysis of the band densities was performed with AlphaImager 2000 software (Alpha Innotech, San Leandro, CA), and bands were normalized to control group.
Complex I (NADH-ubiquinone oxidoreductase) activity assay.
Complex I ETC enzyme activity was measured in solubilized mitochondria isolated from the four different groups. Mitochondria were solubilized with 2% cholic acid and diluted to a final concentration of 100 μg/ml in the experimental buffer [220 mM d-mannitol, 70 mM sucrose, 5 mM MOPS, 2 mM ethylenediaminetetraacetic acid solution (EDTA), and 0.2% fatty acid free BSA (pH 7.4)]. Activity was determined by the rotenone-sensitive reduction of NADH absorbance using decylubiquinone as acceptor. The assay mixture contained 20 μg/ml mitochondrial protein, 50 mM KH2PO4, 0.1 mM EDTA, 0.1% BSA, 0.15 mg/ml asolectin, 2 mM antimycin A, and 0.2 mM NADH in a spectrophotometer cuvette. The reaction was initiated by addition of 75 mM decylubiquinone. The change in absorbance of NADH was measured at 340 nm (ϵ = 6.22 mM−1·cm−1).
Measurement of tissue ATP level and mitochondrial ATP synthesis.
Frozen left ventricular heart tissue samples were homogenized and lysed with Tris-EDTA buffer at pH 7.4. Cardiac ATP concentrations were measured using a Modulus luminometer (Turner Biosystems, Sunnyvale, CA) and a commercially available ATP chemiluminescence assay kit utilizing the reaction of firefly luciferase and luciferin with ATP (Invitrogen).
Mitochondrial ATP synthesis was determined in freshly isolated mitochondria. Reaction solution contained respiration buffer (130 mM KCl, 5 mM K2HPO4, 20 mM MOPS, 2.5 mM EGTA, 1 μM Na4P2O7, and 0.1% BSA, pH 7.4 adjusted with KOH), 0.2 μM diadenosine pentaphosphate, 30 μM ADP, 5 μg/ml mitochondria, 0.1 mg/ml luciferin, and 1.25 μg/ml luciferase. The reaction was initiated by the addition of 5 mM pyruvate and 5 mM malate or 5 mM succinate. The blank was obtained in the absence of substrate. Chemiluminescence was measured in the luminometer at room temperature for 120 s. For both assays, the standard curve was obtained with defined ATP concentrations, from which the level and the rate of mitochondrial ATP production were calculated.
Statistical analysis for Western blotting and activity assays.
Data are reported as means ± SD, and n refers to the number of experiment with samples from different animals. Comparisons between groups were performed by one-way ANOVA and use of Tukey test for post hoc analysis. Differences with P < 0.05 were considered significant.
RESULTS
In our mass spectral analysis of the cardiac mitochondrial samples, a total of 218 proteins were positively identified by SEQUEST with a false discovery rate of <5%, as described in our methods. Each experimental sample pair was analyzed in at least three replicate experiments. See Supplementary Material for a detailed list of complete results.1 Of the 218 proteins identified, 98 are currently annotated as mitochondrial proteins in the UniProt database, while 10 currently have no available annotation. Using ZoomQuant to analyze protein abundances, we quantified the 18O/16O ratio for each peptide identified by SEQUEST on 31% of proteins annotated as mitochondrial proteins (10). The top 12 proteins identified all had a sum of >100 scans when the three replicate experiments were combined. Eleven of the 12 most abundant proteins are annotated in the UniProt database as mitochondrial proteins, and most belong to either the ATP synthase or ADP-ATP translocase. The most abundant protein identified in all three replicates was ATPA, part of ATP synthase alpha chain, with almost 500 scans in total and 31 unique peptides.
Using the ratio data generated by ZoomQuant, we identified 14 mitochondrial proteins that are consistently and significantly up- or downregulated in the APC group compared with control samples. We also found 13 mitochondrial proteins in ischemia-treated rats that showed a significant change in abundance compared with control samples. Lastly, a total of seven mitochondrial proteins were altered, when the APC + ischemia group was compared with rats that just received APC treatment (Table 1).
Table 1.
Protein ratios from 18O-labeled peptides isolated from rat cardiac mitochondria
| APC vs. Control |
Control vs. Ischemia |
APC vs. APC+Ischemia |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Name | Gene Symbol | Uniprot ID | Unique Peptides | Ratio APC/Control | SE | Unique Peptides | Ratio Control/Ischemia | SE | Unique Peptides | Ratio APC/APC+Ischemia | SE |
| 2,4-dienoyl-CoA reductase | Decr1 | Q64591 | 4 | 2.66 | 0.289 | ||||||
| Aconitate hydratase | Aco2 | Q9ER34 | 10 | 1.12 | 0.054 | 3 | 1.44 | 0.156 | |||
| ADP/ATP translocase | Slc25a4 | Q05962 | 3 | 2.13 | 0.116 | 2 | 2.65 | 0.387 | |||
| ATP synthase subunit alpha | Atp5a1 | P15999 | 10 | 0.98 | 0.023 | 4 | 1.85 | 0.098 | 4 | 0.52 | 0.056 |
| ATP synthase subunit b | Atp5f1 | P19511 | 2 | 0.46 | 0.003 | ||||||
| ATP synthase subunit beta | Atp5b | P10719 | 9 | 1.02 | 0.074 | 8 | 1.96 | 0.132 | 14 | 0.45 | 0.002 |
| ATP synthase subunit d | Atp5h | P31399 | 4 | 0.69 | 0.369 | 2 | 1.24 | 0.002 | 0.51 | 0.032 | |
| ATP synthase subunit delta | Atp5d | P35434 | 2 | 0.89 | 0.007 | ||||||
| ATP synthase subunit O | Atp5o | Q06647 | 2 | 0.85 | 0.372 | ||||||
| Citrate synthase | Cs | Q8VHF5 | 2 | 1.79 | 0.076 | ||||||
| Cytochrome b-c1 complex subunit 1 | Uqcrc1 | Q68FY0 | 2 | 0.58 | 0.168 | ||||||
| Cytochrome b-c1 complex subunit 2 | Uqcrc2 | P32551 | 2 | 0.95 | 0.399 | 1 | 1.27 | 0.627 | 2 | 1.84 | 0.529 |
| Cytochrome c | Cycs | P62898 | 3 | 0.89 | 0.006 | ||||||
| Cytochrome c oxidase polypeptide 7A2 | Cox7a2 | P35171 | 2 | 1.24 | 0.568 | ||||||
| Cytochrome c oxidase subunit 2 | Mtco2 | P00406 | 2 | 0.75 | 0.001 | 1 | 2.47 | 0.034 | 2 | 0.92 | 0.061 |
| Cytochrome c oxidase subunit 5B | Cox5b | P12075 | 2 | 1.65 | 0.096 | ||||||
| Electron transfer flavoprotein subunit alpha | Etfa | P13803 | 2 | 1.96 | 0.194 | 0.005 | |||||
| Electron transfer flavoprotein oxidoreductase | Etfdh | Q6UPE1 | 1 | 1.18 | |||||||
| Glutamate dehydrogenase 1 | Glud1 | P10860 | 2 | 1.92 | 0.005 | ||||||
| Isocitrate dehydrogenase [NADP] | Idh2 | P54071 | 8 | 1.27 | 0.784 | 2 | 1.17 | 0.236 | |||
| Malate dehydrogenase | Mdh2 | P04636 | 11 | 1.18 | 0.002 | 2 | 0.63 | 0.007 | |||
| NADH dehydrogenase 1 alpha subunit 4 | Ndufa4 | Q62425 | 2 | 0.34 | 0.007 | 1 | 0.52 | 0.045 | |||
| NADH dehydrogenase 1 alpha subunit 9 | Ndufa9 | Q9DC69 | 1 | 0.45 | 0.002 | 3 | 0.78 | 0.318 | |||
| NADH dehydrogenase 1 subunit C2 | Ndufc2 | Q9CQ54 | 2 | 0.67 | 0.209 | ||||||
| NADH dehydrogenase flavoprotein 2 | Ndufv2 | P19234 | 3 | 1.97 | 0.178 | 2 | 0.57 | 0.194 | 2 | 0.66 | 0.093 |
| NADH oxidoreductase 75 kDa subunit | Ndufs1 | Q66HF1 | 4 | 1.45 | 0.085 | 2 | 1.35 | 0.005 | 2 | 1.46 | 0.063 |
| Pyruvate dehydrogenase E1 subunit beta | Pdhb | P49432 | 3 | 0.59 | 0.003 | ||||||
| Stress-70 protein | Hspa9 | P48721 | 1 | 0.15 | 0.001 | ||||||
| Succinyl-CoA ligase subunit alpha | Suclg1 | P13086 | 2 | 0.34 | 0.002 | ||||||
| Long-chain specific acyl-CoA dehydrogenase | Acadvl | P45953 | 2 | 1.03 | 0.003 | ||||||
Peptides were run on LC-MS/MS and identified using SEQUEST. Proteins enzymatically labeled with 18O and 16O were averaged over 3 replicates, and all identified peptides for each treatment pair. Treatments were: control rats (Control), rats preconditioned with isoflurane (APC), rats subjected to ischemia and reperfusion (Ischemia), and rats preconditioned with isoflurane before being subjected to ischemia and reperfusion (APC + Ischemia). Ratios in boldface indicate that the protein is significantly up- or downregulated based on the ratio being at least 2 SD away from a mean calculated from comparing 2 control rats against each other.
From these analyses, it is apparent that most of the change in protein abundance during both APC and ischemia treatments compared with control rats occur in the complexes of the electron transport chain (ETC): NADH dehydrogenase (complex I), cytochrome bc1 (cty bc1, complex III), cytochrome c oxidase (cty c oxidase, complex IV), and ATP synthase. Several subunits of NADH dehydrogenase were upregulated after ischemia, except for the 75 kDa subunit, which showed downregulation. In contrast, both cty bc1 and cty c oxidase subunits were shown to be present at lower levels after ischemia. Likewise, subunits of the ATP synthase as well as ADP/ATP translocase (ANT) were mostly downregulated after ischemia (Table 1). APC itself had mixed effects on the presence of NADH dehydrogenase subunits, though the largest subunit (75 kDa subunit) containing the active NADH oxidation side is upregulated after APC. APC also showed mixed effects on the two subunits identified as cty bc1. APC itself did increase ANT in mitochondria, but ATP synthase remained unchanged. Overall, fewer changes for ETC complex subunits were detected, when the APC group was compared with the APC + ischemia group. Notably three subunits of ATP synthase were increased in the APC + ischemia group over the APC group. Another interesting protein found to be regulated in our experiment was mortalin (mt-hsp70), which was found to be significantly reduced in mitochondria after isoflurane exposure (Table 1).
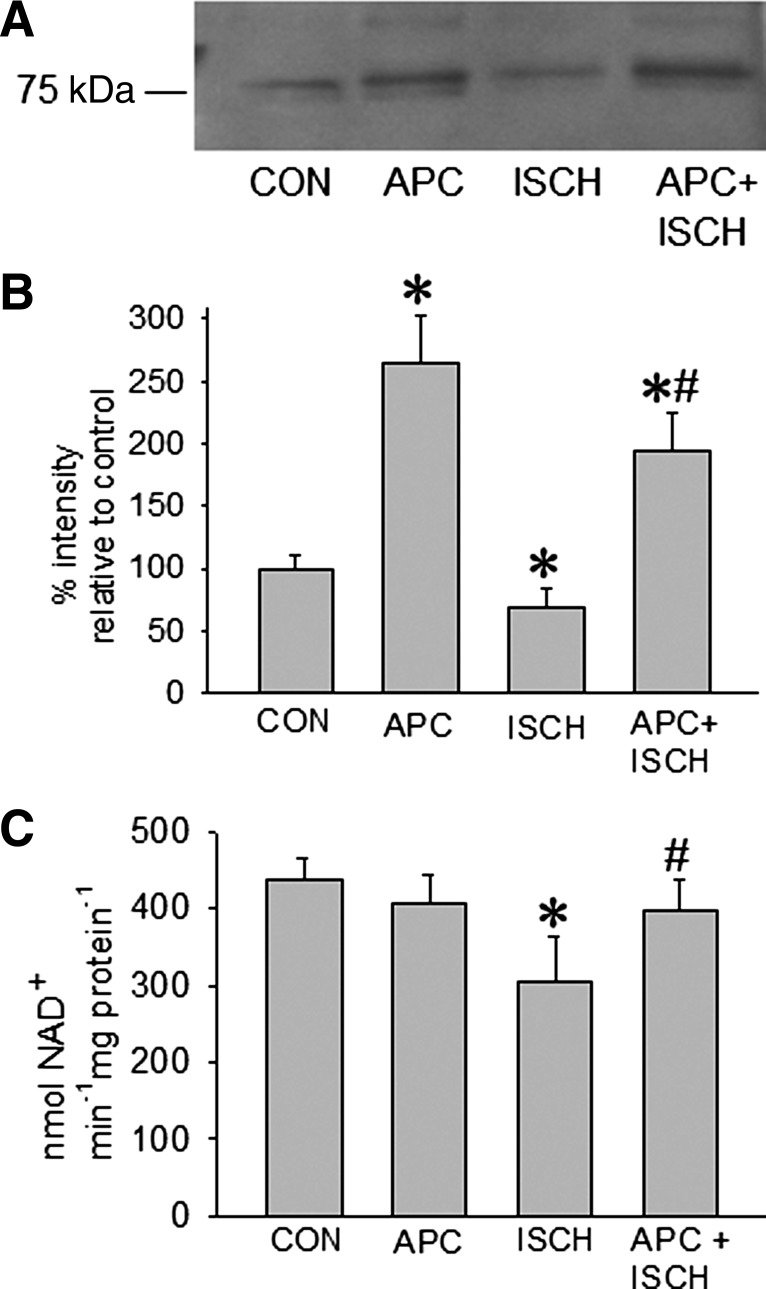
To verify data from mass spectrometry, we performed Western blotting against the 75 kDa subunit of NADH dehydrogenase. Similarly as described above, the level of this subunit was lower in the ischemic group compared with the control but higher in the APC group (Fig. 3, A and B). To assess consequences on complex NADH dehydrogenase activity after isoflurane exposure, we quantified the relative activities of the ETC complexes in cholic acid-solubilized mitochondria (Fig. 3C). After ischemia, NADH dehydrogenase activity was decreased (306 ± 56 nmol NADH/min−1·mg protein−1) compared with control (437 ± 28 nmol NADH/min−1·mg protein−1). On the other hand, APC alone or APC after ischemia stabilized NADH dehydrogenase activity close to control levels (405 ± 38 and 397 ± 41 nmol NADH/min−1·mg protein−1).
Fig. 3.
Western blot (A) against the 75 kDa subunit (NDUFS1) of NADH dehydrogenase (complex I) in control (CON), APC, ischemia and reperfusion (ISCH), and APC followed by ischemia and reperfusion (APC+ISCH) groups. B: the data (means ± SD) summary resulting from densitometry of 3 different blots. C: attenuated enzymatic activity of NADH dehydrogenase (complex I) in the ISCH group that was restored by APC. NADH dehydrogenase activity was measured in solubilized cardiac mitochondria isolated from the four experimental groups. Data are means ± SD, n = 4/group. *Significantly (P < 0.05) different from CON; #significantly different from APC.
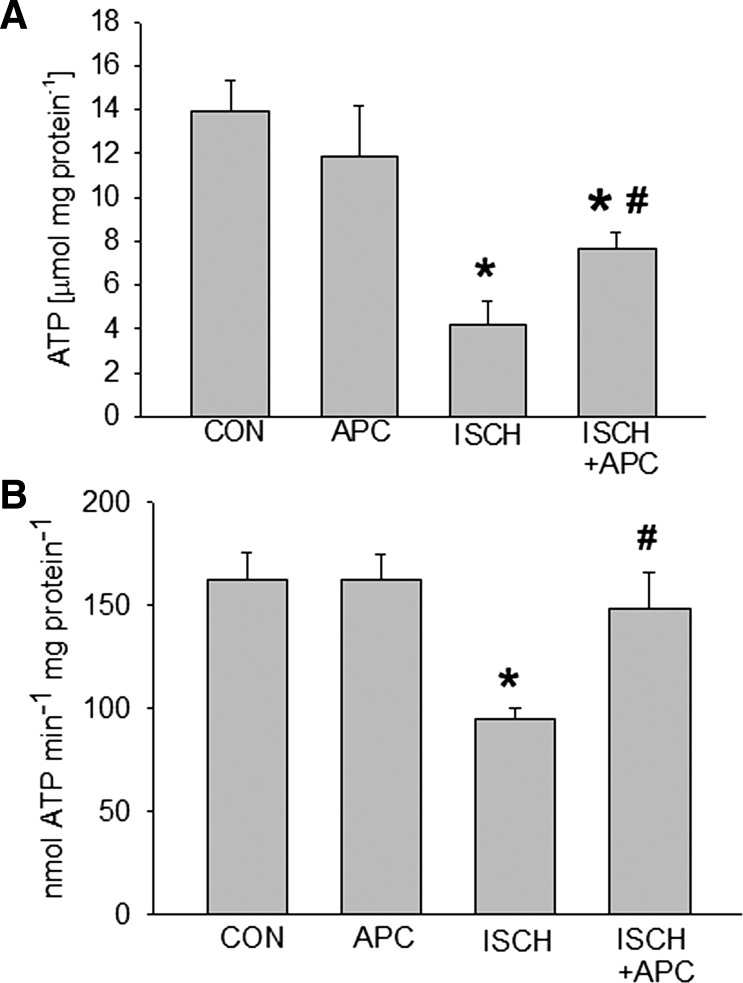
We further measured both ATP levels and rate of mitochondrial ATP generation in our experimental groups. While APC itself did not alter ATP levels significantly (13.9 ± 1.4 μmol/mg protein) compared with control (11.9 ± 2.3 μmol/mg protein) in left ventricular tissue, it prevented the decline of ATP that was observed in the ischemia group (7.6 ± 1.4 μmol/mg protein compared with 4.1 ± 1.8 μmol/mg protein) (Fig. 4A). Similarly, APC did not affect mitochondrial ATP generation in the absence of ischemia (164 ± 13 nmol ATP·min−1·mg protein−1 in APC group compared with 155 ± 21 nmol ATP·min−1·mg protein−1 in control) but reversed partially ischemia and reperfusion induced decline of ATP generation (95 ± 5 nmol ATP·min−1·mg protein−1 in APC group compared with 148 ± 17 nmol ATP·min−1·mg protein−1) (Fig. 4B).
Fig. 4.
Left ventricular ATP level (A) and mitochondrial ATP production rate (B) were quantified by luciferase-based assays in CON, APC, ISCH, and APC+ISCH groups. APC restored ATP level and production after ischemia and reperfusion. Data are means ± SD, n = 4/group. *Significantly (P < 0.05) different from CON; #significantly different from APC.
DISCUSSION
In this study, we analyzed the consequence of APC and/or ischemia on changes in the mitochondrial proteome. APC is a powerful strategy against ischemia and reperfusion injury, and mitochondria emerged as the pivotal players in the complex signaling pathways leading to cardioprotection by volatile anesthetics. Besides evidence suggesting better preservation of mitochondrial function after ischemia and reperfusion in the preconditioned heart, mitochondria also actively contribute as triggers to the mechanism of cardiac protection by APC and other forms of preconditioning. In fact, manipulation of the ETC at different complexes during ischemia and/or reperfusion has been suggested to be a common theme of different cardioprotective strategies (3).
In our mass spectral analysis of changes in the proteome between control and APC, control and ischemia, and APC and APC + ischemia groups, a total of 31 mitochondrial proteins were identified to be up- or downregulated. Thirteen of these proteins are directly involved in electron transport, and seven proteins play a pivotal role in ATP synthesis.
Volatile anesthetics have been suggested to affect mitochondrial function through ETC complexes and mitochondrial cation channels. We and others have shown that isoflurane inhibits the ETC at the level of complex I, which potentially may lead to a small rise in ROS production (13, 24). In fact, a small burst of ROS as signaling molecules, originating from the mitochondria, was reported to be essential for triggering the preconditioning mechanism (13). On the other hand, the large amounts of ROS produced by the ETC during ischemia-reperfusion injury in the untreated heart have been shown to be deleterious and are reduced by APC, likely through changes at the level of the ETC (13, 16). Cardiac mitochondria represent a major source of ROS, but the specific sites and mechanisms of electron leak and O2·− generation along the ETC remain controversial. The use of different substrates and ETC inhibitors has led to the assumption that complexes I and III are the primary sites of O2·− generation (4). Complex III is known to release O2·− into the intermembrane space through the oxidation of ubisemiquinone, a radical intermediate formed in the Q-cycle of the complex and is coupled to the reduction of the bc1 segment (5). In contrast, O2·− generated from complex I is released to the matrix side. APC may affect electron flow through the ETC either through direct interaction of the volatile anesthetic with ETC complexes or through modifications of their composition. In our study of the mitochondrial proteome, we found changes in the levels of ETC complexes after APC. However, we did not detect an increase of ROS production in mitochondria from APC-treated rat hearts as we reported previously when mitochondria were measured directly in the presence of isoflurane (13). This suggests that the isoflurane-induced increase in ROS required for triggering prosurvival pathways responsible for APC may be caused by a direct action of isoflurane on ETC complex I and/or III. On the other hand, changes observed in content of ETC complex subunits after ischemia may very well be leading to the damaging increase of ROS observed after ischemia and reperfusion of the heart since we were able to detect complex I activity differences after ischemia. For example, a reduction in the content of cytochrome c oxidase (complex IV) subunit 2 in ischemia group vs. control could cause a back-up of electrons in the ETC and generation of ROS. Interestingly, no change in any complex IV subunit was observed when APC and APC + ischemia groups were compared. Overall, when APC and APC + ischemia groups were compared, we found only one subunit of the ETC complexes changed (NADH dehydrogenase 75 kDa subunit). Fewer changes in ETC composition may contribute to the reduced ROS production in the heart during reperfusion after APC compared with the untreated heart, ultimately leading to preserved mitochondrial bioenergetics. Preservation of tissue ATP level and mitochondrial ATP generation after ischemia may partially also be connected to subunit alterations of ATP synthase discovered in our proteomics study. The decline in ATP level and activity after ischemia and reperfusion compared with control could be explained by the lower level of several ATP synthase subunits after ischemia. On the other hand, after APC and ischemia and reperfusion three of those subunits were increased compared with the APC group. Regulation of ATP content is likely not only explained by the composition and activity of ATP synthase since other components of the ETC and/or ATP consuming enzymes all contribute to ATP level regulation.
Alterations of protein abundance observed in our experimental APC groups compared with groups without isoflurane are more likely due to changes in protein stabilization or degradation rather than changes in the rate of protein synthesis. Volatile anesthetics are known to alter protein stability (14). Changes in protein synthesis during 30 min isoflurane exposure and 15 min memory period would be of limited significance. Indeed, cardioprotection by pharmacological preconditioning is not blocked by inhibition of protein synthesis (19). In addition, APC, like other preconditioning stimuli, is well known to induce various signaling kinases that also target mitochondria. Such signaling events include PKC isoforms, mitogen-activated kinases and GSK3β (23). While changes in protein phosphorylation are known to influence protein stability, the protein targets of these kinases in mitochondria are not well established. It may involve components and/or regulators of the mitochondrial permeability transition pore, such as voltage-dependent anion channels and ANT, which were shown to bind PKCϵ (2). In fact, we found that ANT was upregulated significantly in the APC and downregulated in the ischemia group compared with control. While the role of ANT in the permeability transition pore remains controversial, an increase in ANT could allow for a more efficient ADP/ATP exchange when energy requirement arises during reperfusion. A phosphorylation site relevant for APC was recently identified within ANT and suggested to contribute to cardioprotection (7). Phosphorylation of other proteins participating in the oxidative phosphorylation have been reported, including complex IV, where phosphorylation of subunit I was associated with a decrease of mitochondrial membrane potential and ROS formation (17). A comprehensive, quantitative comparison of phosphorylation of mitochondrial proteins has yet to be performed in ischemia and reperfusion injury and cardioprotective preconditioning strategies (8).
Similar to our study presented here, various other preconditioning strategies have been examined for alterations in the mitochondrial proteome. In a comparison of control and adenosine or diazoxide preconditioned cardiomyocytes, mitochondrial energetic proteins comprised the majority of identified changes, including seven subunits involved in oxidative phosphorylation (1). In another study, Wong et al. (28) compared the cardiac mitochondrial proteome from ischemic preconditioned mouse hearts and from hearts treated with a GSK3 inhibitor with control hearts. While numerous proteins involved in oxidative phosphorylation were altered in both treated heart groups compared with control, only five proteins were found to be consistently changed when comparing ischemic preconditioning and GSK3 inhibition. Overall, the overlap of specific protein changes detected between various preconditioning strategies is low. Among the proteins commonly regulated between APC and other pharmacological preconditioning strategies is the NADH dehydrogenase flavoprotein 2 subunit, which was increased after treatment with isoflurane, adenosine, diazoxide, and GSK treatment, but decreased after ischemic preconditioning. Similarly, the NADH dehydrogenase 75 kDa subunit was upregulated after isoflurane and GSK treatment and downregulated after ischemic preconditioning.
While the majority of proteins found to be altered by APC is directly linked to oxidative phosphorylation, other changes are also worth to be addressed. We found citrate synthase and isocitrate dehydrogenase, two enzymes of the citrate cycle, increased after APC. The mitochondrial presence of mortalin (mitochondrial HSP70, GRP75) was significantly reduced in mitochondria after isoflurane exposure. Mortalin has been demonstrated to be an important contributor to protection in preconditioning (26).
Some of these apparent differences may be due to differences in protein isolation or mass spectral analysis strategies or instrumentation, while others may reflect underlying functional and mechanistic differences. While mass spectral proteomic profiling has emerged as one of the most reproducible and reliable tools for the comprehensive characterization of quantitative protein changes, the methodology primarily identifies differences in abundant proteins, especially utilizing the ion trap instrument used in this study. Low-abundance proteins such as some mitochondrial ion channels, kinases or other signaling molecules are difficult to detect, due to the low expression levels and the transient and rapid changes in abundance. Thus, our analysis (and the mass spectral characterization of proteomic changes using other preconditioning approaches) is only beginning to characterize the complex changes in mitochondria, and this analysis will require the further examination of both short-term and long-term alterations. However, our analysis and the results of other similar studies illustrate the potential of these proteomic mass spectral approaches to identify quantitative changes without prior knowledge of proteins involved in the cellular response, a significant advantage over traditional antibody-mediated protein quantification approaches.
Overall we have demonstrated that liquid chromatography-tandem mass spectrometry and enzymatic 18O labeling provides a powerful tool to characterize changes of mitochondrial proteins under various conditions of ischemia and reperfusion injury. Changes were mostly observed in subunits of the oxidative phosphorylation complexes in a manner consistent with models of APC-induced conservation of energy maintenance during reperfusion. Future studies will help validate the functional effect of these changes, and further examine proteomic changes at different time points during APC, ischemia, and reperfusion in our rat model.
GRANTS
This work was supported in part by the National Institutes of Health Grants P01GM-066730 (to Z. J. Bosnjak), R01HL-098490 (to M. Bienengraeber), and N01-HV-28182 (to M. Olivier)] and by the Advancing a Healthier Wisconsin Program (to M. Bienengraeber and M. Olivier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.B. and M.O. conception and design of research; M.B., M.P.-H., N.H., and T.M.B. performed experiments; M.B., M.P.-H., T.M.B., and M.O. analyzed data; M.B., M.P.-H., N.H., T.M.B., and M.O. interpreted results of experiments; M.B. and M.P.-H. drafted manuscript; M.B., M.P.-H., Z.J.B., and M.O. approved final version of manuscript; M.P.-H. prepared figures; Z.J.B. and M.O. edited and revised manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mary B. Ziebell, Research Technologist, for technical assistance.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Arrell DK, Elliott ST, Kane LA, Guo Y, Ko YH, Pedersen PL, Robinson J, Murata M, Murphy AM, Marban E, Van Eyk JE. Proteomic analysis of pharmacological preconditioning: novel protein targets converge to mitochondrial metabolism pathways. Circ Res 99: 706–714, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase C epsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res 92: 873–880, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol 46: 804–810, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294: C460–C466, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278: 36027–36031, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Ducret A, Van Oostveen I, Eng JK, Yates JR, 3rd, Aebersold R. High throughput protein characterization by automated reverse-phase chromatography/electrospray tandem mass spectrometry. Protein Sci 7: 706–719, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng J, Zhu M, Schaub MC, Gehrig P, Roschitzki B, Lucchinetti E, Zaugg M. Phosphoproteome analysis of isoflurane-protected heart mitochondria: phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc Res 80: 20–29, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Foster DB, Van Eyk JE, Marban E, O'Rourke B. Redox signaling and protein phosphorylation in mitochondria: progress and prospects. J Bioenerg Biomembr 41: 159–168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall GM, Kirtland SJ, Baum H. The inhibition of mitochondrial respiration by inhalational anaesthetic agents. Br J Anaesth 45: 1005–1009, 1973. [DOI] [PubMed] [Google Scholar]
- 10. Halligan BD, Slyper RY, Twigger SN, Hicks W, Olivier M, Greene AS. ZoomQuant: an application for the quantitation of stable isotope labeled peptides. J Am Soc Mass Spectrom 16: 302–306, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hanley PJ, Ray J, Brandt U, Daut J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol 544: 687–693, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hicks WA, Halligan BD, Slyper RY, Twigger SN, Greene AS, Olivier M. Simultaneous quantification and identification using 18O labeling with an ion trap mass spectrometer and the analysis software application “ZoomQuant”. J Am Soc Mass Spectrom 16: 916–925, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirata N, Shim YH, Pravdic D, Lohr NL, Pratt PF, Jr, Weihrauch D, Kersten JR, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane differentially modulates mitochondrial reactive oxygen species production via forward versus reverse electron transport flow: implications for preconditioning. Anesthesiology 115: 531–540, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johansson JS, Zou H, Tanner JW. Bound volatile general anesthetics alter both local protein dynamics and global protein stability. Anesthesiology 90: 235–245, 1999. [DOI] [PubMed] [Google Scholar]
- 15. Kalenka A, Maurer MH, Feldmann RE, Kuschinsky W, Waschke KF. Volatile anesthetics evoke prolonged changes in the proteome of the left ventricule myocardium: defining a molecular basis of cardioprotection? Acta Anaesthesiol Scand 50: 414–427, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Kevin LG, Novalija E, Riess ML, Camara AK, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg 96: 949–955, 2003. [DOI] [PubMed] [Google Scholar]
- 17. Lee I, Bender E, Kadenbach B. Control of mitochondrial membrane potential and ROS formation by reversible phosphorylation of cytochrome c oxidase. Mol Cell Biochem 234–235: 63–70, 2002. [PubMed] [Google Scholar]
- 18. Ludwig LM, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Protein kinase C translocation and Src protein tyrosine kinase activation mediate isoflurane-induced preconditioning in vivo: potential downstream targets of mitochondrial adenosine triphosphate-sensitive potassium channels and reactive oxygen species. Anesthesiology 100: 532–539, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Matsuyama N, Leavens JE, McKinnon D, Gaudette GR, Aksehirli TO, Krukenkamp IB. Ischemic but not pharmacological preconditioning requires protein synthesis. Circulation 102: III312–III318, 2000. [DOI] [PubMed] [Google Scholar]
- 20. Mirza SP, Olivier M. Methods and approaches for the comprehensive characterization and quantification of cellular proteomes using mass spectrometry. Physiol Genomics 33: 3–11, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mueller LN, Brusniak MY, Mani DR, Aebersold R. An assessment of software solutions for the analysis of mass spectrometry based quantitative proteomics data. J Proteome Res 7: 51–61, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. [DOI] [PubMed] [Google Scholar]
- 23. Pratt PF, Jr, Wang C, Weihrauch D, Bienengraeber MW, Kersten JR, Pagel PS, Warltier DC. Cardioprotection by volatile anesthetics: new applications for old drugs? Curr Opin Anaesthesiol 19: 397–403, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Pravdic D, Hirata N, Baber L, Sedlic F, Bosnjak ZJ, Bienengraeber M. Complex I and ATP synthase mediate membrane depolarization and matrix acidification by isoflurane in mitochondria. Eur J Pharmacol 690: 149–157, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanaka K, Weihrauch D, Kehl F, Ludwig LM, LaDisa JF, Jr, Kersten JR, Pagel PS, Warltier DC. Mechanism of preconditioning by isoflurane in rabbits: a direct role for reactive oxygen species. Anesthesiology 97: 1485–1490, 2002. [DOI] [PubMed] [Google Scholar]
- 26. Tupling AR, Bombardier E, Vigna C, Quadrilatero J, Fu M. Interaction between Hsp70 and the SR Ca2+ pump: a potential mechanism for cytoprotection in heart and skeletal muscle. Appl Physiol Nutr Metab 33: 1023–1032, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Warltier DC, al-Wathiqui MH, Kampine JP, Schmeling WT. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology 69: 552–565, 1988. [DOI] [PubMed] [Google Scholar]
- 28. Wong R, Aponte AM, Steenbergen C, Murphy E. Cardioprotection leads to novel changes in the mitochondrial proteome. Am J Physiol Heart Circ Physiol 298: H75–H91, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.