Abstract
Exercise training ameliorates age-related impairments in endothelium-dependent vasodilation in skeletal muscle arterioles. Additionally, exercise training is associated with increased superoxide production. The purpose of this study was to determine the role of superoxide and superoxide-derived reactive oxygen species (ROS) signaling in mediating endothelium-dependent vasodilation of soleus muscle resistance arterioles from young and old, sedentary and exercise-trained rats. Young (3 mo) and old (22 mo) male rats were either exercise trained or remained sedentary for 10 wk. To determine the impact of ROS signaling on endothelium-dependent vasodilation, responses to acetylcholine were studied under control conditions and during the scavenging of superoxide and/or hydrogen peroxide. To determine the impact of NADPH oxidase-derived ROS, endothelium-dependent vasodilation was determined following NADPH oxidase inhibition. Reactivity to superoxide and hydrogen peroxide was also determined. Tempol, a scavenger of superoxide, and inhibitors of NADPH oxidase reduced endothelium-dependent vasodilation in all groups. Similarly, treatment with catalase and simultaneous treatment with tempol and catalase reduced endothelium-dependent vasodilation in all groups. Decomposition of peroxynitrite also reduced endothelium-dependent vasodilation. Aging had no effect on arteriolar protein content of SOD-1, catalase, or glutathione peroxidase-1; however, exercise training increased protein content of SOD-1 in young and old rats, catalase in young rats, and glutathione peroxidase-1 in old rats. These data indicate that ROS signaling is necessary for endothelium-dependent vasodilation in soleus muscle arterioles, and that exercise training-induced enhancement of endothelial function occurs, in part, through an increase in ROS signaling.
Keywords: hydrogen peroxide, superoxide, peroxynitrite, nitric oxide, acetylcholine
endothelial function in the skeletal muscle resistance vasculature declines with age primarily due to decreased nitric oxide (NO) bioavailability (10, 38, 51, 55). In feed arteries from soleus muscle, reductions in NO-dependent vasodilation are accompanied by reduced expression of endothelial NO synthase (eNOS) (65). In contrast, our laboratory has previously reported that NO-mediated vasodilation of soleus muscle arterioles declines with advancing age, despite an increase in eNOS protein levels (51). Thus the age-related decline in bioavailability of NO may be dependent on numerous other factors that regulate both NO production and degradation. eNOS activity, and subsequent NO production, is regulated by availability of substrate and cofactors, by protein-protein interactions, and by coordinated phosphorylation and dephosphorylation of eNOS (8, 17–19). The availability of NO is also dependent on the presence of cellular superoxide (O2−), a by-product of cellular respiration, which reacts readily with NO to produce peroxynitrite (ONOO−) (50).
In contrast to the effects of O2− to inhibit NO-mediated vasodilation, both O2− and O2−-derived reactive oxygen species (ROS) have also been shown to exhibit vasodilator properties. For example, H2O2 has been reported to produce hyperpolarization and relaxation of vascular smooth muscle (33, 36). H2O2 also upregulates eNOS protein expression and activity (58, 68). Therefore, old age-related changes in ROS levels could conceivably have both positive and negative effects on endothelium-dependent vasodilation in the skeletal muscle microcirculation.
Exercise training has been shown to reverse old age-related reductions in NO bioavailability and improve endothelium-dependent vasodilation. In skeletal muscle arterioles of both young and aged rats, exercise training increases eNOS protein expression and enhances endothelium-dependent vasodilation (51). Additionally, exercise training has been reported to increase vascular expression of superoxide dismutase (SOD), the predominant regulator of cellular O2−, in large conduit arteries. Rush et al. (46) demonstrated that in aortic endothelial cells from young pigs, SOD-1 protein abundance and activity increased with training. Although SOD-1 expression declines with age in skeletal muscle (65) and mesentery (53) resistance arteries, the interactive effects of age and exercise training on the expression of antioxidant proteins have not been thoroughly investigated in the skeletal muscle resistance vasculature.
Therefore, the purpose of this study was to determine the role of O2−-derived ROS in mediating endothelium-dependent vasodilation in skeletal muscle resistance arterioles from young and old, sedentary and exercise-trained rats. We tested the hypothesis that age-related increases in O2−-derived ROS would contribute to endothelium-dependent vasodilation of skeletal muscle arterioles, despite an overall decline in endothelial function. We further hypothesized that exercise training would improve endothelium-dependent vasodilation, in part, through improved regulation of O2−-derived ROS signaling.
MATERIALS AND METHODS
Animals.
All procedures in this study were approved by the Institutional Animal Care and Use Committees at West Virginia University and University of Florida. All methods complied fully with guidelines set in the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), revised 1996]. Young (3 mo) and old (22 mo) male Fischer 344 rats were obtained from Harlan (Indianapolis, IN), housed under a 12:12-h light-dark cycle, and given food and water ad libitum. Fischer 344 rats were chosen because cardiovascular function decreases with age in these rats, without the development of atherosclerosis or hypertension.
Exercise training.
Rats were habituated to treadmill exercise by walking on a motor-driven treadmill at 5 m/min (0° incline), 5 min/day, for 3 days. Young and old rats were randomly assigned to either a control sedentary (SED) group (young SED, n = 55, and old SED, n = 49) or an exercise-trained (ET) group (young ET, n = 48, and old ET, n = 35). ET rats performed treadmill exercise at 15 m/min (15° incline), 5 days/wk, for 10–12 wk. The duration of exercise was gradually increased in the first 3 wk until a 60-min duration was reached. The rats continued to exercise 5 days/wk for 60 min/day for the remainder of the 10- to 12-wk training period. Only rats that completed the entire 10-wk training protocol were used to study vascular responses. Vascular responses were determined at least 24 h after the last exercise bout in ET rats (51). To determine the efficacy of the training protocol, the soleus muscle was stored at −80°C for determination of citrate synthase activity, a measure of muscle oxidative capacity.
Microvessel preparation.
Rats were anesthetized with isoflurane (3%/O2 balance) and euthanized by decapitation. The gastrocnemius-plantaris-soleus muscle group was dissected free from both hindlimbs and placed in a cold (4°C), filtered physiological saline solution (PSS), as previously described (38, 51). With the aid of a dissecting microscope, first-order (1A) arterioles were dissected free of the soleus muscle, which is composed primarily of high-oxidative fibers, as described previously (38). The arterioles were then transferred to a chamber containing PSS with 1% albumin (pH 7.4) equilibrated with room air. Each end of the arteriole was cannulated with micropipettes filled with PSS-albumin solution and secured with nylon suture. The chamber was placed on the stage of an inverted microscope, equipped with a video camera and video caliper. Arterioles were pressurized via two independent reservoirs and checked for leaks. If leaks were present, the arterioles were discarded. Vessels that were free from leaks were pressurized to 70 cmH2O, gradually warmed to 37°C, and allowed to develop spontaneous tone during an initial 1-h equilibration period. A minimal level of 20% spontaneous tone was required to assess vasodilatory responses. The bathing solution was changed every 20 min during the course of each experiment. At the end of all experiments, arterioles were placed in Ca2+-free PSS with 100 μM of the NO donor, sodium nitroprusside, for 1 h to determine maximal passive diameter (35, 37, 38).
Evaluation of vasodilator responses.
Once steady tone was achieved, vasodilator responses to the cumulative addition of the endothelium-dependent vasodilator acetylcholine (ACh, 10−9-10−4 M, 3-min exposure at each consecutive dose) were determined. To evaluate vascular smooth muscle responsiveness to exogenous NO, a concentration-response relationship for diethylamineNONOate (Dea-NONOate, 10−9-10−3 M) was determined.
Evaluation of inhibitory effects of tempol, catalase, tempol + catalase, deferoxamine, tempol + deferoxamine, FeTPPS, and NADPH oxidase.
To determine the role O2− and O2−-derived ROS in endothelium-dependent vasodilation, responses to ACh were evaluated after a 20-min incubation with the following: 1) SOD mimetic tempol (100 μM) (7); 2) H2O2 scavenger, catalase (100 U/ml) (14, 15); or 3) tempol (100 μM) plus catalase (100 U/ml). To determine the impact of NADPH oxidase-derived ROS, ACh-induced vasodilation was determined following a 20-min incubation with the NADPH oxidase inhibitors 1) apocynin (100 μM) (47, 69) or 2) gp91ds-tat (50 μM, gift from Dr. Robert Brock) (45). To determine the contribution of OH−, H2O2, and ONOO− to endothelium-dependent vasodilation, arteriolar responses to ACh were determined following incubation for 20 min with the following: 1) iron chelator and inhibitor of OH− formation deferoxamine (100 μM) (57); 2) deferoxamine (100 μM) plus tempol (100 μM); or 3) ONOO− decomposition catalyst [5,10,15,20-tetrakis-(4-sulfonatophenyl)-porphyrinato-fer(III) (FeTPPS), 10 μM] (54). To determine the role of O2− in scavenging of NO, responses to Dea-NONOate were evaluated after a 20-min incubation with the SOD mimetic, tempol (100 μM) (7).
Evaluation of vasoreactivity to H2O2 and O2−.
To determine the reactivity of soleus muscle arterioles to H2O2 and a O2− generator, concentration-response curves were generated using authentic H2O2 (10−6-10−2 M) and pyrogallol, an O2− generator (10−8-10−4 M).
Evaluation of OONO− with hydroxyphenyl fluorescein.
ACh-induced increases in OONO− were detected using the cell-permeable fluorescent probe, hydroxyphenyl fluorescein (HPF). HPF (100 μM), in albumin-free PSS, was introduced into the arteriolar lumen via flow, followed by a 1-h incubation. At the beginning of each experiment, a baseline image of the loaded vessel was recorded. ACh (100 μM) was administered intraluminally, and fluorescence images were captured at 0-, 3-, 6-, 10-, 30-, 60-, 120-, and 180-s time points following introduction to the lumen. Vessels were then reincubated with HPF (100 μM) for 30 min. Dea-NONOate (100 μM) and pyrogallol (10 μM) were added to the vessel simultaneously to assess the HPF fluorescence signal generated by exogenous ONOO−. Responses to ACh and exogenous ONOO− (Dea-NONOate + pyrogallol) were reevaluated following incubation with FeTPPS (10 μM). To demonstrate that the lack of increase in HPF fluorescence in the presence of FeTPPs was due to scavenging of ONOO− and not photo-bleaching of HPF, vessels were reexposed to exogenous ONOO− after washing away FeTPPS.
A user-defined region of interest (ROI) of the central portion of each vessel was analyzed for fluorescence intensity using ImageJ software (NIH). Basal fluorescence levels varied among ROIs and in different experiments. Therefore, after background subtraction, changes in fluorescence intensity were defined as (F − F0)/F0, where F is peak fluorescence intensity and F0 is baseline fluorescence intensity. For each vessel, baseline and peak fluorescence intensity values were measured within the same ROI, in the same focal plane.
Immunoblot analysis of soleus muscle arteriolar protein.
Differences in SOD-1, catalase, and glutathione peroxidase-1 (GPx-1) protein content in soleus muscle feed arteries were assessed by immunoblot analysis. Feed arteries were used in these analyses due to limitations of tissue volume in soleus muscle 1A arteriolar samples; however, aging- and training-induced adaptations of feed arteries generally parallel those of 1A arterioles (59, 66). Vessels were isolated, snap frozen, and stored at −80°C. Vessels were lysed in sample buffer, electrophoresed (10% SDS-PAGE), and transferred to nitrocellulose membranes. Membranes were blocked for 1 h at room temperature (5% nonfat dry milk) and then incubated overnight at 4° with primary antibodies for SOD-1 1:5,000 (Stressgen), catalase 1:6,000 (Chemicon), and GPx-1 1:4,000 (Abcam) or β-actin 1:2,000 (Cell Signaling Technologies). After washing, membranes were incubated with respective horseradish peroxide-conjugated secondary antibody for 1 h at room temperature. Peroxidase activity was determined using SuperSignal West Femto (Pierce), with image analysis performed using ImageJ (NIH). Loading differences were normalized by expressing all data as densitometric units for protein of interest relative to β-actin.
Data analysis.
Spontaneous tone was calculated as a percent constriction in relation to maximal diameter, as determined by the following equation:
where DM is the maximal diameter recorded at 70 cmH2O, and DT is the steady-state baseline diameter recorded at the same pressure. The vasodilator responses are expressed as percent relaxation as calculated by the formula:
where DS is the arteriolar diameter measured after addition of each dose of the drug being tested; DB is the diameter recorded immediately before initiation of the concentration-diameter curves; and DM is the maximal diameter for the arteriole. Concentration-diameter curves were evaluated by a three-way ANOVA with repeated measures on one factor, to detect differences within concentrations and between experimental groups. Group differences in animal and vessel characteristics, citrate synthase activity, and protein content of SOD-1, catalase, or GPx-1 were assessed by two-way ANOVA. In all experiments, n indicated the number of animals studied. Data are expressed as means ± SE. Statistical significance was defined as P ≤ 0.05.
RESULTS
Animals.
Body mass increased with age (Table 1). Exercise training resulted in lower body mass in both young and old rats. Soleus muscle mass increased with age, but was unchanged by exercise training (Table 1). Soleus muscle mass-to-body mass ratio decreased with age and increased with exercise training (Table 1). Exercise training increased citrate synthase activity by 18.3% in soleus muscle of young rats, and by 20.1% in soleus muscles of old rats, confirming the efficacy of the exercise training regimen, as previously reported (51).
Table 1.
Animal and vessel characteristics
| Young SED | Young ET | Old SED | Old ET | |
|---|---|---|---|---|
| n | 55 | 48 | 49 | 35 |
| Body weight, g | 362 ± 4 | 351 ± 4 | 448 ± 6* | 388 ± 6*† |
| Soleus muscle weight, mg | 151 ± 2.7 | 161.9 ± 3† | 167.7 ± 3.4* | 176.5 ± 4.7* |
| Soleus weight/body weight, mg/g | 0.42 ± 0.01 | 0.44 ± 0.02 | 0.38 ± 0.01* | 0.44 ± 0.02† |
| Vessel characteristics maximal diameter, μm | 125 ± 3 | 129 ± 5 | 126 ± 4 | 123 ± 4 |
| Spontaneous tone, % | 45 ± 2 (50) | 45 ± 2 (33) | 42 ± 2 (43) | 42 ± 2 (27) |
| with tempol | 46 ± 4 (11) | 46 ± 4 (8) | 40 ± 4 (9) | 40 ± 3 (13) |
| with tempol + catalase | 50 ± 4 (11) | 46 ± 5 (7) | 49 ± 3 (11) | 41 ± 2 (7) |
| with catalase | 48 ± 3 (12) | 49 ± 5 (9) | 43 ± 4 (8) | 43 ± 6 (8) |
| with deferoxamine | 32 ± 2 (5) | 32 ± 2 (5) | 38 ± 6 (5) | 39 ± 3 (4) |
| with deferoxamine + tempol | 35 ± 4 (5) | 45 ± 4 (8) | 33 ± 6 (5) | 24 ± 2# (5) |
| with FeTPPS | 41 ± 7 (6) | 46 ± 5 (4) | 30 ± 4 (4) | 32 ± 1 (4) |
| with apocynin | 54 ± 3 (8) | 55 ± 7 (8) | 43 ± 5 (7) | 45 ± 4 (8) |
| with gp91ds-tat | 60 ± 13 (3) | 54 ± 8 (13) | 47 ± 3 (4) | 43 ± 3 (4) |
Values are means ± SE; n, no. of rats. Nos. in parentheses, no. of vessels. SED, sedentary; ET, exercise trained.
Age effect,
ET effect, and
treatment effect compared with corresponding subgroup of vessels: P < 0.05.
Vessel characteristics.
Maximal diameter and spontaneous tone of soleus muscle arterioles were not different among groups (Table 1). Drug treatments did not alter tone in any group, with the exception of tempol + deferoxamine, which reduced tone in arterioles from aged ET rats (Table 1).
Endothelium-dependent vasodilation to ACh.
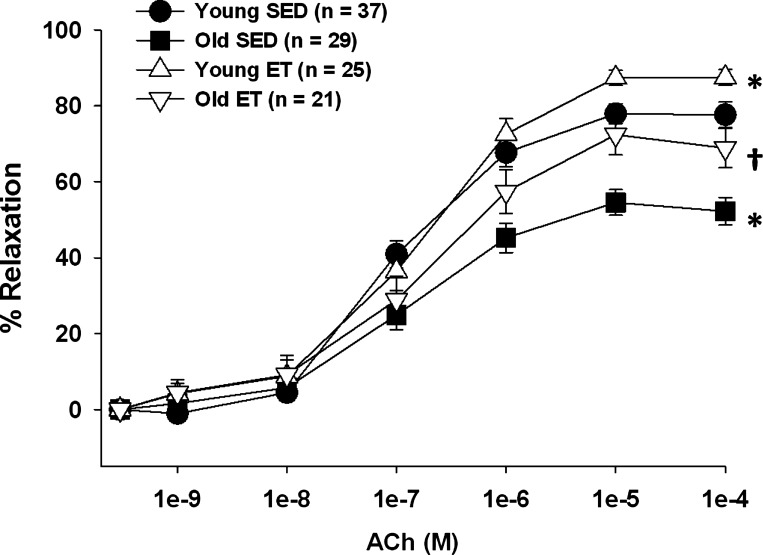
ACh-induced vasodilation was impaired in soleus muscle arterioles from old SED rats, confirming previous results (38, 51) (Fig. 1). Exercise training restored ACh-induced vasodilation in soleus muscle arterioles from old rats to that of young SED rats and increased maximal ACh-induced dilation in arterioles from young rats (Fig. 1).
Fig. 1.
Acetylcholine (ACh)-induced vasodilation of soleus muscle arterioles from young and old, sedentary (SED) and exercise-trained (ET) rats. Age significantly reduced vasodilator responses to ACh in soleus muscle arterioles. Exercise training restored vasodilator responses to ACh in soleus muscle arterioles from old rats. Values are means ± SE. *P < 0.05 vs. young SED. †P < 0.05 vs. old SED.
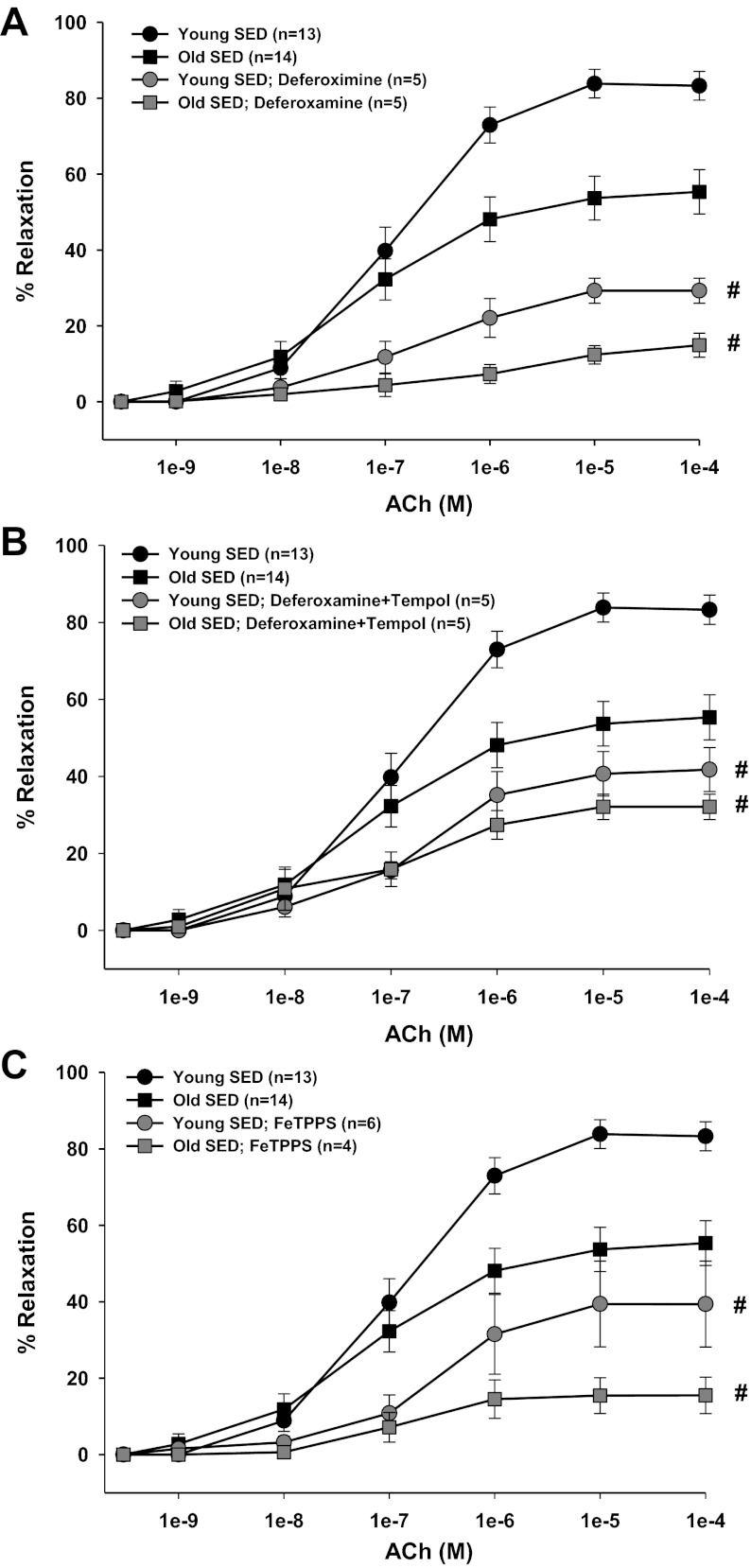
Contribution of O2− and H2O2 to ACh-induced vasodilation.
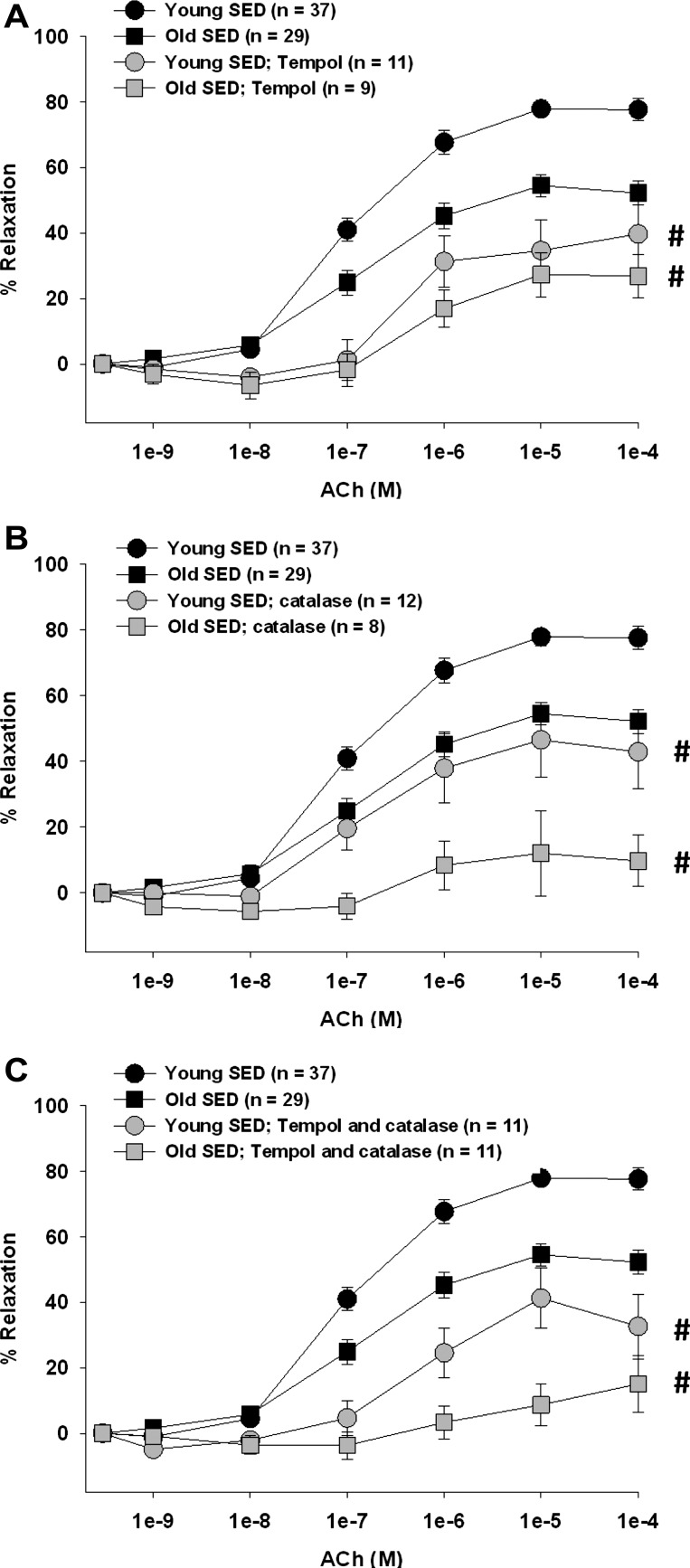
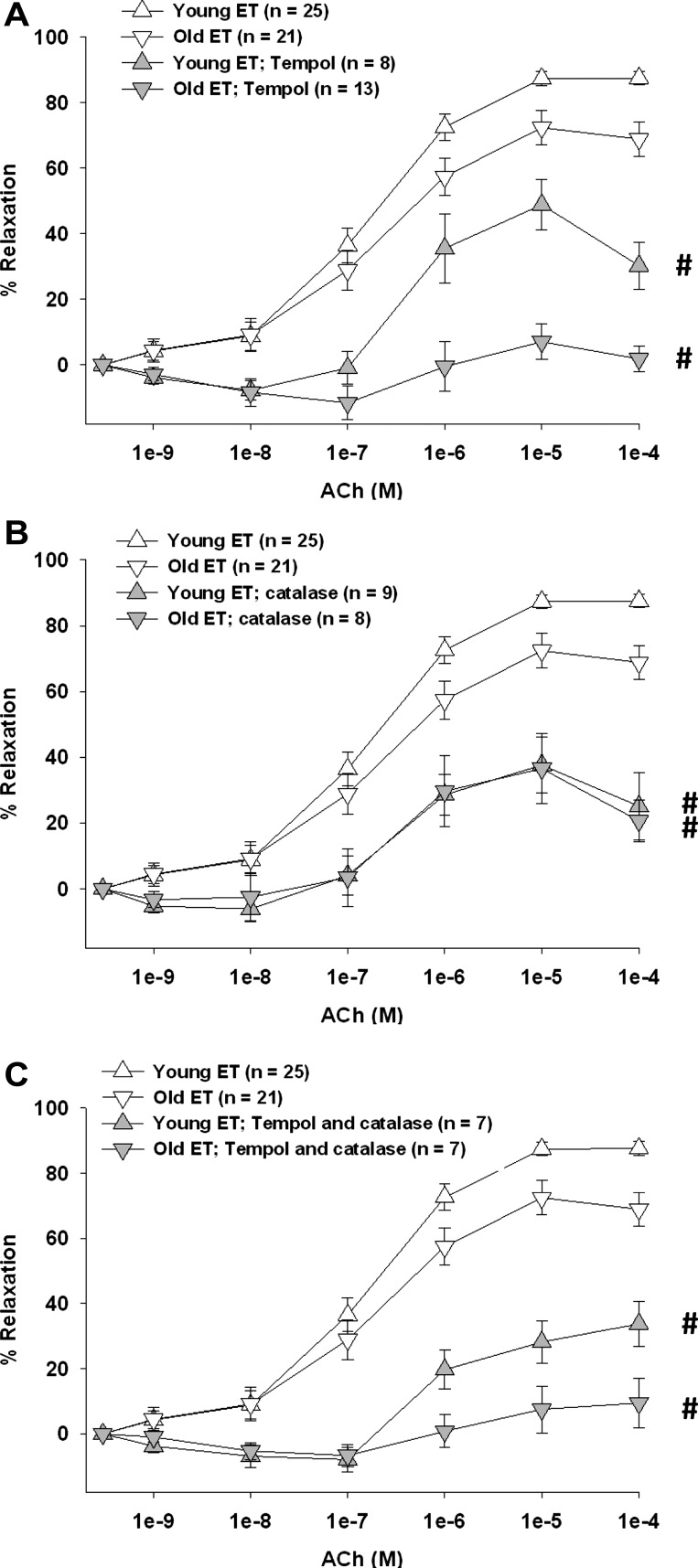
Increasing the dismutation of O2− to H2O2 with tempol reduced ACh-induced vasodilation in young and old, SED and ET rats (Figs. 2A and 3A). Furthermore, tempol eliminated the age-related differences in SED rats (Fig. 2A), suggesting that a reduction of O2-mediated signaling contributes to the old age-associated impairment in endothelium-dependent vasodilation. Tempol reduced ACh-induced vasodilation in arterioles from young and old ET rats, nearly eliminating the dilation in arterioles from old ET rats. These results suggest that the age-related reduction of O2−-mediated signaling is reversed by exercise training (Fig. 3A). Removal of H2O2 by catalase reduced ACh-induced vasodilation in all groups of rats (Figs. 2B and 3B), indicating that H2O2 contributes significantly to endothelium-dependent vasodilation in skeletal muscle arterioles. In arterioles from old, but not young, SED rats, catalase nearly eliminated vasodilation, suggesting that the contribution of H2O2 to endothelium-dependent vasodilation increases with age (Fig. 2B). This old age-related increase in H2O2 signaling was reversed by exercise training (Fig. 3B). Simultaneous scavenging of O2− and H2O2 also reduced ACh-induced vasodilation in all groups (Figs. 2C and 3C).
Fig. 2.
Contribution of O2− and/or hydrogen peroxide (H2O2) to ACh-induced vasodilation of soleus muscle arterioles from young and old SED rats. A: tempol inhibited the vasodilator response to ACh in arterioles from all groups and abolished the age differences in ACh-induced vasodilation. B: catalase inhibited the vasodilator response to ACh in arterioles from all rats; however, the age difference in ACh-induced vasodilation remained. C: combination of tempol and catalase inhibited vasodilator responses to ACh in arterioles from all rats. Values are means ± SE. #P < 0.05, inhibitor + ACh vs. ACh alone.
Fig. 3.
Contribution of O2− and/or H2O2 to ACh-induced vasodilation of soleus muscle arterioles from young and old ET rats. A: tempol inhibited the vasodilator response to ACh in arterioles from all groups. B: catalase inhibited the vasodilator response to ACh in arterioles from all rats and abolished the age differences in ACh-induced vasodilation. C: combination of tempol and catalase inhibited vasodilator responses to ACh in arterioles from all rats. Data are means ± SE. #P < 0.05, inhibitor + ACh vs. ACh alone.
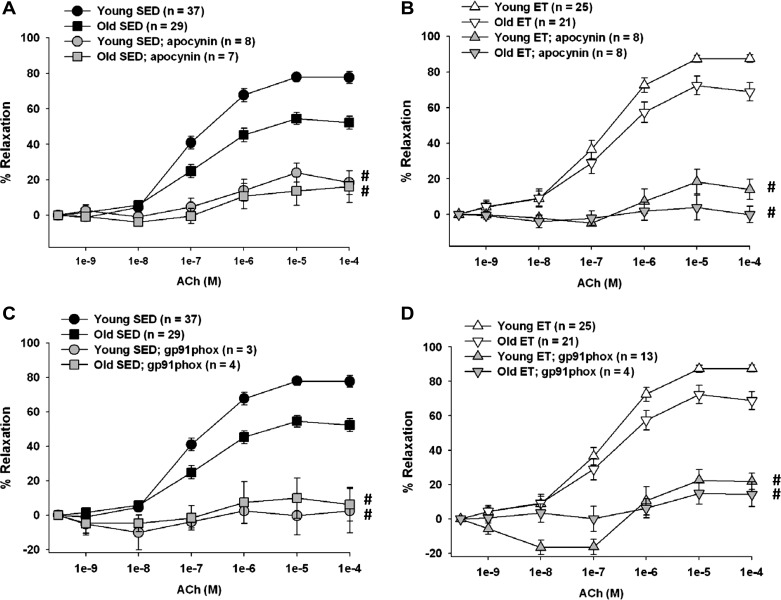
Contribution of NADPH oxidase-derived ROS to ACh-induced vasodilation.
Inhibiting NADPH oxidase-derived ROS with apocynin reduced ACh-induced vasodilation in young and old, SED and ET rats (Fig. 4, A and B). A specific inhibitor of the gp91phox subunit of NADPH oxidase, gp91ds-tat (45), also reduced ACh-induced dilation (Fig. 4, C and D). These data suggest that O2−-derived ROS are necessary for endothelium-dependent vasodilation to proceed in skeletal muscle arterioles.
Fig. 4.
Contribution of NADPH oxidase-derived ROS to ACh-induced vasodilation of soleus muscle arterioles from young and old, SED and ET rats. A: apocynin inhibited the vasodilator response to ACh in arterioles from young and old SED rats and abolished the age differences in ACh-induced vasodilation. B: apocynin inhibited the vasodilator response to ACh in arterioles from young and old ET rats and abolished the age differences in ACh-induced vasodilation. C: gp91ds-tat, a more specific inhibitor of NADPH oxidase, reduced the vasodilator response to ACh in arterioles from young and old SED rats and abolished the age differences in ACh-induced vasodilation. D: gp91ds-tat reduced the vasodilator response to ACh in arterioles from young and old ET rats and abolished the age differences in ACh-induced vasodilation. Values are means ± SE. #P < 0.05, inhibitor + ACh vs. ACh alone.
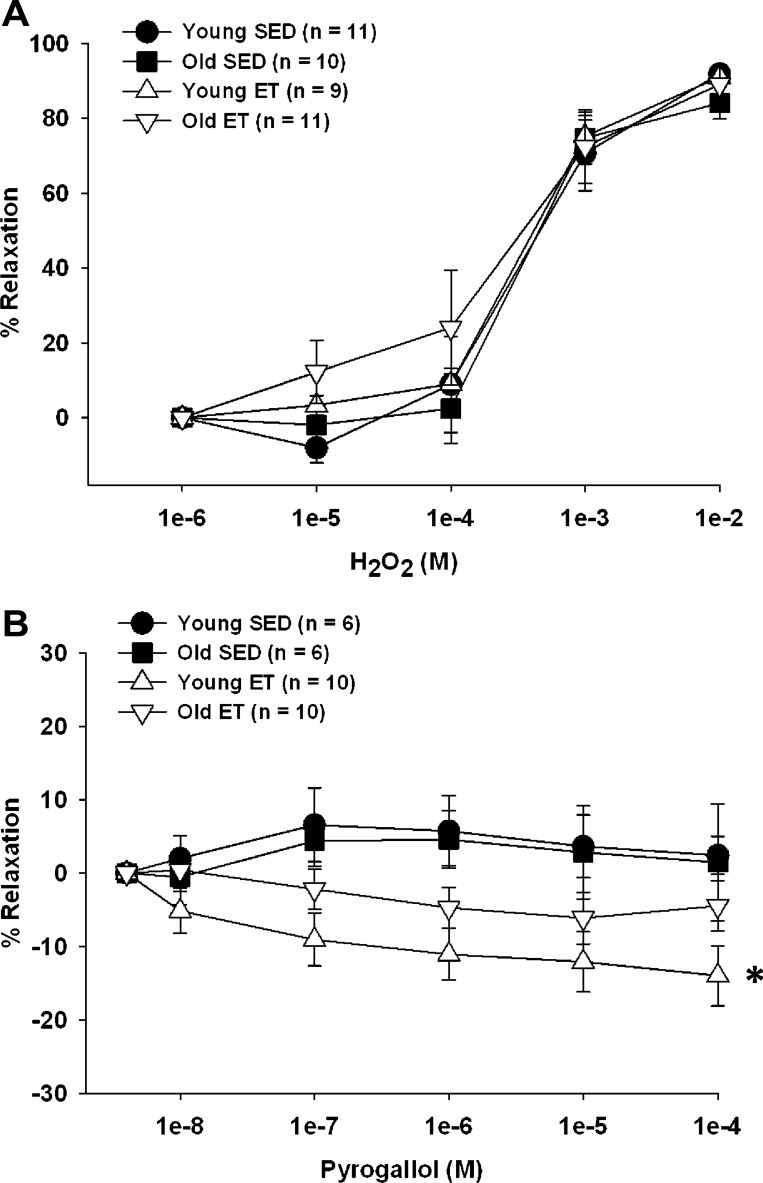
Vasoreactivity to H2O2 and O2− in soleus muscle arterioles.
At concentrations above 10−4 M, H2O2 elicited vasodilation in skeletal muscle arterioles, and these vasodilator responses were not affected by age or training status (Fig. 5A). O2− generated by the auto-oxidation of pyrogallol (29, 30) did not elicit a vasoactive response in arterioles from either young or old SED rats (Fig. 5B). In arterioles from young ET, but not old ET rats, exogenous generation of extracellular O2− elicited a slight vasoconstriction, suggesting that basal NO release increases with exercise training in arterioles from young rats and is scavenged by pyrogallol-released O2−.
Fig. 5.
ROS-induced vasoreactivity in young and old, SED and ET rats. A: vasodilation to exogenous H2O2 was preserved with age and was unchanged by exercise training. B: pyrogallol induced vasoconstriction in young ET rats. Values are means ± SE. *P < 0.05, young SED vs. young ET.
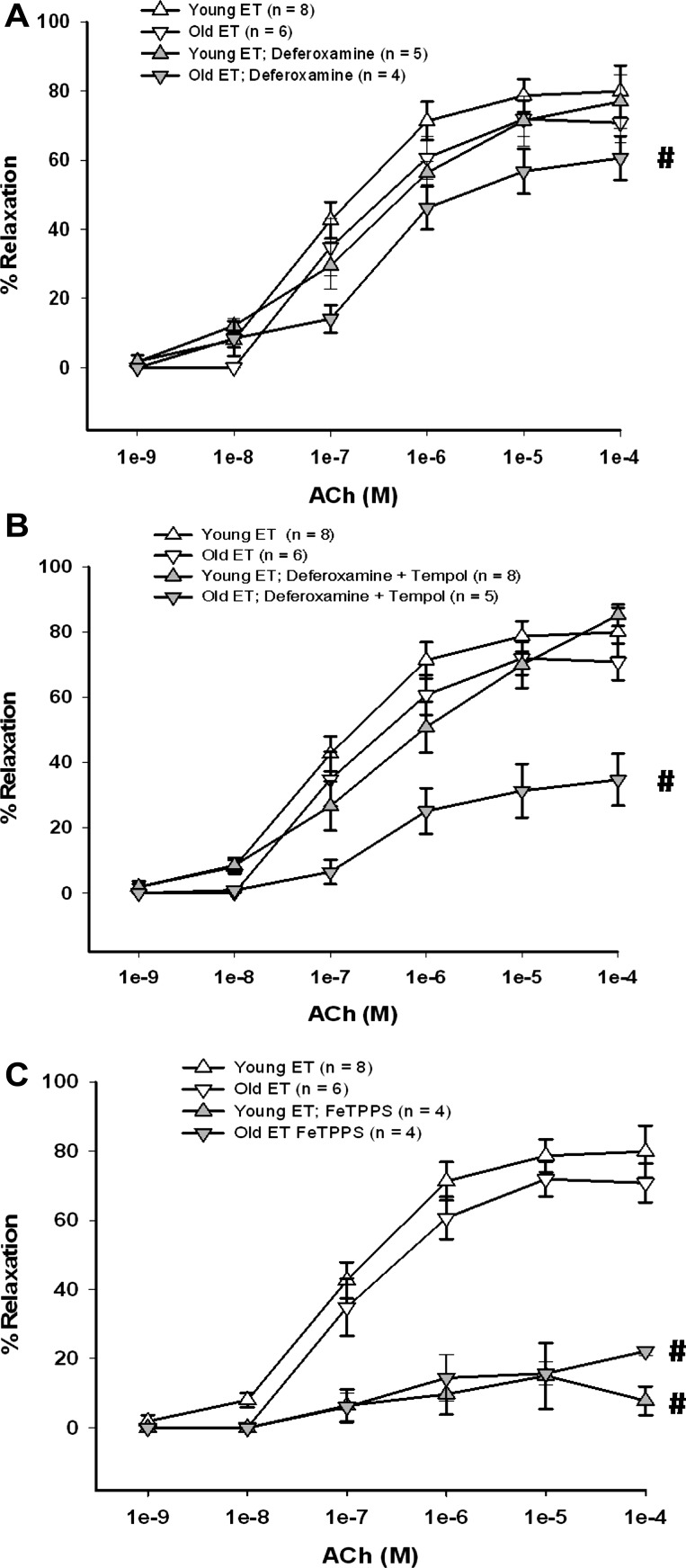
Contribution of OH−, H2O2, and ONOO− to ACh-induced vasodilation.
The iron chelating agent deferoxamine reduces vascular oxidative stress through inhibition of hydroxyl radical production via the Fenton/Haber-Weiss reaction and through inhibition of NADPH oxidase activity (56). Treatment of soleus muscle arterioles with deferoxamine alone inhibited ACh-induced vasodilation in arterioles from young and old SED rats (Fig. 6A) and in those from old ET rats, but not in arterioles from young ET rats (Fig. 7A). Deferoxamine reversed tempol-induced inhibition of ACh-mediated dilation in arterioles from young ET rats (Fig. 7B), but did not alter the inhibitory effects of tempol in arterioles from the other groups of rats (Figs. 6B and 7B). Thus, in arterioles from young ET rats, deferoxamine appears to primarily prevent OH− formation from H2O2, whereas, in arterioles from old ET rats and from SED rats of either age, deferoxamine may inhibit NADPH oxidase activity (56), contributing to a reduction of ACh-induced vasodilation, despite prevention of OH− formation (Figs. 6B and 7B).
Fig. 6.
Effect of iron chelation and peroxynitrite (ONOO−) decomposition on ACh-induced vasodilation of soleus muscle arterioles from young and old SED rats. A: deferoxamine inhibited the vasodilator response to ACh in arterioles from young and old SED groups. B: combination of deferoxamine + tempol inhibited the vasodilator response to ACh in arterioles from all rats. C: FeTTPS (ONOO− decomposition catalyst) inhibited vasodilator responses to ACh in arterioles from young and old rats. Values are means ± SE. #P < 0.05, inhibitor + ACh vs. ACh alone.
Fig. 7.
Effect of iron chelation and ONOO− decomposition on ACh-induced vasodilation of soleus muscle arterioles from young and old ET rats. A: deferoxamine inhibited the vasodilator response to ACh in arterioles from old ET rats. B: combination of deferoxamine + tempol inhibited the vasodilator response to ACh in arterioles from old ET rats. C: FeTTPS (ONOO− decomposition catalyst) inhibited vasodilator responses to ACh in arterioles from young and old ET rats. Values are means ± SE. #P < 0.05, inhibitor + ACh vs. ACh alone.
Decomposition of ONOO− with FeTPPS reduced ACh-induced vasodilation in young and old, SED and ET rats (Figs. 6C and 7C), suggesting endothelial NO and O2− react to form ONOO−, which may act as an NO donor to the underlying smooth muscle (40, 54).
ACh-stimulated ONOO− production.
Following intraluminal application of 100 μM ACh, peak HPF fluorescence increased by 9% above baseline values (Fig. 8), indicating that ACh stimulates significant production of ONOO− in soleus muscle arterioles. Generation of exogenous ONOO−, by simultaneous addition of pyrogallol and Dea-NONOate, increased peak HPF fluorescence by ∼7%. FeTPPS eliminated the ONOO−-induced increase in HPF fluorescence, indicating the specificity of its action in decomposing ONOO− (Fig. 8).
Fig. 8.
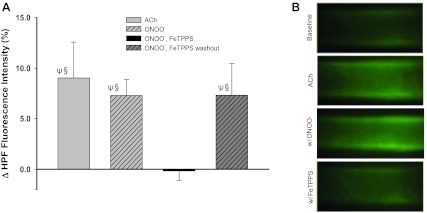
Detection of ONOO− with hydroxyphenyl fluorescein (HPF) fluorescence. A: ACh- and exogenous ONOO−-induced changes (Δ) in HPF fluorescence (from baseline) in soleus muscle arterioles in the absence and presence of FeTTPS (n = 8). Values are means ± SE. ΨSignificantly different from baseline, P < 0.05. §Significantly different from ONOO−, FeTPPS, P < 0.05. B: representative images illustrating the HPF fluorescence in soleus muscle arterioles at baseline and peak fluorescence following addition of ACh (100 μM) or diethylamineNONOate (Dea-NONOate; 100 μM) and pyrogallol (10 μM), to generate exogenous ONOO.
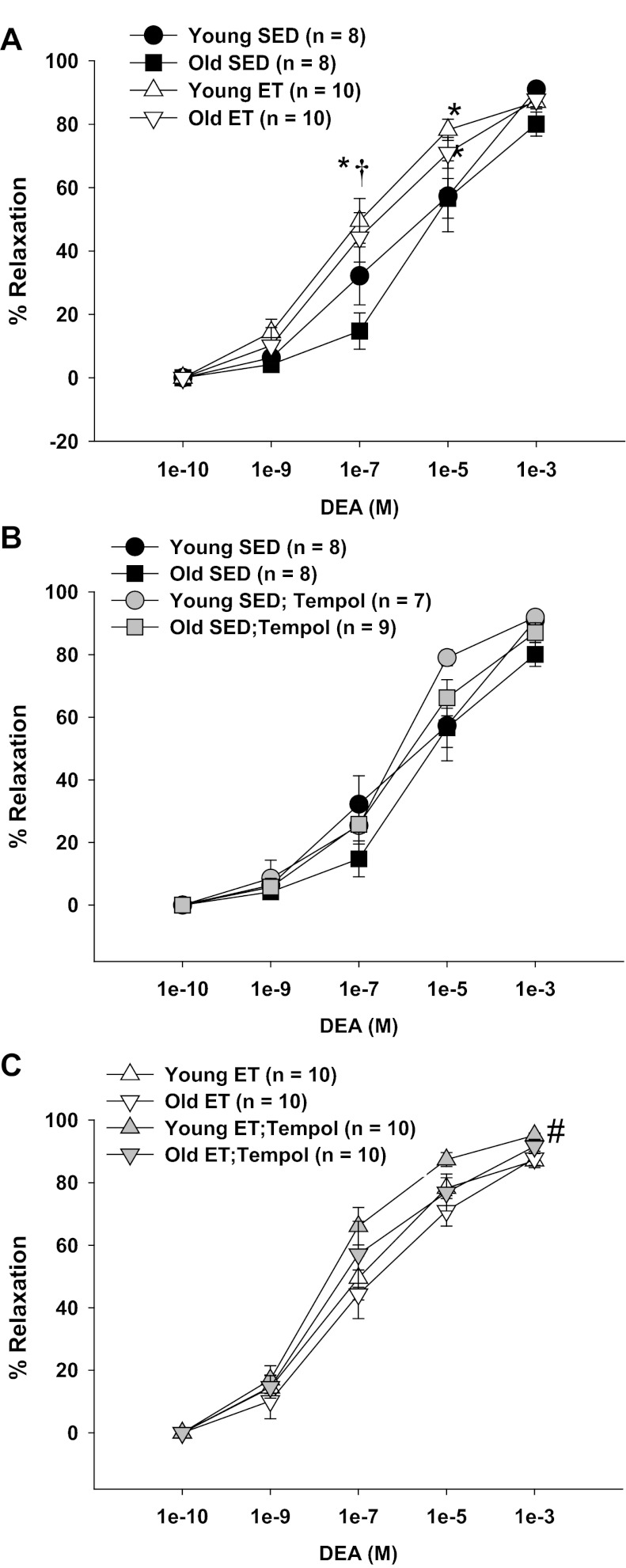
Vasodilation to exogenous NO.
Confirming previous findings (38), endothelial-independent vasodilation to exogenous NO was preserved with age. Exercise training increased vasodilation of skeletal muscle arterioles at concentrations of 10−7 M Dea-NONOate in both young and old rats (Fig. 9A). Scavenging of O2− with tempol had no effect on endothelium-independent vasodilation in arterioles from young and old SED rats or old ET rats, but improved endothelium-independent vasodilation in young ET rats (Fig. 9, B and C). Thus the old age-related reduction of NO bioavailability in skeletal muscle arterioles is not likely the result of increased scavenging of extracellular NO by excess extracellular O2−.
Fig. 9.
Vasodilation to exogenous NO in soleus muscle arterioles from young and old, SED and ET rats. A: age did not alter responses to DEA. Exercise training increased responses to DEA in arteriole from both young and old rats. B: tempol did not affect responses to DEA in arterioles from young and old SED rats. C: tempol increased vasodilation to DEA in arterioles from young, but not old ET rats. Data are presented as means ± SE. *P < 0.05 vs. YSED; †P < 0.05 vs. OSED. #P < 0.05 tempol + DEA vs. DEA alone.
SOD-1, catalase, and GPx-1 protein levels.
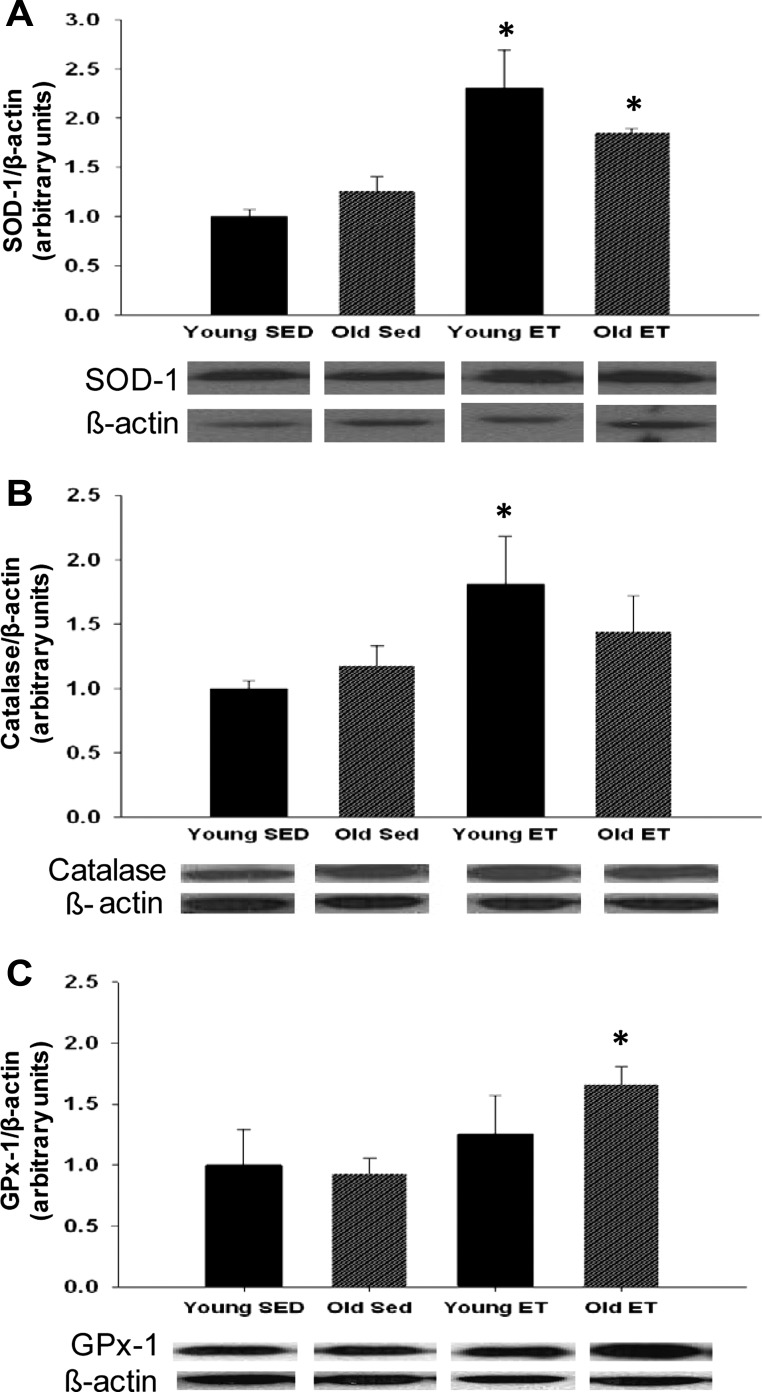
SOD-1 protein levels were not different between arterioles from young and old SED rats. Exercise training increased SOD-1 protein levels by 130% in arterioles from young rats and by 85% in arterioles from old rats (Fig. 10A). Catalase protein levels were not different between arterioles from young and old SED rats. Exercise training increased catalase protein by 81% in arterioles from young rats (Fig. 10B). GPx-1 protein levels in arterioles were unaffected by age; however, exercise training increased GPx-1 protein levels in arterioles from old rats by 66% (Fig. 10C).
Fig. 10.
Superoxide dismutase (SOD-1; A), catalase (B), and glutathione peroxidase-1 (GPx-1; C) protein content in soleus muscle arterioles of young and old, SED and ET rats (n = 6 per group) are shown. Values are means ± SE. *P < 0.05 indicates exercise training effect.
DISCUSSION
This study confirms previous reports of age-induced impairment of endothelium-dependent vasodilation (8, 38) and restoration of endothelial function by exercise training (51). New findings from this study demonstrate that ROS are essential mediators of endothelium-dependent vasodilation in skeletal muscle arterioles. The results of this study confirm our previous observations that alterations of ROS signaling are critical to age- and training-induced adaptations of endothelial function in the skeletal muscle resistance vasculature. Importantly, our present data provide novel data implicating ONOO− as a significant contributor to endothelium-dependent vasodilation in skeletal muscle arterioles and indicate that signaling mediated by both ONOO− and H2O2 relies on upstream production of endothelial O2−. Furthermore, the dependence of endothelium-dependent vasodilation on H2O2 signaling increases with age, whereas the contribution of NO and ONOO− to endothelium-dependent vasodilation increases with exercise training. In addition, our data indicate that, although aging does not reduce the expression of antioxidant proteins in soleus muscle arterioles, exercise training increases the expression of SOD-1, catalase, and GPx-1 protein in arterioles from young and old rats. When considered in aggregate, the present data reveal the critical role of ROS signaling in endothelium-dependent vasodilation and emphasize the importance of adaptations of ROS regulatory mechanisms in the endothelium of skeletal muscle arterioles.
Age-related differences in ACh-induced vasodilation were eliminated by scavenging O2− with the exogenous SOD mimetic tempol (Fig. 2A), indicating that alterations of O2− signaling contribute to old age-associated impairment of endothelium-dependent vasodilation. The finding that tempol reduced vasodilation of skeletal muscle arterioles from all groups of rats was not unexpected; our laboratory has previously shown that tempol reduces flow-induced vasodilation in skeletal muscle arterioles from old SED and young and old ET rats (47). In addition, Marvar et al. (32) have reported that locally generated ROS contribute to functional vasodilation. In coronary arterioles, tempol-induced impairment of flow-induced vasodilation is reversed by treatment with deferoxamine (22), suggesting that addition of exogenous SOD results in excessive production of H2O2 and cytotoxic OH− through activation of Fenton chemistry (42). In the present study, tempol inhibition of ACh-induced vasodilation was reversed by deferoxamine in arterioles from young ET rats, suggesting a similar overproduction of H2O2 and OH− occurs when exogenous SOD overwhelms endogenous catalase in these arterioles. In contrast, deferoxamine did not reverse the effects of tempol in arterioles from old rats, and deferoxamine alone inhibited ACh-induced vasodilation in arterioles from young and old SED rats and in arterioles from old ET rats. This effect of deferoxamine may be due to direct impairment of NADPH oxidase (56) rather than reduction of OH− production.
In the present study, H2O2 generated by tempol was catalyzed to H2O and O2 with the addition of catalase, thus scavenging both O2− and O2−-derived H2O2 (Fig. 11). This simultaneous scavenging of both O2− and H2O2 eliminated endothelium-dependent vasodilation in old SED rats (Fig. 2C), supporting our hypothesis that, although endothelium-dependent vasodilation is diminished with old age, O2−-derived ROS are necessary for vasodilation in old SED rats. Our laboratory's previous study indicated that age diminishes authentic NO-mediated vasodilation in skeletal muscle arterioles (47), and this finding is reinforced by our present finding that, in arterioles from young SED and ET rats, vasodilation to ACh remained during simultaneous scavenging of both O2− and H2O2 (Figs. 2C and 3C), indicating a contribution of O2−-independent signaling that is most likely due to direct NO-mediated vasodilation. Direct measures of NO in ACh-stimulated vessels treated with ROS inhibitors will be needed to verify that this residual dilation results from authentic NO signaling.
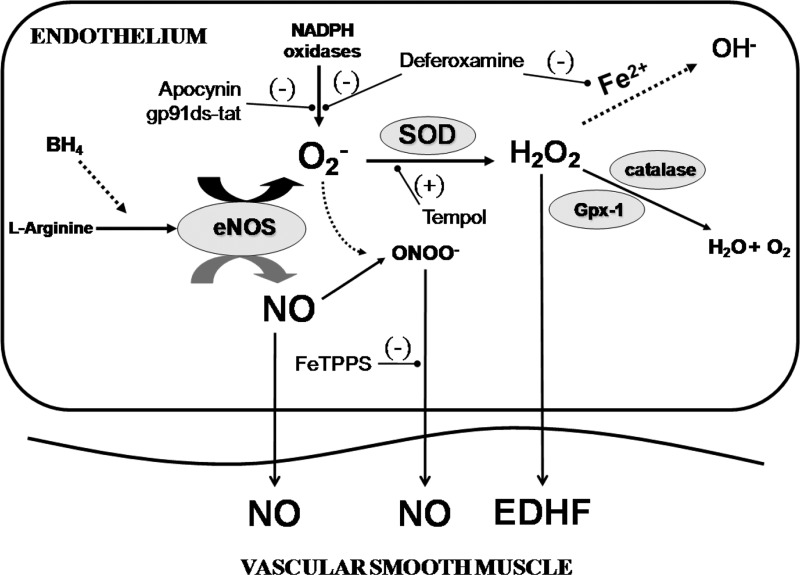
Fig. 11.
The proposed effects of ROS generation in the vascular endothelium. Superoxide is produced by endothelial nitric oxide synthase (eNOS) uncoupling and NADPH oxidases. Superoxide quickly reacts with nitric oxide (NO) to produce ONOO−. Endogenous SOD scavenges superoxide, which produces the vasodilator H2O2. In the presence of catalytic transition metals (i.e., Fe2+), H2O2 can produce the potent vasoconstrictor OH−. In the presence of catalase or Gpx-1, H2O2 is converted to H2O and O2. ONOO− is a potential NO donor; however, this capacity is inhibited when ONOO− is decomposed with FeTPPS or when tempol scavenges O2−, especially in old animals. H2O2 acts as an endothelial-derived hyperpolarization factor (EDHF); however, this capacity is blunted with tempol, which increases O2−-derived H2O2 increasing OH− production, or with tempol plus catalase, which drives the formation of H2O and O2 formation.
O2− is derived from several sources in the endothelium, including eNOS (14, 62), mitochondria (27), xanthine oxidase (64), and NADPH oxidase (5). NADPH oxidase-derived ROS increase with age in animals (25) and humans (11) and are associated with decreases in endothelial function in large conduit arteries (11, 28). In contrast, our present data show that inhibition of NADPH oxidase-derived ROS impaired endothelium-dependent vasodilation in skeletal muscle arterioles from all groups (Fig. 4, A–D). Our results are, however, in agreement with reports that NADPH oxidase-derived ROS are required for endothelium-dependent vasodilation in skeletal muscle and coronary arterioles (16, 47). Trott et al. (60) reported that inhibition of NADPH oxidase reduced ACh-induced vasodilation in soleus feed arteries from young, but not old, rats. In carotid arteries, inhibition of NADPH oxidase also reversed the old age-related impairment in ACh-induced vasodilation (48), suggesting that vascular bed specificity may be an important determinant of ROS signaling. Together, these findings support the notion that NADPH oxidase-derived ROS may act as an important signaling molecule necessary for maintaining endothelium-dependent vasodilation in small resistance arterioles.
H2O2 is emerging as an important vasodilator and signaling molecule in the vasculature (7). Previous work has shown in isolated coronary arterioles that endothelium-derived H2O2 contributes to flow-induced vasodilation (21, 22). In the present study, the contribution of H2O2 to endothelium-derived vasodilation was more pronounced in arterioles from old SED rats (Fig. 2B). This increased dependence on H2O2 was reversed by exercise training in arterioles from old rats (Fig. 3B). H2O2 has been labeled as an endothelium-derived hyperpolarizing factor because it is produced by the endothelium and elicits vascular smooth muscle relaxation through activation of Ca2+-dependent K+ channel activation (31, 34). In pulmonary (4), cerebral (64), and mesenteric (33) vascular beds, exogenous H2O2 causes vasodilation. Although the present results do not indicate the mechanism through which H2O2 induces vasodilation in the vascular smooth muscle, the findings that neither age nor exercise training altered responsiveness of soleus muscle arterioles to exogenous H2O2 (Fig. 5A) suggest that age-related adaptations in H2O2 signaling are confined to the endothelium and occur due to changes in endogenous production and/or generation of H2O2.
Previous findings indicate that old age reduces NO-mediated vasodilation, whereas exercise training promotes endothelial signaling through NO (51). In arterioles from old rats, exercise training appears to promote less dependence on H2O2 signaling and greater dependence on NO signaling, similar to signaling in arterioles from young rats. In addition, this study reveals that in arterioles from both young and old rats, ONOO− may function as an endogenous NO donor, similar to reports in coronary arteries and the feline hindlimb vasculature (26, 40). Although ONOO− is less potent than NO in producing relaxation of vascular smooth muscle (26), our results indicate that decomposition of ONOO− with FeTPPS reduced endothelium-dependent vasodilation in arterioles from all groups of rats. We confirmed the generation of ONOO− in response to ACh in arterioles and the efficacy of FeTPPS as an inhibitor of ONOO− using the fluorescent dye FHP. In isolated coronary arteries and aortic rings, ONOO− produces rapid and dose-dependent relaxation, but is less potent than NO (24, 26). ONOO− appears to induce vascular relaxation by at least two mechanisms. Chemiluminescence analysis indicates that ONOO− initially induces relaxation by generation of small quantities of NO (23, 44). Prolonged relaxation to ONOO− is likely to occur when it reacts with tissue sulfhydryls to form nitrosothiols, which can serve as NO donors, stimulating guanylate cyclase in vascular smooth muscle (26, 67). Vasodilatory responses to ONOO− in coronary, pulmonary, and systemic vascular beds are inhibitible by NO scavengers and blockers of guanulate cyclase, suggesting that relaxation to ONOO− is mediated by NO or NO donors (6, 24, 40, 41, 44, 63). Our novel findings support the idea that endothelium-dependent vasodilators activate a signaling pathway in which O2− and NO combine to produce ONOO− (Fig. 11) and subsequent relaxation of the vascular smooth muscle in skeletal muscle resistance arterioles (61). Surprisingly, our present experiments suggest that ONOO− increases similarly in the vascular wall in response to either ACh stimulation or through exogenous generation of ONOO−. Further experiments will be needed to determine the cellular mechanisms by which ONOO− stimulates smooth muscle relaxation of these arterioles.
Although O2− generation and oxidant stress increase with exercise (1), exercise training restores endothelium-dependent vasodilation in old rats [present study (51)]. The increase in oxidant stress associated with exercise likely contributes to enhanced O2− and O2−-derived ROS signaling in the endothelium of skeletal muscle arterioles of ET rats (Fig. 3). The enhanced regulation of O2− and O2−-derived ROS may depend on protein levels and activities of antioxidant enzymes. For example, Rush et al. (46) demonstrated in aortic endothelial cells from young pigs that SOD-1 protein abundance and activity increased with training, but had no effect on catalase content. In the present study, exercise training increased SOD-1 protein levels in skeletal muscle arterioles from young and old rats (Fig. 10A), indicating that more H2O2 was produced in the vasculature of ET rats. Exercise training also increased catalase levels in young rats (Fig. 10B), conceivably in response to increased SOD-mediated generation of H2O2. Similarly, exercise training increased GPx-1 content in old rats (Fig. 10C), possibly in response to training-induced increases in SOD content and generation of H2O2. Thus enhancement of endothelial function by exercise training may result, in part, from a training-induced upregulation of antioxidant proteins that regulate intracellular levels of necessary ROS signaling molecules.
Reduction of O2−, whether through inhibition of NADPH oxidase or scavenging with tempol, significantly reduced ACh-mediated vasodilation, suggesting that O2− may act as a direct vasodilator of soleus muscle arterioles. In cerebral arterioles, O2− acts as a direct vasodilator (49). To test the possibility that O2− directly dilates soleus muscle arterioles, we assessed responses of soleus muscle arterioles to increasing concentrations of pyrogallol, which produces O2− by auto-oxidation (30). The addition of exogenous O2− had no vasodilator effect in any group of rats (Fig. 5B), suggesting that O2− is not acting directly to cause vasodilation of vascular smooth muscle; however, since we did not have the means to measure O2− in the bathing medium, we cannot assume that the levels of O2− generated by pyrogallol did not surpass the physiological range, producing mixed vasodilatory and vasoconstrictor effects. Tempol did not alter responsiveness to Dea-NONOate in any group except young ET (Fig. 9, B and C), suggesting that tempol did not produce nonspecific impairment of vascular smooth muscle function. These results also suggest that reduced NO bioavailability that occurs with old age in skeletal muscle arterioles is probably not the result of increased scavenging of NO by extracellular O2−. Together, these data indicate that O2− is necessary for intracellular endothelial signaling to proceed, possibly through direct vasodilatory effects, or through O2−-derived H2O2 and/or ONOO− production.
Our study provides insight into mechanisms that contribute to alterations of blood flow regulation in skeletal muscle with aging and regular exercise training; however, the interpretation of our data may be limited by our experimental approach in which we studied the role of NO and ROS in vascular signaling only in arterioles from the highly oxidative soleus muscle (1). Aging is associated with a redistribution of blood flow away from highly oxidative muscles such as the soleus and red portion of the gastrocnemius muscle during walking exercise toward the more highly glycolytic muscle, such as the white portion of gastrocnemius muscle (39). This flow redistribution is mediated, at least in part, by diminished NO contribution in the vasculature of highly oxidative muscles (20). Our present data would suggest that this loss of NO contribution may be due, in part, to the loss of authentic NO, but may also be due to the loss of vasodilation mediated by other sources of NO, namely ONOO−. However, these vascular data from a highly oxidative muscle may not be applicable to other muscles of varying fiber composition and oxidative capacity. In our laboratory's recent work (3), exercise training increased vascular conductance and blood flow to the red, oxidative portion of the gastrocnemius muscle of old rats, whereas vascular conductance to the highly glycolytic white portion of the gastrocnemius decreased. In resistance arteries isolated from highly oxidative soleus muscle, age- and exercise training-induced adaptations of endothelium-dependent vasodilation are mediated predominantly by alterations in NO synthase (NOS) regulation and signaling (8, 38, 47, 51, 65), whereas alterations in prostanoid signaling contribute significantly to changes in endothelium-dependent vasodilation in arterioles isolated from the white glycolytic portion of the gastrocnemius muscle (38, 52).
Blood flow distribution within and among muscles composed of different fiber types is influenced by numerous factors at rest and during exercise (9). For example, recent work has shown that the magnitude of control of muscle perfusion through the sympathetic nerves and adrenergic receptors is relatively small across a range of flows at rest and during exercise in the highly oxidative soleus and red gastrocnemius muscles, whereas modulation of sympathetic nerve activity has the potential to control the entire range of perfusion in highly glycolytic white muscle (2). With old age, adrenergic receptor-mediated vasoconstriction is enhanced due to an impairment of the endothelial cell α-adrenoreceptor-NOS signaling mechanism in soleus muscle arterioles, but not in arterioles from highly glycolytic white portion of the gastrocnemius muscle (12). Exercise training, however, was shown to attenuate adrenergic vasoconstriction through augmented endothelial α-adrenoreceptor-mediated NO vasodilator function in both muscle types (12). Other vasoconstrictor mechanisms that are likewise modulated by endothelial cell NOS signaling, such as through endothelin and angiotensin II, are also differentially affected by aging and exercise training in muscles composed of different fiber types (13, 43). Thus, further work will be needed to determine whether signaling that occurs through ROS and NO contributes to age- and exercise training-induced adaptations of vascular reactivity that occur in glycolytic muscles and that are critical to altered distribution of blood flow at rest and during exercise.
This study implicates O2−-derived H2O2 and ONOO− as signaling molecules required for endothelium-dependent vasodilation in soleus muscle arterioles. The dependence of endothelium-dependent vasodilation on H2O2 increases with age and decreases with exercise training. Our results suggest that the relative levels of NO, O2−, and the product of the reaction of these two reactive species, ONOO−, are regulated by exercise training. The balance between the vasodilation produced by NO, H2O2, and ONOO− is critical to maintenance of endothelial function with advancing age.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute awards HL-077224 and R01 HL-090937.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.L.S., R.A.R., B.C., P.G., A.N.G., L.S.K., and J.M.M.-D. performed experiments; A.L.S., R.A.R., B.C., P.G., A.N.G., L.S.K., A.J.C., M.D.D., and J.M.M.-D. analyzed data; A.L.S., R.A.R., P.G., A.N.G., L.S.K., A.J.C., M.D.D., and J.M.M.-D. interpreted results of experiments; A.L.S., B.C., P.G., A.N.G., and J.M.M.-D. prepared figures; A.L.S. drafted manuscript; A.L.S., B.C., P.G., A.J.C., M.D.D., and J.M.M.-D. edited and revised manuscript; P.G., M.D.D., and J.M.M.-D. conception and design of research; A.J.C., M.D.D., and J.M.M.-D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Robert Brock for the kind gift of the gp91ds-tat, and Yanduan Hu and Zachary Grimm for technical assistance.
REFERENCES
- 1. Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sports Exerc 32: 1576–1581, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Behnke BJ, Armstrong RB, Delp MD. Adrenergic control of vascular resistance varies in muscles composed of different fiber types: influence of the vascular endothelium. Am J Physiol Regul Integr Comp Physiol 301: R783–R790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Behnke BJ, Ramsey MW, Stabley JN, Dominguez JM, 2nd, Davis RT, 3rd, McCullough DJ, Muller-Delp JM, Delp MD. Effects of aging and exercise training on skeletal muscle blood flow and resistance artery morphology. J Appl Physiol 113: 1699–1708, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol Heart Circ Physiol 252: H721–H732, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Cai H, Li Z, Dikalov S, Holland SM, Hwang J, Jo H, Dudley SC, Jr, Harrison DG. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin II. J Biol Chem 277: 48311–48317, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Casey DB, Pankey EA, Badejo AM, Bueno FR, Bhartiya M, Murthy SN, Uppu RM, Nossaman BD, Kadowitz PJ. Peroxynitrite has potent pulmonary vasodilator activity in the rat. Can J Physiol Pharmacol 90: 485–500, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol Heart Circ Physiol 293: H2085–H2092, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand 162: 411–419, 1998 [DOI] [PubMed] [Google Scholar]
- 10. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappa B. Circ Res 100: 1659–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol 579: 115–125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res 66: 393–401, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Drouin A, Thorin-Trescases N, Hamel E, Falck JR, Thorin E. Endothelial nitric oxide synthase activation leads to dilatory H(2)O(2) production in mouse cerebral arteries. Cardiovasc Res 73: 73–81, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Emsley AM, Jeremy JY, Gomes GN, Angelini GD, Plane F. Investigation of the inhibitory effects of homocysteine and copper on nitric oxide-mediated relaxation of rat isolated aorta. Br J Pharmacol 126: 1034–1040, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng J, Damrauer SM, Lee M, Sellke FW, Ferran C, Abid MR. Endothelium-dependent coronary vasodilatation requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol 30: 1703–1710, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem 276: 16587–16591, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Hirai DM, Copp SW, Hageman KS, Poole DC, Musch TI. Aging alters the contribution of nitric oxide to regional muscle hemodynamic control at rest and during exercise in rats. J Appl Physiol 111: 989–998, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Kang LS, Chen B, Reyes RA, Leblanc AJ, Teng B, Mustafa SJ, Muller-Delp JM. Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Circ Physiol 300: H2105–H2115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kikuchi K, Nagano T, Hayakawa H, Hirata Y, Hirobe M. Detection of nitric oxide production from a perfused organ by a luminol-H2O2 system. Anal Chem 65: 1794–1799, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Li J, Li W, Altura BT, Altura BM. Peroxynitrite-induced relaxation in isolated rat aortic rings and mechanisms of action. Toxicol Appl Pharmacol 209: 269–276, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Li L, Smith A, Hagen TM, Frei B. Vascular oxidative stress and inflammation increase with age: ameliorating effects of alpha-lipoic acid supplementation. Ann N Y Acad Sci 1203: 151–159, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Liu S, Beckman JS, Ku DD. Peroxynitrite, a product of superoxide and nitric oxide, produces coronary vasorelaxation in dogs. J Pharmacol Exp Ther 268: 1114–1121, 1994 [PubMed] [Google Scholar]
- 27. Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Lopez-Sepulveda R, Gomez-Guzman M, Zarzuelo MJ, Romero M, Sanchez M, Quintela AM, Galindo P, O'Valle F, Tamargo J, Perez-Vizcaino F, Duarte J, Jimenez R. Red wine polyphenols prevent endothelial dysfunction induced by endothelin-1 in rat aorta: role of NADPH oxidase. Clin Sci (Lond) 120: 321–333, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Ma X, Li YF, Gao Q, Ye ZG, Lu XJ, Wang HP, Jiang HD, Bruce IC, Xia Q. Inhibition of superoxide anion-mediated impairment of endothelium by treatment with luteolin and apigenin in rat mesenteric artery. Life Sci 83: 110–117, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47: 469–474, 1974 [DOI] [PubMed] [Google Scholar]
- 31. Marvar PJ, Hammer LW, Boegehold MA. Hydrogen peroxide-dependent arteriolar dilation in contracting muscle of rats fed normal and high salt diets. Microcirculation 14: 779–791, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Marvar PJ, Nurkiewicz TR, Boegehold MA. Reduced arteriolar responses to skeletal muscle contraction after ingestion of a high salt diet. J Vasc Res 42: 226–236, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Matoba T, Shimokawa H. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Pharm Sci 92: 1–6, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCurdy MR, Colleran PN, Muller-Delp J, Delp MD. Effects of fiber composition and hindlimb unloading on the vasodilator properties of skeletal muscle arterioles. J Appl Physiol 89: 398–405, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96: 81–88, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Nossaman BD, Bivalacqua TJ, Champion HC, Baber SR, Kadowitz PJ. Analysis of vasodilator responses to peroxynitrite in the hindlimb vascular bed of the cat. J Cardiovasc Pharmacol 50: 358–366, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Nossaman BD, Kadowitz PJ. Potential benefits of peroxynitrite. Open Pharmacol J 2: 31–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park Y, Prisby RD, Behnke BJ, Dominguez JM, 2nd, Lesniewski LA, Donato AJ, Muller-Delp J, Delp MD. Effects of aging, TNF-alpha, and exercise training on angiotensin II-induced vasoconstriction of rat skeletal muscle arterioles. J Appl Physiol 113: 1091–1100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Radi R, Cosgrove TP, Beckman JS, Freeman BA. Peroxynitrite-induced luminol chemiluminescence. Biochem J 290: 51–57, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sobey CG, Heistad DD, Faraci FM. Potassium channels mediate dilatation of cerebral arterioles in response to arachidonate. Am J Physiol Heart Circ Physiol 275: H1606–H1612, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Somers MJ, Harrison DG. Reactive oxygen species and the control of vasomotor tone. Curr Hypertens Rep 1: 102–108, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spier SA, Delp MD, Stallone JN, Dominguez JM, 2nd, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292: H3119–H3127, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249–H2256, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662–680, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Tajima S, Ikeda Y, Sawada K, Yamano N, Horinouchi Y, Kihira Y, Ishizawa K, Izawa-Ishizawa Y, Kawazoe K, Tomita S, Minakuchi K, Tsuchiya K, Tamaki T. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. Am J Physiol Endocrinol Metab 302: E77–E86, 2012 [DOI] [PubMed] [Google Scholar]
- 57. Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol 26: 2035–2042, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 277: 6017–6024, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 106: 1925–1934, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trott DW, Seawright JW, Luttrell MJ, Woodman CR. NAD(P)H oxidase-derived reactive oxygen species contribute to age-related impairments of endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol 110: 1171–1180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Villa LM, Salas E, Darley-Usmar VM, Radomski MW, Moncada S. Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc Natl Acad Sci U S A 91: 12383–12387, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wei EP, Kontos HA. H2O2 and endothelium-dependent cerebral arteriolar dilation. Implications for the identity of endothelium-derived relaxing factor generated by acetylcholine. Hypertension 16: 162–169, 1990 [DOI] [PubMed] [Google Scholar]
- 65. Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93: 1685–1690, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Woodman CR, Price EM, Laughlin MH. Selected Contribution: Aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol 95: 2164–2170, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Wu M, Pritchard KA, Jr, Kaminski PM, Fayngersh RP, Hintze TH, Wolin MS. Involvement of nitric oxide and nitrosothiols in relaxation of pulmonary arteries to peroxynitrite. Am J Physiol Heart Circ Physiol 266: H2108–H2113, 1994 [DOI] [PubMed] [Google Scholar]
- 68. Zhen J, Lu H, Wang XQ, Vaziri ND, Zhou XJ. Upregulation of endothelial and inducible nitric oxide synthase expression by reactive oxygen species. Am J Hypertens 21: 28–34, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res 44: 382–390, 2007 [DOI] [PubMed] [Google Scholar]