Abstract
Normal activity of neurons within the medullary ventral respiratory column (VRC) in or near the pre-Bötzinger Complex (preBötC) is dependent on the balance of inhibitory and excitatory neuromodulators acting at their respective receptors. The role of cholinergic neuromodulation during awake and sleep states is unknown. Accordingly, our objective herein was to test the hypotheses that attenuation of cholinergic modulation of VRC/preBötC neurons in vivo with atropine would: 1) decrease breathing frequency more while awake than during non-rapid-eye-movement (NREM) sleep and 2) increase other excitatory neuromodulators. To test these hypotheses, we unilaterally dialyzed mock cerebrospinal fluid (mCSF) or 50 mM atropine in mCSF in or near the preBötC region of adult goats during the awake (n = 9) and NREM sleep (n = 7) states. Breathing was monitored, and effluent dialysate was collected for analysis of multiple neurochemicals. Compared with dialysis of mCSF alone, atropine increased (P < 0.05) breathing frequency while awake during the day [+10 breaths (br)/min] and at night (+9 br/min) and, to a lesser extent, during NREM sleep (+5 br/min). Atropine increased (P < 0.05) effluent concentrations of serotonin (5-HT), substance P (SP), and glycine during the day and at night. When atropine was dialyzed in one preBötC and mCSF in the contralateral preBötC, 5-HT and SP increased only at the site of atropine dialysis. We conclude: 1) attenuation of a single neuromodulator results in local changes in other neuromodulators that affect ventilatory control, 2) effects of perturbations of cholinergic neuromodulation on breathing are state-dependent, and 3) interpretation of perturbations in vivo requires consideration of direct and indirect effects.
Keywords: neuromodulators, control of breathing, acetylcholine, non-rapid-eye-movement sleep
the neuromodulator acetylcholine (ACh) is involved in a variety of central and peripheral neural circuits, including the control of breathing and sleep (7, 12, 16, 19, 25, 39). The effects of ACh are mediated through either nicotinic or muscarinic receptors coupled to second messenger pathways (6, 10, 13, 42). There are five known muscarinic ACh receptor (mAChR) subtypes (M1 to M5) expressed in the brain stem, differing in expression profiles and in effects on breathing (2, 9, 11, 13, 33, 34). For example, the pontine respiratory group (PRG) nuclei express M1-M3 mAChRs (34). The predominant effect of cholinergic modulation of PRG neurons within the Kölliker-Fuse Nucleus (KFN) appears to be excitatory, since reverse dialysis of the nonselective mAChR antagonist atropine reduced minute ventilation (V̇I) and breathing frequency in both the awake state and during non-rapid-eye-movement (NREM) sleep at night, but not while awake during the day in unanesthetized goats (8). These effects were specific to the KFN, since breathing was not decreased during atropine dialysis in three other rostral pontine nuclei. Atropine also decreased concentrations of ACh and choline in effluent dialysate collected from the KFN and other pontine nuclei. These data suggest a role for the KFN in the control of breathing, particularly in the regulation of breathing frequency, at night through a cholinergic mechanism.
Data from multiple studies suggest an important role for mAChRs in intracranial chemosensitivity, upper airway respiratory motoneuron activity, and respiratory rhythm generation. For example, in anesthetized cats, atropine or a M1-specific receptor antagonist applied to the ventral lateral medulla decreases baseline phrenic nerve activity and the phrenic response to hypercapnia (39, 41). Likewise, based on data from carbachol-induced rapid-eye-movement (REM) sleep and in vitro studies, it has been hypothesized that direct and indirect excitatory M2 receptor modulation of hypoglossal motoneurons maintains a patent upper airway during REM sleep (4, 5). Furthermore, strong support for a role for mAChRs in respiratory rhythmogenesis comes from studies using a medullary slice preparation from neonatal rats that found that local application of ACh excites 88% of inspiratory neurons within the pre-Bötzinger Complex (preBötC), a site critical for respiratory rhythm generation in some (55, 57), but not all (51, 53), studies. These preBötC responses to ACh were blocked by atropine and by specific blockers of M3 receptors, but not by specific blockers of M1, M2, or M5 receptors, leading to the conclusion that ACh is excitatory to preBötC neurons (45, 46), likely through the activation of postsynaptic M3 receptors.
Because of a lack of information on the topic, the goal of the present study was to determine the effects on breathing and effluent neurochemical concentrations of reverse dialysis of atropine in or near the preBötC region in awake and sleeping adult goats. Studies were done in both the awake state and during natural NREM sleep to determine whether the effects of atropine were state-dependent. Given the importance of the preBötC region to respiratory rhythmogenesis (48, 49, 55, 57) and evidence for an excitatory role of mAChRs in respiratory control (4, 5, 45, 47), we hypothesized that dialysis of atropine in the preBötC region will decrease V̇I secondary to a decrease in breathing frequency. In addition, because there is evidence of a withdrawal of excitatory input to respiratory centers and a reduction in respiratory output during sleep (5), we further hypothesized that atropine dialysis will attenuate breathing more in the awake state than during NREM sleep. Finally, because there is evidence that attenuation of one excitatory neuromodulator will be compensated by an increase in other excitatory neuromodulators (18), we postulated that dialysis of atropine would result in an increase in multiple excitatory neurochemicals, such as serotonin (5-HT) and substance P (SP), in the effluent dialysate.
METHODS
Goats.
A total of 12 adult, nonpregnant female goats weighing 44.4 ± 1.8 kg were studied. Goats were housed and studied in an environmental chamber with a fixed ambient temperature and 12:12-h light-dark cycle (lights on 7:00 A.M.). All goats were allowed ad libitum access to food and water except during study periods and during a 24-h fasting period before surgery. All aspects of the study were reviewed and approved by the Medical College of Wisconsin Animal Care Committee before studies were initiated.
Surgical procedures.
Before all surgical procedures, goats were anesthetized with ketamine (5.0 ml iv), intubated, and mechanically ventilated with 2% isoflurane in 100% oxygen. The nonsteroidal anti-inflammatory analgesic flunixin meglumine (Banamine, 1 mg/kg im) was administered one time before every surgery. Rectal body temperature (Tr), heart rate, and blood oxygen saturation were continuously monitored throughout and immediately after each surgery. To minimize infection, ceftiofur sodium (Naxcel, 4 mg/kg im) was administered daily, and all surgical sites were treated daily with triple antibiotic for ≥7 days postoperatively. Buprenorphine hydrochloride (Buprenex, 0.005 mg/kg im) was administered two times daily for 2 days postoperatively to alleviate pain following all surgeries.
Two surgeries were performed, each under sterile conditions. In the initial surgery, electroencephalogram (EEG) and electrooculogram (EOG) electrodes were implanted in the midline cranium and superior orbital ridge, respectively, to monitor and score sleep state, as previously described (21, 28). After ≥1 wk of recovery, goats underwent a second surgery for chronic bilateral (n = 11) or unilateral (n = 1) implantation of stainless steel microtubules (MTs, 70 mm length, 1.27 mm outer diameter, 0.84 mm inner diameter) in the preBötC region of the medulla (29, 57). An occipital craniotomy was created, the dura mater excised, and the posterior cerebellum and dorsal medulla exposed for visualization of obex. As in previous studies (29, 57), the target site for implanting MTs was 2.5–3.5 mm rostral to obex, 4.0–5.0 mm lateral to midline, and 4.0–6.0 mm from the dorsal surface of the medulla (ventral to nucleus ambiguous). However, in some goats, the site of implantation had to be adjusted to avoid blood vessels on the dorsal medullary surface. After implantation, the MTs were permanently secured with dental acrylic, and the skin incisions were sutured closed. Stainless steel stylets of the same length as the MTs were inserted in the MTs such that they did not penetrate the tissue and were removed only during study periods. Postoperative monitoring and medications were administered as previously described (29, 57).
Physiological variables.
Goats were trained to become accustomed to the equipment used in dialysis studies and given a minimum of 2 wk to recover from the second surgery before beginning studies. For all studies, a custom-made mask was secured to the goat's muzzle, to which was attached a one-way breathing valve connected to inspiratory and expiratory tubing. Ventilatory variables, inspiratory flow (l/min, V̇I), breathing frequency [breaths (br)/min, f], and tidal volume (l/br, VT), were measured with a pneumotachograph attached in series to the inspiratory side of the breathing valve. Expired air was collected at regular intervals in a spirometer (day studies only) for determination of the expired volume and carbon dioxide and oxygen concentrations, allowing for calculation of oxygen consumption (V̇o2, l/min) and carbon dioxide excretion (V̇co2, l/min). During night studies, EEG and EOG activity was recorded for subsequent analysis of sleep state. Tr was measured at regular intervals by insertion of a thermocouple in the rectum. Ventilatory data were continuously recorded using a Windaq data acquisition system and later analyzed.
In vivo dialysis.
Dialysis probes (Harvard Apparatus, formerly CMA Microdialysis, Holliston, MA) were 72 mm in total length, consisting of a 70-mm shaft and a 2-mm membrane (0.5 mm membrane diameter, 20 kDa cut-off, 3 μl internal volume). Probes were inserted through the MTs such that only the membrane penetrated the tissue. The perfusate was either mock cerebrospinal fluid (mCSF: 124 mM NaCl, 2.0 mM KCl, 2.0 mM MgCl2, 1.3 mM K2PO4, 2.0 mM CaCl2, 11 mM glucose, 26 mM NaHCO3−, and pH 7.32 in sterile distilled H2O) alone or atropine (50 mM; Sigma) dissolved in mCSF. Previous in vitro studies in the laboratory have established that atropine crosses the membrane (8). Dialyzed solutions were warmed to 39°C and equilibrated with 6.4% CO2 and 21% O2 balanced with N2 in a tonometer. The syringe pump (Harvard Apparatus) used for delivery of solutions to the probe was outside the animal chamber. Thus, a 150- or 180-cm length of polypropylene tubing was needed to connect the pump to the probe, resulting in an approximate delay of 20 min between the start of the pump and when fresh perfusate reached the probe. Effluent dialysate was collected in modified cryotubes, separated into aliquots, and then frozen at −80°C for subsequent analyses.
Thirty minutes after probe insertion, control data were collected for 30 min followed by three consecutive 1-h dialysis periods (flow rate 25 μl/min) in order: 1) mCSF alone, 2) either mCSF alone or with atropine, and 3) mCSF alone. Effluent dialysate was continuously collected in each hour in separate vials.
Three groups of studies were completed. In group 1 time control studies, mCSF was unilaterally dialyzed in each of the three dialysis periods during the day (goats 1–7; Fig. 1). Group 2 studies differed from group 1 only in that atropine was dialyzed in hour 2 (goats 4–12 during the day and goats 4–10 during the night; Fig. 1). Duplicate dialysis studies (one on each side) were done in three group 1 goats, five group 2 goats during the day, and three group 2 goats during the night. In group 3 studies (goat 10; Fig. 1), mCSF was dialyzed during the day for three consecutive hours on one side while on the contralateral side, mCSF was dialyzed during hours 1 and 3, and atropine was dialyzed during hour 2. Four days later, the study was repeated with the side receiving either mCSF or atropine reversed. The maximum number of probe insertions in the majority of goats was four.
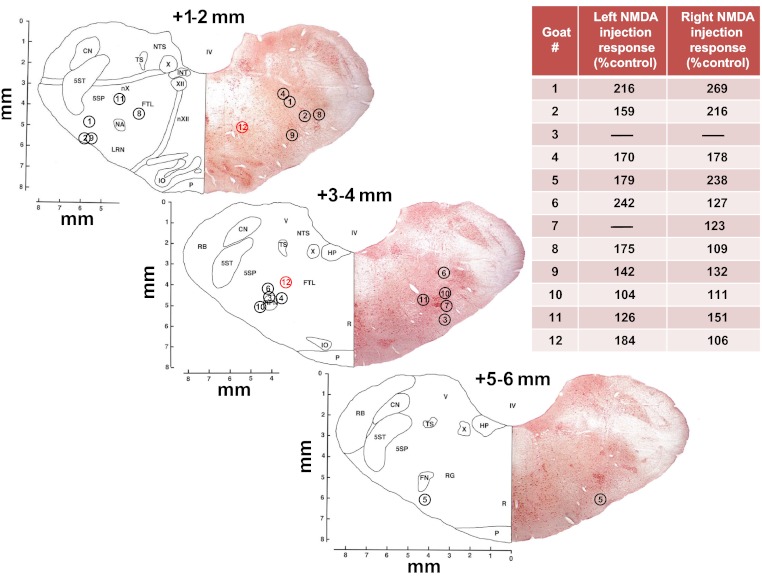
Fig. 1.
Sections from the goat brain stem atlas (14) at different distances (mm) rostral to obex. Inserted are nos. assigned to each goat identifying the site of implanted microtubules (MTs). The locations are based on histological analysis of each goat's brain stem identifying the center of the distal tip of the implanted MT. From previous data (29, 57), we have estimated the goat pre-Bötzinger Complex (preBötC) to be about 2.5–3.5 mm rostral to obex, 4.0–5.0 mm from the midline, and ventral to nucleus ambiguous. The inset table lists the peak increase in breathing frequency when N-methyl-d-aspartic acid (NMDA, 100 mM, 500 nl) was injected at the distal tip of the MT. Based on the histologically identified site and/or the response to NMDA, we conclude that in all goats the MTs were to a variable degree within the preBötC region of the ventral respiratory group. x- and y-Axes are in mm.
Daytime dialysis studies were completed within the hours of 9:00 A.M. to 2:00 P.M., and night studies were completed within the hours of 8:00 P.M. to 1:00 A.M. There was a minimum of 36 h between all consecutive studies.
mCSF and glutamate receptor agonist injections.
Administration of glutamate receptor agonists to the preBötC elicits a tachypneic ventilatory response (29, 36, 50, 57). Thus, as in past studies, we used injections of a glutamate receptor agonist as physiological evidence that MT placement approximated the preBötC region (29, 57). Breathing was continuously measured over 2 h, and, at 30-min intervals, mCSF or N-methyl-d-aspartic acid (NMDA, 100 mM) was injected (500 nl) in the MTs.
Neurochemical analyses.
Glycine (GLY) and γ-aminobutyric acid (GABA) were measured by reverse-phase high-performance liquid chromatography with fluorescent detection using the following parameters: Waters Resolve C18 column (150 × 3.9), a fluorescent detector with excitation at 229 nm and emission at 470 nm, β-alanine internal standard, and o-phthaldialdehyde derivitization. For detection and measurement of 5-HT, electrochemical detection with the same type of column was used, but with a potential setting of 0.6 volts vs. Ag/AgCl reference electrode and a N-methylserotonin internal standard. Measurement of epinephrine (Epi) and dopamine (DA) was accomplished by using a Waters μBondapak C18 column (300 × 3.9), potential setting of 0.65 volts vs. Ag/AgCl reference electrode, and a 2,5-dihydroxybenzylamine hydrobromide internal standard. A commercially available immunoassay (Assay Designs 900–018, range 9.76–10,000 pg/ml) was used to measure SP via a microplate reader at 405 nm. The ACh/choline analysis was performed using a Bioanalytical Systems analytical column utilizing a polymer packing coupled in series with a postcolumn immobilized enzyme reactor and potential setting of 0.5 volts vs. Ag/AgCl.
Data and statistical analysis.
V̇I, f, VT, and, in the case for night studies, EEG and EOG signals, were analyzed on a breath-by-breath basis. For each study, the inspiratory flow signal was calibrated against known air flow rates. For night studies, EEG and EOG signals were used to visually categorize each breath as being in either the awake, REM sleep, or NREM sleep states.
Breath-by-breath values of ventilatory variables were averaged into 1-, 5-, and 15-min bins and expressed as raw values for statistical analyses and graphical presentation. Analysis of each bin size led to the same general conclusions, and, thus, the 15-min bin size is presented herein (means ± SE). In four goats, data obtained on separate days in the left and right preBötC were analyzed by two-way repeated-measures (RM) ANOVA. Because no significant differences (P > 0.05) were found, data were averaged for each goat. For group 1 and 2 studies, a one-way RM ANOVA was used to determine whether changes in breathing occurred within each of the 210-min protocols. A two-way ANOVA (time and treatment as factors, Holm-Sidak post hoc, when appropriate) was used to compare the effects on breathing of mCSF or atropine dialysis. A two-way RM ANOVA (Holm-Sidak post hoc, when appropriate) was used to compare the effects on breathing of time of day (time and time of day as factors) and the effects of sleep state (time and sleep state as factors). Similarly, a two-way RM ANOVA (time and treatment as factors, Holm-Sidak post hoc, when appropriate) was used to determine whether there was a difference in time spent in the awake state and time spent in NREM sleep in night studies, and a one-way RM ANOVA was used to determine whether atropine dialysis affected time in each state.
Neurochemical values of dialysate concentrations of 5-HT, SP, GLY, GABA, Epi, and DA obtained from measurements from duplicate studies for any goat were averaged. In group 1 and 2 studies, a one-way RM ANOVA was used to determine whether significant changes in neurochemicals occurred within the 3 h of dialysis. A two-way ANOVA (Holm-Sidak post hoc, when appropriate) was used to compare the effects on effluent neurochemicals of mCSF or atropine dialysis (time and treatment as factors). A two-way RM ANOVA (Holm-Sidak post hoc, when appropriate) was used to compare the effects of time of day (time and time of day as factors) on effluent neurochemicals. Paired t-tests were used to determine if there were differences in neurochemicals in hour 1 between day and night studies in four group 2 goats. Because of a limited number of samples for analysis of ACh and choline, values from day and night dialysis studies were combined and averaged for each animal. A one-way RM ANOVA was used to determine whether significant changes in ACh/choline occurred within the 3 h of dialysis. Finally, paired t-tests were used to determine the effects of mCSF and atropine dialysis on Tr.
Histological analysis.
Upon completion of the protocol, goats were anesthetized with ketamine (2.3 ml iv), circulation to the head was isolated, and the goat was killed (B-euthanasia, 10 ml iv). The head was perfused with PBS, followed by 4% paraformaldehyde in PBS. The brain stem was extracted for processing as previously described (29). Nissl-stained 4,000 dots/inch-scanned images of the entire MT tract were captured (Nikon Super Coolscan 9000). Image software (Metamorph) was used to calibrate and measure MT placement in millimeters (relative to midline and the ventral medullary surface) near the middle of the rostral-caudal MT damage range.
RESULTS
Histological and physiological evidence of MT placements.
Our previous studies on goats provided evidence that the preBötC in goats extends from ∼2.5 to 3.5 mm rostral to obex, 4.0–5.0 mm lateral to the midline, and ventral to the nucleus ambiguous (29, 57). For the goats studied herein, histological studies indicated that the center of the distal tip of the implanted MTs was within this range (Fig. 1). Regarding the physiological marker of the preBötC, there were variable degrees of increased breathing after injection of a glutamate receptor agonist (NMDA, 100 mM, 500 nl) at the distal end of the MT (see inset in Fig. 1). The sites of greater NMDA responses corresponded in proximity to the preBötC. For example, for goat 11 left side and goat 12 right side, the MTs were placed relatively caudal and medial, respectively, to preBötC, and there was a low response to NMDA (Fig. 1). On the other hand, for goat 11 right side and goat 12 left side, the MT placements were in the preBötC region, and there was a substantial response to the NMDA injection. There was no significant (P > 0.10) change in breathing when mCSF was injected. Even though the histological and physiological data for each goat did not indicate identical placements of the left and right MTs, we averaged the responses to atropine dialysis in the right and left sides to obtain a single response for each goat, and these values were used for statistical analyses and graphical presentation of the data.
Effects of atropine on ventilatory variables.
As determined by a one-way RM ANOVA, unilateral dialysis of mCSF in the preBötC for three consecutive hours during the day (group 1, time control) did not affect V̇I (F = 1.53, P = 0.124), but there was a significant increase in f (F = 7.05, P < 0.001) and a decrease in VT (F = 2.593, P = 0.005; Fig. 2). These changes may reflect normal physiological variation over time and/or an effect of washout of endogenous neurochemicals.
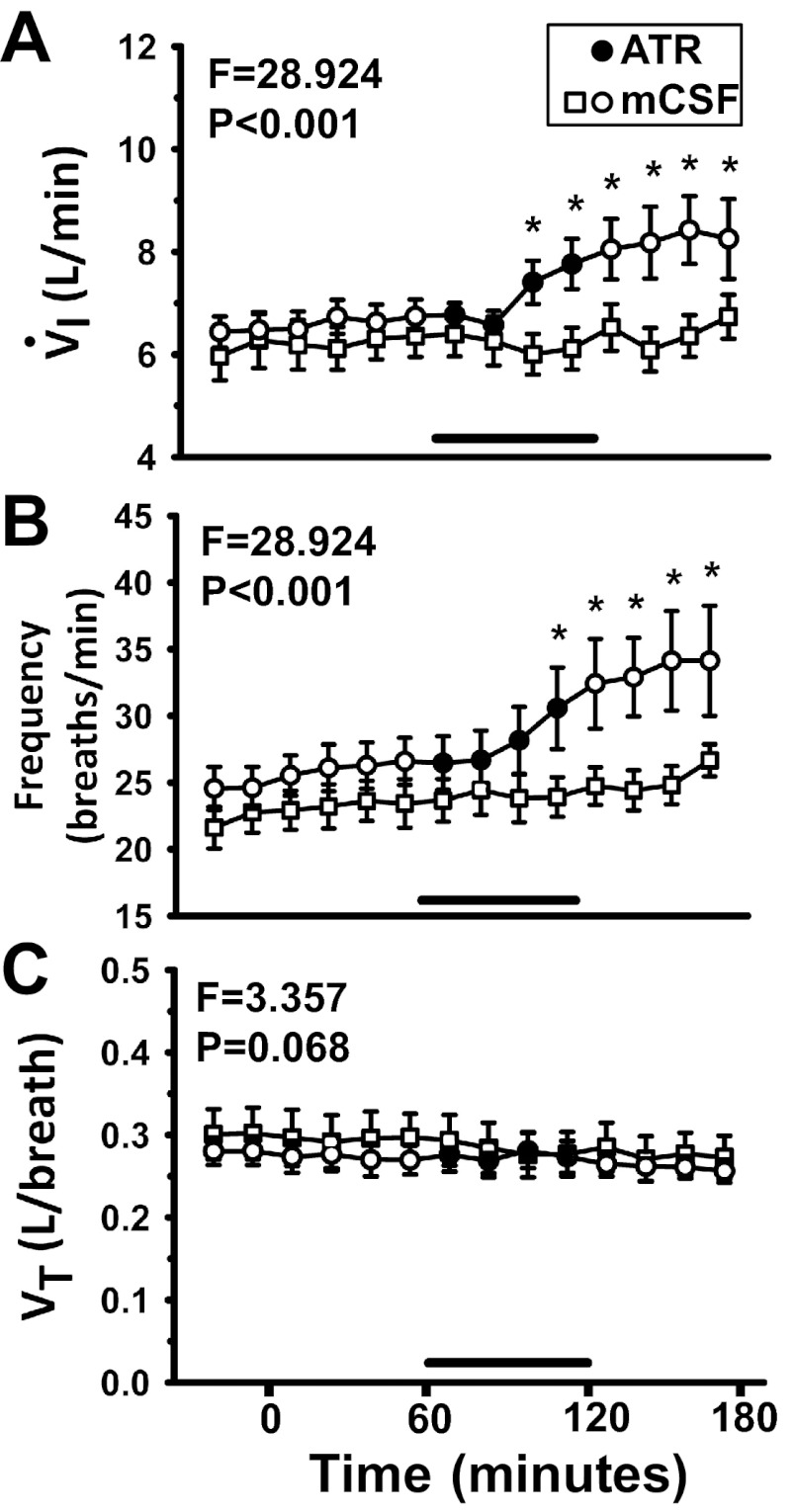
Fig. 2.
Dialysis of atropine in the preBötC during the day significantly (P < 0.001) increased inspiratory flow (V̇I, A) and breathing frequency (f, B), and decreased tidal volume (VT, C) (P = 0.016), as determined by 1-way repeated-measures (RM) ANOVA. x-Axis is time from start of dialysis (min); symbols left of 0 min indicate predialysis period. Open squares represent 3 continuous hours of mock cerebrospinal fluid (mCSF) dialysis (n = 7); circles (n = 9) represent studies in which atropine (ATR) was dialyzed between 60 and 120 min into the study, indicated by horizontal bars and closed circles. F and P values are taken from 2-way ANOVA (time and treatment as factors). *P < 0.05 between treatment groups, as determined by post hoc analysis (Holm-Sidak). Power of performed tests for atropine dialysis studies for V̇I and f were 1.0, and, for VT, power ranged between 0.32 and 0.66.
When atropine was dialyzed during hour 2, V̇I was significantly elevated (+1.0–1.8 l/min) over the last 15 min of atropine dialysis and throughout hour 3 (Fig. 2A). The increase was compared with the predialysis control period (F = 6.94, P < 0.001, 1-way RM ANOVA) and with the time control study (F = 28.9, P < 0.001, 2-way ANOVA). Similarly, over the last 15 min of atropine dialysis and throughout hour 3, f was significantly elevated (+10 br/min) compared with the predialysis control (F = 10.737, P < 0.001, 1-way RM ANOVA) and compared with the time control study (F = 28.9, P < 0.001, 2-way ANOVA; Fig. 2B). VT decreased significantly during atropine dialysis compared with the predialysis control (F = 2.17, P = 0.016, 1-way RM ANOVA). VT tended to be lower throughout the protocol when atropine was dialyzed during hour 2 compared with time control studies, but this was not significant (F = 3.36, P = 0.068, 2-way ANOVA; Fig. 2C).
During dialysis of atropine at night in the awake state, V̇I was significantly increased (+1.7 l/min, F = 2.13, P = 0.021, 1-way RM ANOVA; Fig. 3A), but there was no significant (F = 3.27, P = 0.121) difference in V̇I in the awake state between day and night atropine studies (2-way RM ANOVA). Atropine dialysis at night in the awake state significantly increased f (+9 br/min, F = 5.01, P < 0.001, 1-way RM ANOVA; Fig. 3B), but there was no significant difference in f in the awake state between day and night atropine dialysis studies (F = 1.522, P = 0.263, 2-way RM ANOVA). At night in the awake state, atropine dialysis did not affect VT significantly (F = 0.538, P = 0.89, 1-way RM ANOVA; Fig. 3C), and there was no significant difference in VT between day and night studies in the awake state (F = 0.002, P = 0.969, 2-way RM ANOVA).
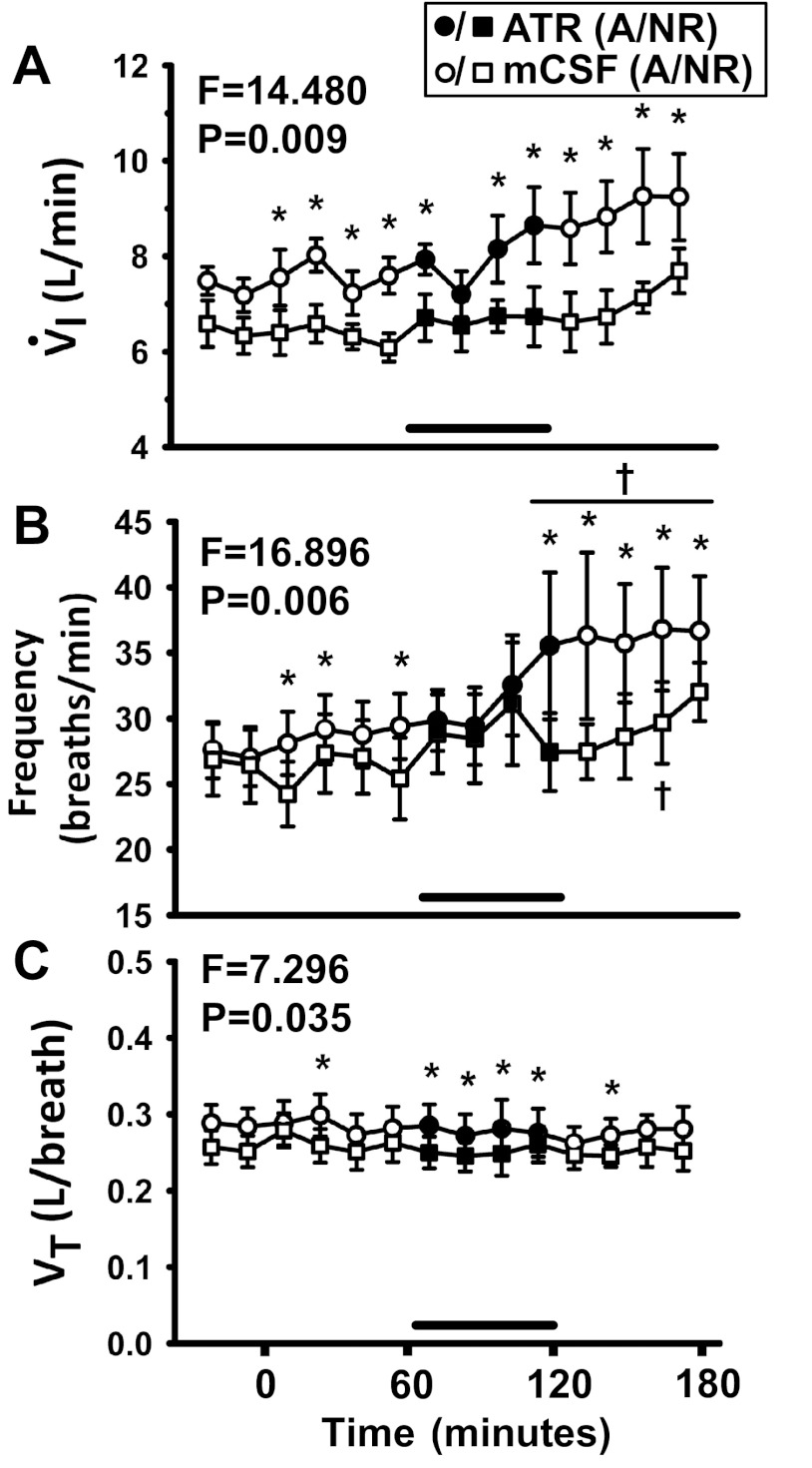
Fig. 3.
There was a significant (P < 0.05) difference in breathing and the effect of atropine dialysis at night on V̇I (A), f (B), and VT (C) in the awake state compared with NREM (n = 7, 2-way RM ANOVA, time and sleep state as factors). x-Axis is time from start of dialysis (min); symbols left of 0 min indicate predialysis period. Squares represent breathing during NREM sleep (NR); circles represent breathing during the awake state at night (“A” in legend). Open symbols indicate dialysis of mCSF; filled symbols and horizontal bar indicate dialysis of atropine. F and P values are taken from 2-way RM ANOVA. *P < 0.05 between states and †P < 0.05 compared with one or more points in the predialysis control period or hour 1, as determined by post hoc analysis (Holm-Sidak). Power of 2-way RM ANOVA tests comparing awake and non-rapid-eye-movement (NREM) sleep were 0.87, 0.92, and 0.56 for V̇I, f, and VT, respectively.
During NREM sleep at night, V̇I was not significantly affected by atropine dialysis (F = 1.207, P = 0.294, 1-way RM ANOVA). Compared with atropine dialysis in the awake state at night, V̇I was significantly lower during NREM sleep (F = 14.48, P = 0.009, 2-way RM ANOVA; Fig. 3A). Atropine dialysis during NREM sleep significantly increased f (F = 4.53, P < 0.001, 1-way RM ANOVA). However, during atropine dialysis studies, f during NREM was significantly lower (F = 16.9, P = 0.006, 2-way RM ANOVA) compared with the awake state at night (Fig. 3B). During NREM sleep, atropine dialysis did not significantly affect VT (F = 1.05, P = 0.42, 1-way RM ANOVA). VT was significantly lower during NREM sleep compared with the awake state at night (F = 7.3, P = 0.035, 2-way RM ANOVA; Fig. 3C).
During the night studies, significantly more time was spent in the awake state than during NREM sleep (F = 25.93, P = 0.002, 2-way RM ANOVA, data not shown). There were no significant effects of atropine dialysis on time spent in either the awake (F = 2.07, P = 0.168) or NREM (F = 1.50, P = 0.26) sleep states (1-way RM ANOVA). In the hour before, during, and after atropine dialysis, the average values for time spent in the awake state ranged between 64 and 76% of total time, whereas average values for time spent in NREM sleep ranged between 21 and 33% of total time. These values are in agreement with previously published data on goats (35). However, in past studies (35) on goats, about 10% of total time was spent in REM sleep, whereas herein only two of seven goats showed periods of REM sleep of any duration. The abnormally low REM sleep time was observed throughout the 3-h protocol and was not unique to the period of atropine dialysis; thus, it appears dialysis per se, and not atropine, reduced time in REM sleep.
Effects of atropine on neurochemicals in effluent mCSF.
Dialysis of mCSF alone in time control studies during the day had no significant effect on effluent concentrations of 5-HT, SP, GLY, GABA, Epi, or DA throughout all three study hours (P > 0.05; Fig. 4), as determined by one-way RM ANOVA. In addition, there were no significant differences (P > 0.05) in hour 1 values for 5-HT, SP, GLY, or GABA between atropine dialysis studies done in the day and atropine dialysis studies done at night (n = 4, data not shown), as determined by paired t-tests.
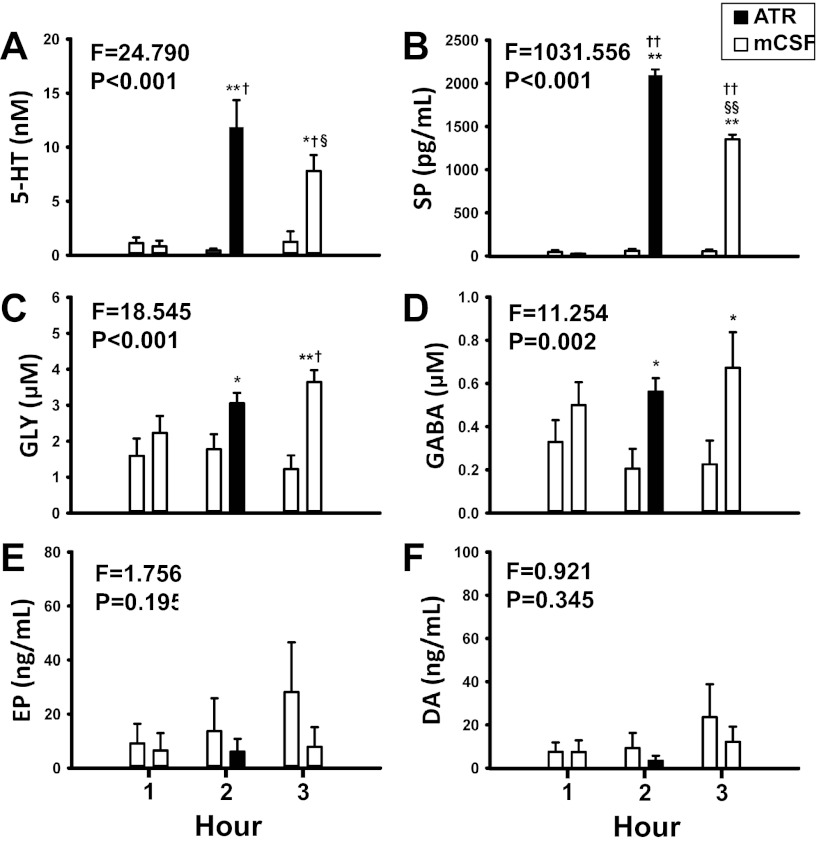
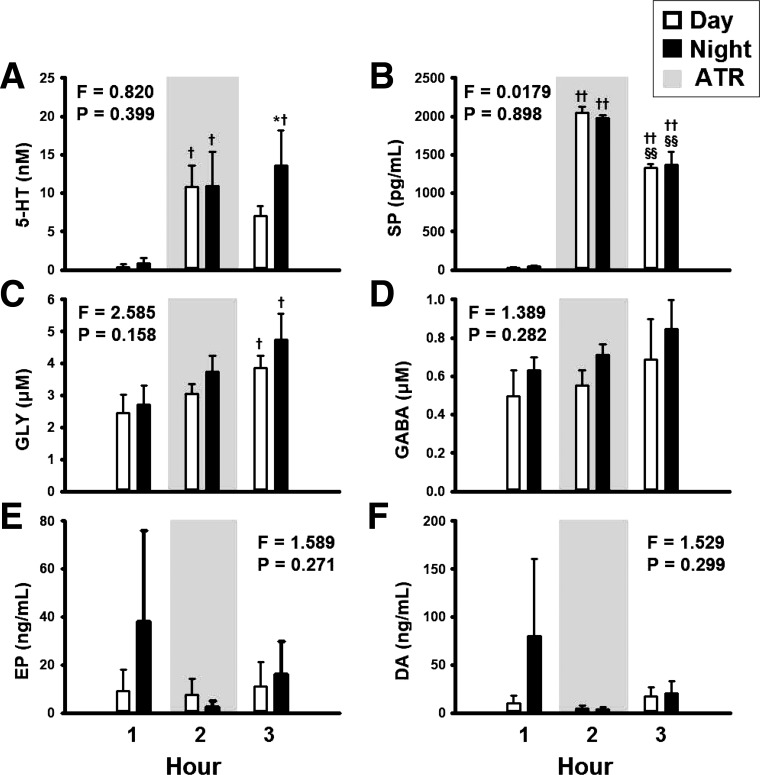
Fig. 4.
The concentrations of serotonin (5-HT, A), substance P (SP, B), glycine (GLY, C), and γ-aminobutyric acid (GABA, D) in the effluent dialysate were significantly (P < 0.05) elevated with daytime dialysis of atropine compared with time control, as determined by 2-way ANOVA (time and treatment as factors). x-Axis indicates hour of dialysis. Open bars indicate dialysis of mCSF; filled bars indicate atropine dialysis. For each hour, open bars on the left are data from time control studies and bars on the right are data from atropine dialysis studies. F and P values are taken from 2-way ANOVA. *P < 0.05 between treatment groups, **P < 0.001 between treatment groups, †P < 0.05 vs. hour 1, ††P < 0.001 vs. hour 1, §P < 0.05 vs. hour 2, and §§P < 0.001 vs. hour 2, as indicated by post hoc analysis (Holm-Sidak). For 5-HT, SP, GLY, GABA: time control, n = 7; atropine studies, n = 9. For epinephrine [Epi (EP)]: time control, n = 6; atropine studies, n = 7. For dopamine (DA): time control and atropine studies, n = 6. Power of the 2-way ANOVAs ranged between 0.9 and 1.0 for 5-HT, SP, GLY, and GABA, whereas for Epi and DA, power was <0.2.
Dialysis of atropine during the day significantly increased the effluent dialysate concentrations of 5-HT (F = 15.99, P < 0.001), SP (F = 530.239, P < 0.001), and GLY (F = 13.7, P < 0.001; Fig. 4, A–C, respectively), as determined by one-way RM ANOVA. These neurochemicals were elevated during atropine dialysis (hour 2) and remained elevated during hour 3. Dialysis of atropine during the day had no significant effect (P > 0.05) on effluent GABA, Epi, or DA concentrations, as determined by one-way RM ANOVA (Fig. 4, D–F, respectively). However, GABA concentrations were significantly (F = 11.254, P = 0.002) higher during and after atropine dialysis (Fig. 4D) compared with time control studies, as determined by two-way ANOVA.
Dialysis of atropine at night significantly increased 5-HT (F = 7.37, P = 0.009), SP (F = 90.04, P < 0.001), and GLY (F = 4.418, P = 0.042) but did not significantly (P > 0.05) increase GABA, Epi, or DA in the effluent dialysate (Fig. 5), as determined by one-way RM ANOVA. The effect of atropine dialysis on any measured neurochemical did not differ between day and night studies (P > 0.05), as determined by two-way RM ANOVA (Fig. 5).
Fig. 5.
The effect of atropine on the effluent concentrations of 5-HT (A), SP (B), GLY (C), GABA (D, n = 7), Epi (E, n = 5), and DA (F, n = 4) did not differ (P > 0.05) between day and night studies, as determined by 2-way RM ANOVA (time and time of day as factors). x-Axis indicates hour of dialysis. Open bars represent atropine dialysis studies done in the day; filled bars represent atropine dialysis studies done at night; gray indicates period of atropine dialysis. F and P values are taken from 2-way RM ANOVA. *P < 0.05 between treatment groups, †P < 0.05 vs. hour 1, ††P < 0.001 vs. hour 1, and §§P < 0.001 vs. hour 2, as determined by post hoc analysis (Holm-Sidak). Power for all 2-way RM ANOVAs were <0.20.
Dialysis of atropine did not affect the concentrations of either ACh (F = 1.250, P = 0.337) or choline (F = 1.244, P = 0.338) when data from day and night dialysis studies were combined (1-way RM ANOVA, data not shown).
Local vs. global effects of atropine dialysis on neurochemicals.
In group 3 studies, during dialysis of atropine, effluent 5-HT concentrations were 6.16 and 5.31 nM on the left and right sides, respectively, but, in the contralateral side that received only mCSF, 5-HT was nondetectable (data not shown). Similarly, SP concentrations were 2,199.7 and 2,114.4 pg/ml on the side that received atropine, but, on the contralateral side, SP was either nondetectable or within the range of time control studies (42.5 pg/ml, data not shown). GLY increased on the side receiving atropine by 0.54 and 0.79 μM, while decreasing by 0.35 μM or increasing by 0.32 μM on the contralateral side (data not shown). GABA tended to decrease in both the side receiving atropine and the side receiving mCSF (data not shown). It is important to note that the agreement in measured effluent neurochemicals on the left and right sides was excellent.
Metabolic rate and body temperature effects of atropine.
There were no significant (P > 0.05) changes in V̇o2 and V̇co2 (Fig. 6, A and B, respectively), as determined by one-way RM ANOVA, or in Tr (paired t-test, data not shown) within time control studies. V̇co2 did not change significantly during atropine dialysis (F = 1.45, P = 0.253, 1-way RM ANOVA). However, during atropine dialysis, V̇o2 was significantly increased (F = 4.38, P = 0.014, 1-way RM ANOVA). In addition, both V̇o2 (F = 14.66, P < 0.001) and V̇co2 (F = 10.99, P = 0.002) were significantly higher during atropine studies in the day compared with time control studies, as determined by two-way ANOVA (Fig. 6, A and B, respectively). In addition, Tr increased significantly (P < 0.05) during daytime atropine dialysis, as determined by paired t-tests (data not shown). Metabolic rate was not measured during night studies. Baseline Tr during night atropine dialysis was significantly higher than in daytime atropine studies by 0.46°C, as determined by paired t-test (P = 0.036, data not shown). Atropine dialysis at night did not significantly affect Tr (P > 0.05, paired t-test, data not shown).
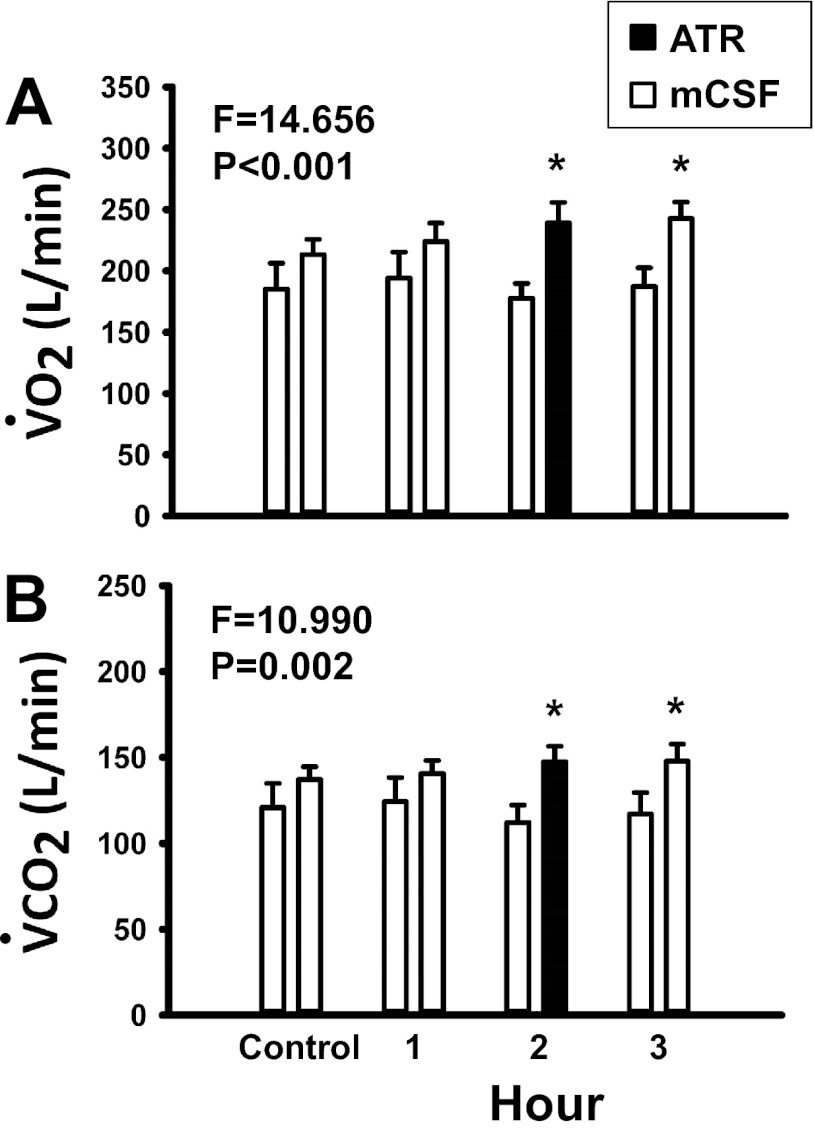
Fig. 6.
Oxygen consumption (V̇o2, A) and carbon dioxide excretion (V̇co2, B) were significantly (P < 0.05) elevated during dialysis of atropine (n = 9) in the day compared with time control studies (n = 7), as determined by 2-way ANOVA (time and treatment as factors). x-Axis indicates predialysis control period and hour of dialysis. Open bars represent dialysis of mCSF; filled bars represent dialysis of atropine. For each hour, open bars on the left are data from time control studies and bars on the right are data from daytime atropine studies. F and P values are taken from 2-way ANOVA. *P < 0.05 between treatment groups, as determined by post hoc analysis (Holm-Sidak). Power for the 2-way ANOVA for V̇o2 was 0.967, and, for V̇co2, power was 0.892.
DISCUSSION
Based on mAChR distribution (32) and functional in vitro evidence that neuromodulation of preBötC function by ACh is predominantly excitatory (45, 47), we first hypothesized that dialysis of atropine in the preBötC region of the ventral respiratory column would decrease breathing of awake and sleeping goats. This hypothesis was not validated, since in awake goats, we found that dialysis of atropine in the preBötC region increased rather than decreased breathing frequency and V̇I. In addition, given that excitatory modulation is reduced during sleep (5), our second hypothesis was that atropine dialysis would decrease breathing more while awake than while asleep. This hypothesis was not validated, since atropine increased breathing during both states, but to a greater extent during the awake state. Finally, based on the observation that reductions in one excitatory neuromodulator leads to compensatory increases in other excitatory neuromodulators (18), our third hypothesis was that blocking mAChRs in the preBötC region with atropine would increase 5-HT and SP in the effluent dialysate. This hypothesis was validated, since preBötC atropine dialysis led to local increases in 5-HT and SP levels as measured in the effluent dialysate.
Anatomic sites affected by atropine.
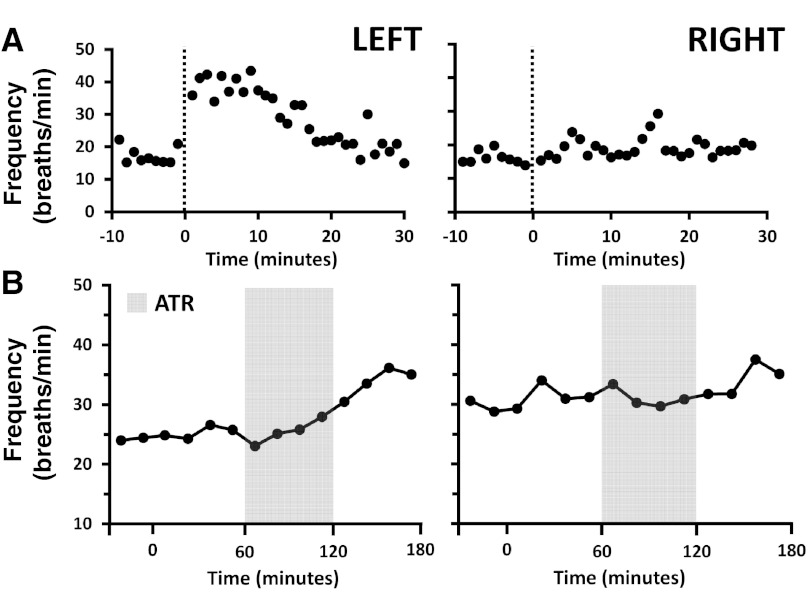
We are unable to state with certainty the degree to which atropine affected preBötC neurons because: 1) the exact anatomic site of the preBötC remains controversial for all species (23, 24, 29, 57), 2) there is no unequivocal physiological marker of the preBötC, and 3) it is problematic to calculate/determine the area of atropine diffusion (31). We have previously provided estimates of the anatomic site of the preBötC and a physiological marker of the preBötC in goats (29, 57). The data on goat 12 are consistent with these estimates, since the right MT was rather caudal and medial to the presumed preBötC region, whereas the left MT was within our estimates of the preBötC region (Fig. 1). As shown in Fig. 7, the breathing frequency response to our physiological marker of the preBötC (NMDA injection) and to atropine dialysis was minimal on the right side and robust on the left side in goat 12. Moreover, although there was substantial variation among goats, the histologically identified MT locations and/or the responses to NMDA (Fig. 1) indicate that the atropine was dialyzed within the preBötC region of the ventral respiratory column in all goats. Accordingly, we conclude it is likely that the atropine reached the preBötC region in all goats to a variable extent and that, in most goats, it is also likely that the atropine affected airway motor neurons in nucleus ambiguous and/or chemoreceptor neurons near the ventral lateral medullary surface (4, 5, 39).
Fig. 7.
Proximity to preBötC is a determinant of ventilatory response (f) to injection of the glutamate agonist NMDA (100 mM, 500 nl) (A) and dialysis of atropine (B) in the day. Data are from goat 12, in whom the right MT was implanted relatively caudal and medial to the preBötC region, whereas the left MT was closer in proximity to the preBötC (Fig. 1). This figure shows that the ventilatory responses to NMDA and atropine were greater on the left side than on the right side. x-Axes indicate time (min). A: symbols left of 0 min indicate pre-NMDA injection period, and dotted lines indicate NMDA injection. B: symbols left of 0 min indicate predialysis control period, and gray indicates dialysis of atropine.
Muscarinic receptor subtypes and their role in preBötC function.
mAChRs are metabotropic G protein-coupled receptors that activate second messenger pathways resulting in an increase or decrease in neuronal excitability, depending on the second messenger pathways activated/inhibited (6, 10, 20). mAChRs are categorized into five subtypes. M1, M3, and M5 mAChRs are preferentially coupled to the Gq/11 subtype, and activation of these receptors leads to an increase in intracellular calcium (10, 20), and can suppress inhibitory currents such as the potassium M current (6, 13), both of which would lead to an increase in neuronal excitability. M2 and M4 mAChRs are preferentially coupled to the Gi/o subtype, and activation of these receptors leads to inhibition of adenylyl cyclase activity, and to a decrease in neuronal excitability and neurotransmitter release (1, 3, 6). mAChRs are extensively distributed in the cortex and brain stem, including respiratory-related nuclei (34, 36, 45), and are found pre- and postsynaptically on cholinergic and noncholinergic neurons (27, 38). Accordingly, the effect of ACh or atropine at any site is dependent on the: 1) subtype of receptors at that site, 2) the balance of pre- vs. postsynaptic expression of the different subtypes, and 3) distribution of these receptors on nerve terminals of neurons that release other excitatory or inhibitory neuromodulators. The latter two factors may determine the capability to compensate for attenuation of one or more excitatory inputs, and thus provide a mechanism by which attenuation of an individual neuromodulator could increase the local levels of other neuromodulators. Differences in the basal levels of the neuromodulators themselves may also be a contributing factor related to the compensatory capacity of each site.
Effects of atropine in the preBötC region while awake.
Findings of Lai et al. suggest that M2 and M3 are the primary mAChR subtypes expressed in the neonatal rat preBötC (32). Other in vitro studies implicate a role for the M3 subtype (45) or nicotinic receptors (47) in mediating excitatory ACh neurotransmission in the preBötC (45), whereas presynaptic activation of the M2 subtype appears to have an inhibitory effect on neurotransmitter release (3, 42, 56). Because dialysis of atropine did not significantly affect ACh or choline, the increase in breathing is unlikely the result of increased ACh release, increased activation of nicotinic receptors (47), or decreased turnover of ACh.
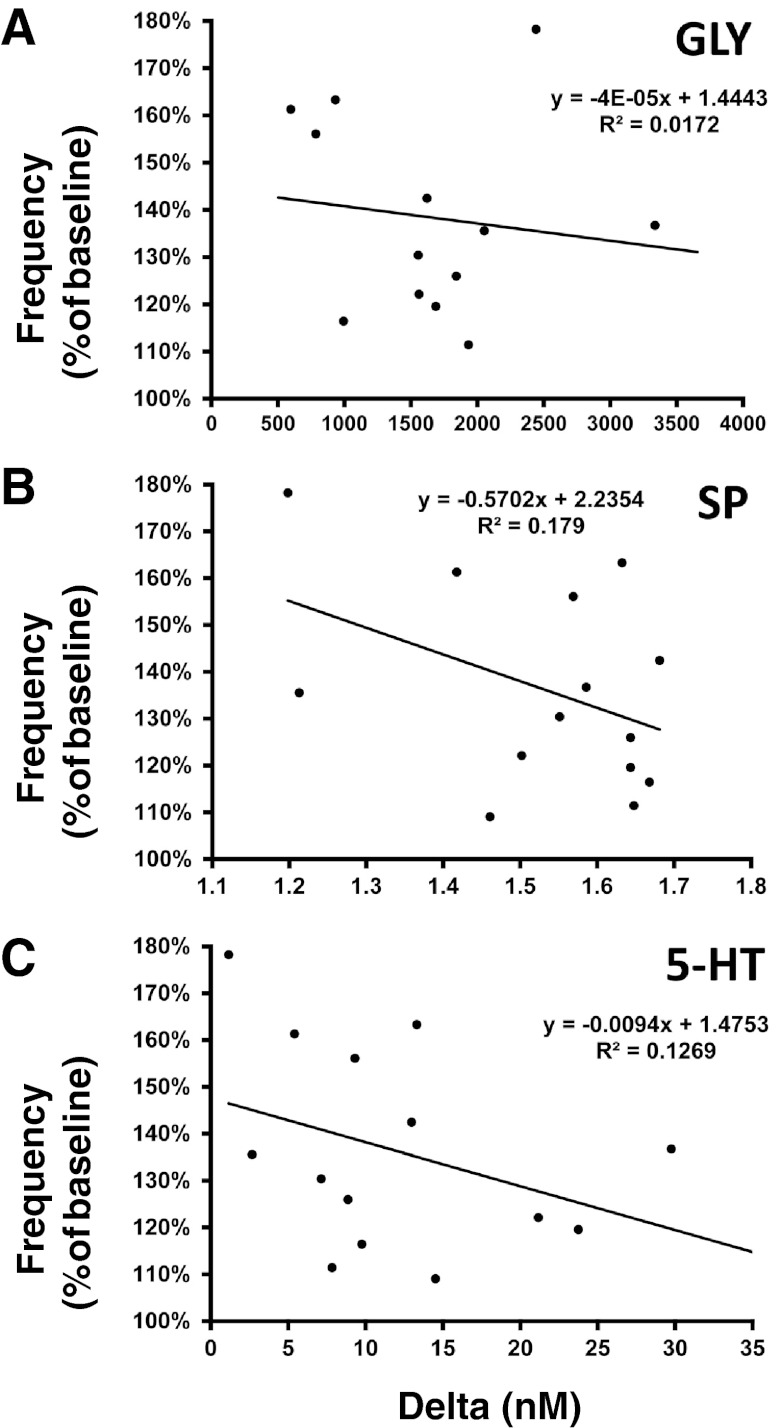
It would seem that the atropine-induced increases in the excitatory neuromodulators, 5-HT and SP (Fig. 4), would contribute to the atropine-induced increase in breathing (Fig. 2). This effect may have been countered by the concurrent increase in GLY (Fig. 4). However, there was no positive correlation between the increase in breathing and the changes in 5-HT, SP, and GLY (Fig. 8). Thus, the increase in these neurochemicals does not readily account for the effect of atropine on breathing.
Fig. 8.
There were no significant correlations between the ventilatory response to atropine dialysis (f as percent of baseline) and the corresponding increases in GLY (A), SP (B), and 5-HT (C). Plotted data are from individual daytime atropine studies showing the percent peak increase in f against the corresponding absolute changes in neurochemicals from baseline (hour 1) to peak values during or after atropine dialysis (hours 2 or 3, respectively).
A potential mechanism by which atropine increased 5-HT and SP is that atropine blocked inhibitory presynaptic M2 receptors on 5-HT/SP terminals, facilitating the release of these neuromodulators. This enhanced release of 5-HT and SP may have led to an increase in GLY release, or atropine had a direct effect on glycinergic neurons. Whatever the mechanism of the changes in neuromodulator levels, the data emphasize that perturbations of a single neuromodulator will result in multiple changes, including what appear to be compensatory (or even overcompensatory) changes, in other neuromodulators (18).
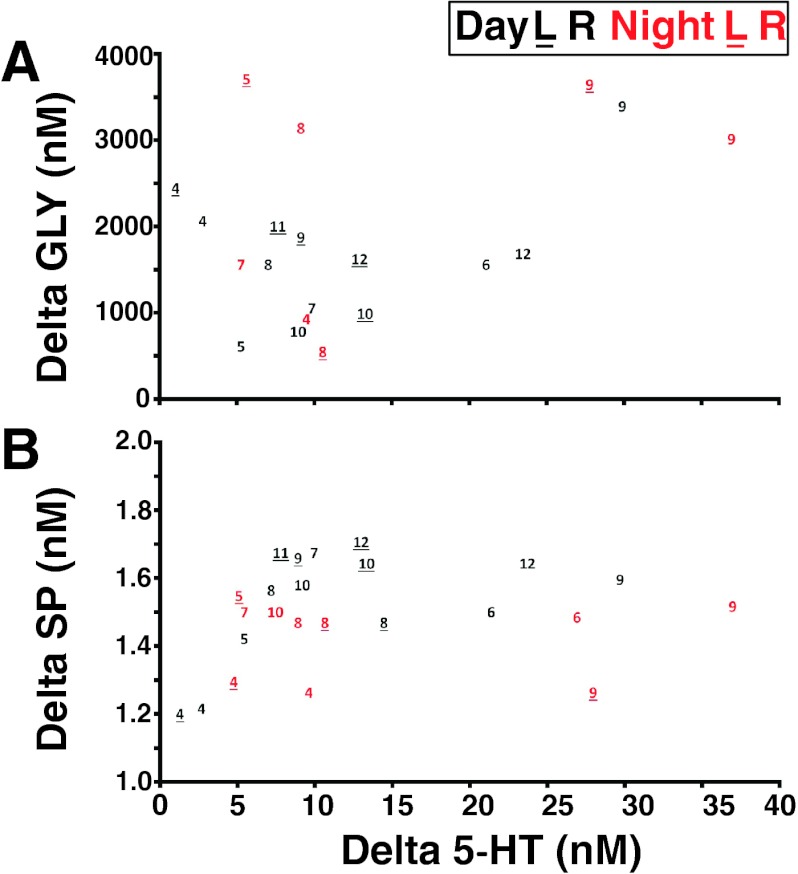
As shown in Fig. 9, the increase in neurochemical release from basal levels with atropine dialysis ranged between 1.16 and 29.8 nM for 5-HT, 1.198 and 1.681 nM for SP, and 595.6 and 3,337.1 nM for GLY. The relatively greater variability in 5-HT and GLY was consistently most extreme in goats 6, 9, and 12 (Fig. 9). The MT placements in these three goats were in close proximity to goats that had a small 5-HT increase with atropine dialysis (compare placement in goats 4 and 6 left side, 3–4 mm rostral to obex; Fig. 1). Note also that there was a large increase in 5-HT (such as in goat 12) even when the left and right MTs were not placed at the same site on each side. For all three neurochemicals, there were generally no significant (P > 0.10) differences between day and night studies (Fig. 5). The greater variability in 5-HT release than in SP release is striking because these neurochemicals are coreleased from 5-HT terminals. Thus, it seems that atropine may have had differential effects on mechanisms regulating 5-HT and SP in the synaptic cleft.
Fig. 9.
There were no trends in the absolute changes in effluent concentration of GLY (A) or SP (B) and the corresponding change in 5-HT (x-axis) with dialysis of atropine. Plotted data are from individual day (black font) and night (red font) atropine studies from goats identified by the assigned nos. shown in Fig. 1. Plots show the absolute change in effluent neurochemical concentration from baseline (hour 1) to peak values during or after atropine dialysis (hours 2 or 3, respectively). Note that values for individual animals tend to cluster (e.g., 5-HT values for goat 9 are consistently high, whereas values for goat 4 are consistently low). Note also that MT placement affected the responses for some, but not all, neurochemicals. For example, in goat 12, the MTs were not symmetrically implanted (Fig. 1), yet GLY and SP values were nearly the same in the left and right MTs, whereas there was considerable difference in 5-HT values between the two MTs. L, left side; R, right side.
Both V̇o2 and V̇co2 were significantly (P ≤ 0.002) higher during dialysis of atropine than during time control studies. It is well known that V̇I is coupled to metabolic rate (15, 22) during exercise, changes in body weight, and other conditions. Thus, it is possible that changes in metabolic rate contributed to the atropine-induced changes in breathing.
Effects of atropine dialysis in the preBötC region during sleep.
During sleep, the activity of neurons that synthesize several excitatory neuromodulators is lower than during wakefulness (5). Precise measurement of neuromodulators during natural sleep has proven to be difficult, but it is conceivable that excitatory neuromodulation of the preBötC is reduced during natural sleep. Similarly, the levels of GLY and GABA at the preBötC during sleep are also unknown. Whereas there were no statistically significant differences in the baseline values of 5-HT, SP, GLY, and GABA between atropine studies done in the day compared with those done at night (Fig. 5), we did note a trend toward higher GLY concentrations at night, although we cannot determine differences in neurochemical measurements between wakefulness and NREM sleep. Nevertheless, our data suggest that at night, and possibly during NREM sleep, there is a shift toward a relatively greater inhibition of breathing. Such a shift may contribute to the lower breathing during NREM sleep compared with wakefulness (Fig. 3) and also to the reduced tachypnea during atropine dialysis in NREM compared with wakefulness.
We also cannot exclude the contribution of other neuromodulators that we did not measure to the state-dependent effects of atropine on breathing. For example, it is well known that the neuropeptide orexin contributes to arousal state (26) and that orexinergic neurons are more active in the awake state than during sleep (54). These neurons have widespread projections to the brain stem, including respiratory-related nuclei (43, 58), and there is evidence that orexin contributes to chemosensitivity, particularly more in the awake state compared with NREM sleep (17). It is possible that the withdrawal of orexinergic input to the preBötC, or to neurons that project to the preBötC, during NREM may underlie the reduced effect of atropine dialysis on breathing in this state, despite an increase in 5-HT and SP. These data highlight the need to further investigate how attenuation of one neuromodulator affects the levels of other neuromodulators, and the conditions under which these interactions affect physiological behavior.
The state-dependent differences in effects of atropine could also be caused by postulated state-dependent differences in the mechanisms of rhythm generation, which is a concept suggested by mathematical models (44) and supported by experimental data (29, 37). Conceivably, pre- and postsynaptic M2 and M3 receptors may not be equally distributed on neurons primarily responsible for rhythm generation while awake vs. neurons responsible for rhythm generation during sleep.
Difference in response to atropine dialysis within the preBötC and PRG.
Mallios et al. found M1 to M3 mAChR subtypes in the lateral and medial parabrachial nuclei (LPBN and MPBN, respectively) and the KFN of cats (34). Generally, the expression of each mAChR subtype was slightly higher in the LPBN and MPBN than in the KFN. We have documented the presence of M2 mAChRs in all three of these nuclei in goats (8). In addition, dialysis of atropine in the LPBN and MPBN had no effect on breathing during the day and night (awake or NREM sleep) (8). However, dialysis of atropine in the KFN decreased breathing frequency at night while awake and during NREM sleep but had no effect during the day while awake (8). This response is clearly different from the response reported herein on atropine dialysis in the preBötC region. These site-specific differences in the response to atropine between the KFN, LPBN/MPBN, and preBötC region are potentially due to site-specific differences in: 1) mAChR subtypes, 2) the balance of pre- vs. postsynaptic expression of the different subtypes, and 3) distribution of mAChRs on nerve terminals of other neurons releasing excitatory or inhibitory neuromodulators, and/or combinations of these differences.
An alternative explanation for the different effects of atropine dialysis in the KFN and preBötC region is that the differences indicate that the site of respiratory rhythm generation is within the rostral pons and not the preBötC region. Notable were previous findings in decerebrate cats that a presumed respiratory rhythm is sustained in the mylohyoid branch of the trigeminal nerve after the phrenic rhythm is abolished by chemical lesions in the medulla (53). These data led to the conclusion that the neurogenesis of eupnea is within rostral pontine nuclei (53). This conclusion is supported by data showing that chemical destruction of the preBötC in vivo only transiently eliminated respiratory rhythm (51). Moreover, data from other studies have shown that a pontine-medullary circuit is necessary for the three-phase eupneic respiratory rhythm and that the medulla in isolation is only capable of generating the gasping rhythm (48, 52, 53). Accordingly, our findings might be interpreted as indicating that the neurons in the KFN and preBötC region have different functions during eupnea, and the KFN seems more likely to generate the eupneic rhythm than the preBötC region.
Implications of present findings.
Our data suggest that inhibition of mAChRs within the preBötC region leads to an increase in breathing frequency potentially through increases in local 5-HT and SP, thus drawing greater attention to the physiological complications of a specific pharmacological intervention in vivo. Multiple past studies were designed (and data interpreted) based on the assumption that the major response of the primary variable (breathing frequency) was due to a direct effect of the perturbation (atropine dialysis). However, we found that the major effect of atropine on breathing (an increase in breathing frequency) is potentially due to an indirect effect of compensatory increases in 5-HT and SP, which may be partially countered by an increase in GLY. These data emphasize that indirect effects complicate interpretation of data obtained in vivo and emphasize the need for attempts to measure and/or account for indirect effects of pharmacological perturbations for more precise conclusions and better foresight in study design.
Caveats and limitations.
Two limitations of the present study are: 1) because of the length of the inlet tubing, there was a delay between when dialysis of atropine was initiated and when it actually reached the tissue, and 2) because of the manual system of switching between dialysate collection vials, we could not separate collection of effluent dialysate during the awake and sleep states. Thus, neurochemical values reported for night dialysis studies are a mixture of neurochemicals collected during both awake and sleep states. A major emphasis in this and past studies was to minimize extraneous factors potentially disturbing the goats during periods of study. Accordingly, the goats were in an environmental chamber isolated as much as possible from all laboratory equipment and the investigators except for ∼1 min required for exchange of the effluent dialysate collection tubes at the end of each hour of dialysis. This physical separation of the equipment and animals necessitated an extended length (150 or 180 cm) of tubing to deliver the perfusate from the pump to the probe. As a result, when a new dialysis period was initiated, there was an estimated 20-min delay before the fresh perfusate reached the probe due to the length of the perfusion line. Thus, it is likely that the atropine did not reach the tissue until ∼20 min into hour 2, continuing through the first 20 min of hour 3 (mCSF dialysis). These delays may explain why breathing during atropine dialysis did not increase until the latter 30 min of hour 2 and why it remained elevated throughout hour 3. Moreover, the delay may also explain why the local neuromodulator concentrations remained elevated during hour 3. Although it was possible to have flushed the inlet line at the beginning of each new dialysis period or to replace the inlet line between dialysis hours, doing so would have disturbed the animal and risked damaging the probe. Furthermore, there would still be a delay because of the probe itself and the time required for exchange with and diffusion in the tissue. For these reasons, we decided to forego these options to minimize the risk of agitating the animal or damaging the probe. The same problems and risks made it undesirable to separate the effluent mCSF collection between wakefulness and sleep as the goats cycled through these states.
Other limitations were the potential tissue damage resulting from repeated probe insertions and/or the potentially chronic effects of multiple periods of atropine dialysis. Although it is likely that probe insertion created some degree of tissue damage, there were no statistical (1-way RM ANOVA) trends for hour 1 (baseline) levels of 5-HT, SP, GLY, or GABA to change from day 1 to day 2, 3, or 4 even after there had been large increases in neurochemicals in preceding days. These data suggest that any potential chronic effects of atropine dialysis and/or tissue damage had relatively small effects on local neurochemical levels during dialysis of mCSF or in response to atropine.
Small sample size and variability between goats also were potential limitations in some parts of our study. These and the limitations stated above restrict the conclusions we can make from our data; thus, our stated conclusions are conservative, but warranted, despite these limitations.
Conclusions.
The major conclusions from the present study are: 1) perturbation of a single neuromodulator system within the preBötC region results in compensatory (or even overcompensatory) changes in other neuromodulators, further supporting the concept that “a modulator's action is determined by the concurrent modulation and interaction with other neuromodulators” (18); 2) disruption of cholinergic neuromodulation within the preBötC region has state-dependent effects on breathing, further supporting the concept that the fundamental mechanisms controlling breathing are state-dependent (18, 44); and 3) interpretation of data from in vivo perturbations within the respiratory control network (and likely elsewhere) must account for direct and indirect effects of the primary perturbation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-25739 and HL-007852 and also by the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.M. and H.V.F. conception and design of research; C.M., S.E.N., J.M., S.J.O., L.G.P., and H.V.F. performed experiments; C.M., S.E.N., M.R.H., and H.V.F. analyzed data; C.M., M.R.H., and H.V.F. interpreted results of experiments; C.M. and H.V.F. prepared figures; C.M., M.R.H., and H.V.F. drafted manuscript; C.M., S.E.N., J.M., S.J.O., M.R.H., and H.V.F. edited and revised manuscript; C.M., S.E.N., M.R.H., and H.V.F. approved final version of manuscript.
REFERENCES
- 1. Baghdoyan HA, Lydic R, Fleegal MA. M2 muscarinic autoreceptors modulate acetylcholine release in the medial pontine reticular formation. J Pharmacol Exp Ther 286: 1446–1452, 1998 [PubMed] [Google Scholar]
- 2. Baghdoyan HA, Mallios VJ, Duckrow RB, Mash DC. Localization of muscarinic receptor subtypes in brain stem areas regulating sleep. NeuroReport 5: 1631–1634, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol 76: 3758–3770, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Bellingham MC, Funk GD. Cholinergic modulation of respiratory brain-stem neurons and its function in sleep-wake state determination. Clin Exp Pharmacol Physiol 27: 132–137, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Bellingham MC, Ireland MF. Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir Physiol Neurobiol 131: 135–144, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Betke KM, Wells CA, Hamm HE. GPCR mediated regulation of synaptic transmission. Prog Neurobiol 96: 304–321, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohmer G, Schmid K, Baumann M. Evidence for a respiration-modulated cholinergic action on the activity of medullary respiration-related neurons in the rabbit. An iontophoretic study. Pflugers Arch 415: 72–80, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV. A role for the Kolliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J Appl Physiol 109: 159–170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonner TI, Young AC, Brann MR, Buckley NJ. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron 1: 403–410, 1988 [DOI] [PubMed] [Google Scholar]
- 10. Brown DA. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms. J Mol Neurosci 41: 340–346, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci 8: 4646–4652, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burton MD, Nouri K, Baichoo S, Samuels-Toyloy N, Kazemi H. Ventilatory output and acetylcholine: perturbations in release and muscarinic receptor activation. J Appl Physiol 77: 2275–2284, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Caulfield MP, Jones S, Vallis Y, Buckley NJ, Kim GD, Milligan G, Brown DA. Muscarinic M-current inhibition via G alpha q/11 and alpha-adrenoceptor inhibition of Ca2+ current via G alpha o in rat sympathetic neurones. J Physiol 477: 415–422, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dean C, Geiger LK, Sprtel BM, Ohtake PJ, Forster HV. An anatomic atlas of the medulla oblongata of the adult goat. J Appl Physiol 87: 1220–1229, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Dempsey JA, Gledhill N, Reddan WG, Forster HV, Hanson PG, Claremont AD. Pulmonary adaptation to exercise: effects of exercise type and duration, chronic hypoxia and physical training. Ann NY Acad Sci 301: 243–261, 1977 [DOI] [PubMed] [Google Scholar]
- 16. Dev NB, Loeschcke HH. A cholinergic mechanism involved in the respiratory chemosensitivity of the medulla oblongata in the cat. Pflugers Arch 379: 29–36, 1979 [DOI] [PubMed] [Google Scholar]
- 17. Dias MB, Li A, Nattie E. The orexin receptor 1 (OX(1)R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir Physiol Neurobiol 170: 96–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci 30: 8251–8262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Douglas CL, Demarco GJ, Baghdoyan HA, Lydic R. Pontine and basal forebrain cholinergic interaction: implications for sleep and breathing. Respir Physiol Neurobiol 143: 251–262, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Eglen RM. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol 26: 219–233, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Feroah TR, Forster HV, Pan L, Schlick NE, Martino P, Rice T. Negative pressure effects on mechanically opposing pharyngeal muscles in awake and sleeping goats. J Appl Physiol 91: 2289–2297, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Forster HV, Haouzi P, Dempsey JA. Control of breathing during exercise. Comp Physiol 2: 743–777, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 286: 1566–1568, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci 22: 3806–3816, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haji A, Furuichi S, Takeda R. Effects on iontophoretically applied acetylcholine on membrane potential and synaptic activity of bulbar respiratory neurones in decerebrate cats. Neuropharmacology 35: 195–203, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Han F, Mignot E, Wei YC, Dong SX, Li J, Lin L, An P, Wang LH, Wang JS, He MZ, Gao HY, Li M, Gao ZC, Strohl KP. Ventilatory chemoresponsiveness, narcolepsy-cataplexy, and human leukocyte antigen HLADQB1*0602 status. Eur Respir J 36: 577–583, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Hersch SM, Levey AI. Diverse pre- and post-synaptic expression of m1-m4 muscarinic receptor proteins in neurons and afferents in the rat neostriatum. Life Sci 56: 931–938, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol 96: 1815–1824, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, Qian B, Pan LG. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Botzinger complex and the surrounding region. J Appl Physiol 106: 605–619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krause KL, Neumueller SE, Marshall BD, Kiner T, Bonis JM, Pan LG, Qian B, Forster HV. Micro-opioid receptor agonist injections into the presumed pre-Botzinger complex and the surrounding region of awake goats do not alter eupneic breathing. J Appl Physiol 107: 1591–1599, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lai J, Shao XM, Pan RW, Dy E, Huang CH, Feldman JL. RT-PCR reveals muscarinic acetylcholine receptor mRNA in the pre-Botzinger complex. Am J Physiol Lung Cell Mol Physiol 281: L1420–L1424, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA. Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience 63: 207–221, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Mallios VJ, Lydic R, Baghdoyan HA. Muscarinic receptor subtypes are differentially distributed across brain stem respiratory nuclei. Am J Physiol Lung Cell Mol Physiol 268: L941–L949, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Martino PF, Forster HV, Feroah T, Wenninger J, Hodges M, Pan LG. Do neurotoxic lesions in rostral medullary nuclei induce/accentuate hypoventilation during NREM sleep? Respir Physiol Neurobiol 138: 59–75, 2003 [DOI] [PubMed] [Google Scholar]
- 36. McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol 27: 126–131, 2000 [DOI] [PubMed] [Google Scholar]
- 37. McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci 8: 1142–1144, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mrzljak L, Levey AI, Rakic P. Selective expression of m2 muscarinic receptor in the parvocellular channel of the primate visual cortex. Proc Natl Acad Sci USA 93: 7337–7340, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nattie EE, Li AH. Ventral medulla sites of muscarinic receptor subtypes involved in cardiorespiratory control. J Appl Physiol 69: 33–41, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Nattie EE, Wood J, Mega A, Goritski W. Rostral ventrolateral medulla muscarinic receptor involvement in central ventilatory chemosensitivity. J Appl Physiol 66: 1462–1470, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Pagnotta SE, Lape R, Quitadamo C, Nistri A. Pre- and postsynaptic modulation of glycinergic and gabaergic transmission by muscarinic receptors on rat hypoglossal motoneurons in vitro. Neuroscience 130: 783–795, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rybak IA, O'Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol 100: 1770–1799, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBotzinger complex inspiratory neurons. J Neurophysiol 83: 1243–1252, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shao XM, Ge Q, Feldman JL. Modulation of AMPA receptors by cAMP-dependent protein kinase in preBotzinger complex inspiratory neurons regulates respiratory rhythm in the rat. J Physiol 547: 543–553, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shao XM, Tan W, Xiu J, Puskar N, Fonck C, Lester HA, Feldman JL. Alpha4* nicotinic receptors in preBotzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J Neurosci 28: 519–528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci 364: 2577–2587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Botzinger complex in vivo. J Neurophysiol 81: 1150–1161, 1999 [DOI] [PubMed] [Google Scholar]
- 51. St-Jacques R, St-John WM. Transient, reversible apnoea following ablation of the pre-Botzinger complex in rats. J Physiol 520: 303–314, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. St-John WM, Paton JF. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Respir Physiol 123: 201–213, 2000 [DOI] [PubMed] [Google Scholar]
- 53. St John WM, Bledsoe TA. Genesis of rhythmic respiratory activity in pons independent of medulla. J Appl Physiol 59: 684–690, 1985 [DOI] [PubMed] [Google Scholar]
- 54. Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience 153: 860–870, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci 11: 538–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang L, Yuan LL. Activation of M2 muscarinic receptors leads to sustained suppression of hippocampal transmission in the medial prefrontal cortex. J Physiol 587: 5139–5147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Botzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol 97: 1629–1636, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol 98: 1387–1395, 2005 [DOI] [PubMed] [Google Scholar]