Abstract
Epidemiological data support the concept that phenols and polyphenols in diet are safe and nontoxic, and have long-lasting beneficial effects on human health. The potential target for complementary and alternative medicine (CAM) research has been on the discovery of natural compounds that can be used in the prevention and treatment of cancer. Propolis is one of the richest sources of plant phenolics (flavonoids and phenolic acids). The ethanolic extract of propolis (EEP) and its polyphenols possess immunomodulatory, chemopreventive, and antitumor effects. Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) is a naturally occurring anticancer agent that preferentially induces apoptosis in cancer cells and is not toxic to normal cells. Endogenous TRAIL plays a significant role in immunosurveillance and defense against cancer cells. However, as more tumor cells are reported to be resistant to TRAIL-mediated death, it is important to develop new strategies to overcome this resistance. EEP and polyphenols isolated from propolis have been shown to sensitize cancer cells to TRAIL-induced apoptosis. In this paper we demonstrate for the first time the crucial role of the main phenolics isolated from propolis in enhancing TRAIL-mediated death in tumor cells for cancer chemoprevention.
1. Introduction
The induction of cancer cell-specific apoptosis via the activation of TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) signaling has become an important focus of cancer research [1, 2]. However, as more tumor cells are reported to be resistant to TRAIL-mediated death, it is necessary to develop new strategies to overcome this resistance [3–6]. Propolis and its phenolic components exert anticancer and chemopreventive properties by multiple mechanism of action affecting apoptotic pathways in cancer cells [7, 8]. Extracts of propolis and polyphenols isolated from propolis have been shown to sensitize cancer cells to TRAIL-induced apoptosis [9–11]. In this paper, we summarize the evidence for the crucial role of the main phenolics isolated from propolis in enhancing TRAIL-mediated death in tumor cells for cancer chemoprevention.
2. Propolis and Its Polyphenolic Constituents as Cancer Chemopreventive Agents
Propolis (bee glue) is a resinous hive product collected by honey bees from many plant sources. The chemical composition of propolis is complex and largely depends on the geographical origin and specific flora at the site of collection [12]. It usually contains a variety of different compounds, including phenolic acids or their esters, flavonoids (flavones, flavanones, isoflavones, flavonols, dihydroflavonols, chalcones), terpenes, aromatic aldehydes and alcohols, fatty acids, stilbenes, and β-steroids [13, 14].
Propolis is one of the richest sources of plant phenolics (flavonoids and phenolic acids) [9, 13]. Cinnamic acid, o-coumaric acid, m-coumaric acid, p-coumaric acid, ferulic acid, isoferulic acid, caffeic acid, caffeic acid phenylethyl ester (CAPE), chrysin, tectochrysin, apigenin, acacetin, naringenin, rhamnetin, pinocembrin, pinostrobin (pinocembrin-7-methylether), pinobanksin, sakuranetin, isosakuranetin, galangin, kaempferol, kaempferide, quercetin have been reported to be identified in European propolis (Croatian, Dutch, Polish, Portuguese, and Slovenian) [8–10, 15–19]. The main constituents of Brazilian green propolis are p-coumaric acid, ferulic acid, cinnamic acid, and its derivative—drupanin, baccharin, and artepillin C, chrysin, tectochrysin, pinocembrin, pinobanksin, isosakuranetin, kaempferol, kaempferide, and quercetin [20–23]. Brazilian red propolis is abundant with pinocembrin, pinobanksin, liquiritigenin, naringenin, daidzein, formonetin, biochanin A, quercetin, rutin, and isoliquiritigenin [24]. Chinese propolis contains CAPE, chrysin, and pinocembrin at high concentration [25].
Propolis as a harmless natural product has been used in folk medicine since ancient times and recently, it became a subject of special interest in the area of oncological research as a source of valuable polyphenolic compounds for the prevention and treatment of cancer [7, 8, 26]. Propolis cannot be used as raw material and it must be purified by extraction to remove the inert material and preserve the polyphenolic fraction [10]. The ethanolic extract of propolis (EEP) and its phenolics possess immunomodulatory, anticancer, and chemopreventive properties [27–35].
Chemoprevention is a means of cancer control in which carcinogenesis is inhibited or reversed by nutritional or pharmacological intervention with natural or synthetic agents [36–38]. When Dr. Michael Sporn for the first time introduced the term “chemoprevention,” referring to the activity of natural forms of vitamin A and its synthetic analogs in preventing the development and progression of epithelial cancer, he originated a novel field in cancer research [39].
Several mechanisms contribute to the overall cancer preventive and antitumor properties of propolis. EEP and its phenolic components suppress proliferation and tumor growth, induce cell-cycle arrest and apoptosis in cancer cells [7–11, 26–29]. The role of propolis in host immune functions against tumor onset has become increasingly recognized in our understanding of the mechanisms of cancer prevention. EEP stimulates nonspecific immunity, activates humoral immunity, and enhances cell-mediated immunity [30]. In our opinion the immunomodulatory effect of propolis and its phenolic components is also evoked by targeting of TRAIL-induced apoptosis in cancer cells for cancer chemoprevention. We have shown that TRAIL-resistant cancer cells can be sensitized by EEP and its phenolic components [9–11].
3. Characteristics of TRAIL and Death Receptors
The death ligand TRAIL (tumor necrosis factor-related apoptosis-inducing ligand), a member of the TNF superfamily, induces apoptosis in cancer cells with no toxicity against normal tissues [40–43]. TRAIL was discovered independently by two teams, both of which reported sequence homology with the extracellular domain of the other TNF family members: FasL (CD95L/Apo2L) and TNF-α [44, 45].
The death ligand is expressed on the T lymphocytes, natural killer cells, dendritic cells, neutrophils, monocytes or macrophages [46–48]. Membrane-bound TRAIL can be cleaved from the cell surface into a soluble secreted form. Soluble or expressed on immune cells TRAIL plays an important role in surveillance and defense mechanisms against tumor cells [46, 49]. Endogenous TRAIL triggers apoptosis via receptor-mediated death (extrinsic pathway) through interaction with the death receptors (DRs) in cancer cells [49–51]. There are two agonistic transmembrane receptors, TRAIL-R1/DR4 and TRAIL-R2/DR5, which bind ligand by extracellular domains. The death receptors contain complete and functional intracellular death domains (DD) responsible for the activation of apoptosis pathway in cancer cells [52, 53].
However, some cancer cells are resistant to TRAIL-induced death [4, 5, 54]. This failure to undergo apoptosis has been implicated in the resistance of cancer cells to TRAIL surveillance and, therefore, in tumor development [49]. The expression of DRs and proapoptotic (Bid, Bax, Bak, Smac/DIABLO) or antiapoptotic (FLIP, Bcl-2, Bcl-xL, Mcl-1, Akt, IAP-1, IAP-2, XIAP, survivin) proteins in cancer cells is involved in TRAIL-resistance [4, 5, 41, 49, 50]. TRAIL-resistant cancer cells can be sensitized to TRAIL-mediated apoptosis by certain polyphenolic compounds [49, 55].
4. TRAIL Signaling Pathways and the Mechanisms of TRAIL-Resistance in Cancer Cells
Binding of TRAIL to DRs is the first step of extrinsic apoptotic pathway, also known as the death receptor pathway [56]. The decreased expression of DRs in cancer cell surface causes TRAIL-resistance [57, 58]. Ligation of trimerized TRAIL to TRAIL-R1/DR4 and/or TRAIL-R2/DR5 leads to conformational change in their DD along with the subsequent oligomerization and clustering of the DRs [59, 60]. This DRs activation allows for the recruitment of the adaptor molecule FADD (Fas-associated death domain) with formation of the DISC (death inducing signaling complex), activation of initiator caspases (caspase-8 and -10), cleavage of effector caspases (caspase-3, -6 and -7), and finally DNA fragmentation [61–64]. The antiapoptotic protein FLIP (FLICE (FADD-like IL-1β-converting enzyme) inhibitory protein) can also be part of DISC to replace caspase-8 and form an inactive complex. Overexpression of FLIP in cancer cells blocks activation of caspase-8 [4, 5].
In some cancer cells activated caspase-8 is sufficient to trigger apoptosis, while other cells require activation of the mitochondrial (intrinsic) pathway to amplify the apoptotic signal. In the mitochondrial pathway, caspase-8 leads indirectly to the activation of effector caspases through the cleavage of the BH3-interacting domain death agonist (Bid), along with the mitochondrial membrane potential (MMP) disruption [49]. Crosstalk between the extrinsic (receptor-dependent) and intrinsic (mitochondrial-dependent) apoptosis pathways is linked by caspase-8-mediated Bid cleavage and subsequent translocation of tBid (truncated Bid) to the mitochondria to initiate the intrinsic apoptosis pathway [1, 2]. Truncated Bid interacts with proapoptotic mitochondrial proteins from Bcl-2 (B-cell leukemia 2) family (Bax, Bak) stimulating the decrease in the MMP [6, 60]. The loss of the integrity of the mitochondrial membrane leads to the release of the cytochrome c and the second mitochondrial activator of caspases/direct inhibitor of apoptosis binding protein with low isoelectric point (Smac/DIABLO) [64]. Among the cellular signaling pathways that promote cell survival, antiapoptotic members of Bcl-2 family (Bcl-2, Bcl-xL, Mcl-1) could inhibit the liberation of cytochrome c from mitochondria [4, 5]. Akt, a serine/threonine protein kinase, is another important factor contributing TRAIL-resistance. Akt can prevent cytochrome c escape to cytosol [47, 48]. Furthermore, cytochrome c, in the presence of Apaf-1 (apoptotic protease-activating factor-1) and procaspase-9 forms the apoptosome. Activated caspase-9 stimulates in turn executioner caspases (caspase-3, -6, -7) leading to cell death [10, 49]. Effector caspases activity is controlled by IAPs (inhibitor of apoptosis proteins): IAP-1, IAP-2, XIAP (X-linked inhibitor of apoptosis protein), and survivin. Smac/DIABLO augments apoptosis by binging to the cellular IAP members, which are potent caspase inhibitors [1–5]. Figure 1 demonstrates the TRAIL-induced apoptotic pathways in cancer cells [65].
Figure 1.
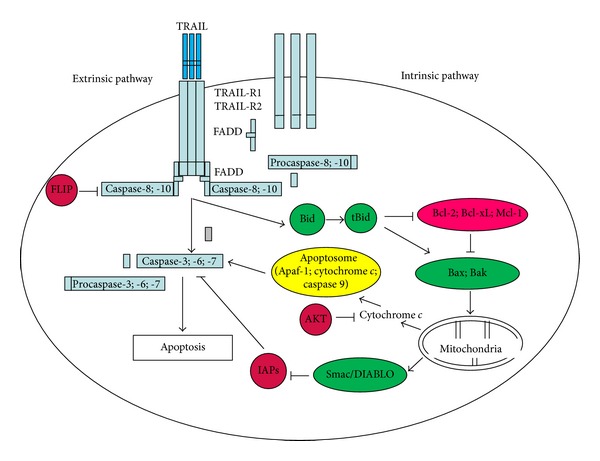
TRAIL-induced apoptotic pathways in cancer cells. TRAIL binds to death receptors, TRAIL-R1 and/or TRAIL-R2 and promotes the recruitment of adaptor molecule FADD (Fas-associated-death domain) to activate caspase-8 and/or caspase-10, which trigger activation of downstream effector caspases (caspase-3, -6, -7). FLIP can block activation of caspase-8 or casapase-10. Caspase-8 mediated also cleavage of Bid (BH3-interacting domain death agonist). Trucated Bid called tBid translocates to the mitochondria where it interacts with proapoptotic Bax and Bak, stimulating disruption of MMP (mitochondrial membrane potential) and the release of cytochrome c and Smac/DIABLO (second mitochondrial activator of caspases/direct inhibitor of apoptosis binding protein with low isoelectric point). Antiapoptotic members of Bcl-2 family (Bcl-2, Bcl-xL, and Mcl-1) could inhibit loss of MMP. Akt may prevent cytochrome c escape to cytosol. Cytochrome c liberated from the mitochondria binds to the adaptor protein Apaf-1 (apoptotic protease-activating factor-1) and procaspase-9, forming the apoptosome and activating caspase-9 which in turn activates executioner caspases (caspase-3, -6, -7) leading to cell death. Activity of executioner caspases is inhibited by IAPs (inhibitor of apoptosis protein): IAP-1, IAP-2, XIAP, and survivin. Smac/DIABLO blocks IAPs.
5. Effect of Polyphenolic Components from Propolis on TRAIL-Induced Apoptosis in Cancer Cells
Propolis is a promising raw mixture of natural compounds that should be studied to discover new pharmaceutical products with anticancer and chemopreventive properties [7, 8, 26]. The major active components of propolis are flavonoids and phenolic acids or their esters [9, 13]. Accumulating data clearly indicate that induction of apoptosis is an essential event for chemoprevention of cancer by polyphenolic compounds [36–38]. Inactivation of the TRAIL pathway and escape from the TRAIL-mediated immunosurveillance might play important roles in tumor onset and progression [49]. TRAIL in combination with propolis extracts or with polyphenolic compounds identified in propolis resulted in the synergistic induction of cancer cell death. Our previous findings demonstrated for the first time that ethanolic extracts of European and Brazilian propolis and its polyphenolic constituents overcome TRAIL-resistance in HeLa cervical, LNCaP, and DU145 prostate cancer cells [9–11]. Propolis significantly augments the anticancer activity of TRAIL in cancer cells due to its phenolics. Cinnamic acid and its derivative artepillin C, o-coumaric acid, m-coumaric acid, p-coumaric acid, caffeic acid and its derivative—caffeic acid phenylethyl ester (CAPE), chrysin, apigenin, acacetin, naringenin, daidzein, biochanin A, galangin, kaempferol, kaempferide, quercetin, isoliquiritigenin have been reported to enhance TRAIL-induced death [9–11, 49]. The chemical structures of the components found in propolis supporting TRAIL-mediated cytotoxicity are shown in Figure 2.
Figure 2.
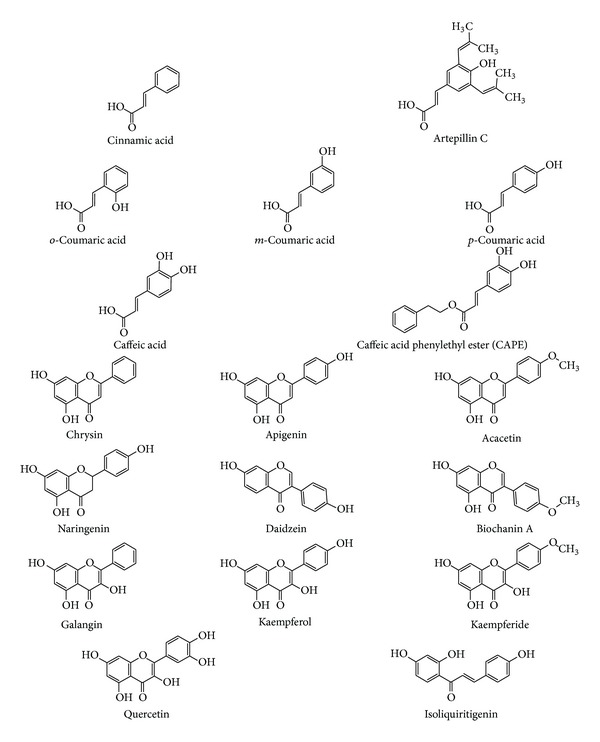
The chemical structures of the main polyphenols from propolis supporting TRAIL-mediated cytotoxicity.
TRAIL is a potent inducer of apoptosis in cancer cells. Numerous studies show that many types of cancer cells are resistant to TRAIL-induced death, but combinatorial approaches based on TRAIL and different chemotherapeutic agents, such as small-molecule inhibitors, drugs, and natural compounds, have been developed to overcome the resistance of cancer cells to TRAIL [66, 67]. The decreased expression of death receptors TRAIL-R1 and TRAIL-R2 and antiapoptotic proteins (Bid, Bax, Bak, Smac/DIABLO) or increased expression of antiapoptotic proteins (FLIP, Bcl-2, Bcl-xL, Mcl-1, Akt, IAP-1, IAP-2, XIAP, survivin) in cancer cells were involved in TRAIL-resistance [4, 5]. Recently the molecular mechanism by which the polyphenols identified in propolis sensitize TRAIL-resistant cancer cells are known for artepillin C, chrysin, apigenin, naringenin, daidzein, biochanin A, kaempferol, quercetin, and isoliquiritigenin. The targets in TRAIL-mediated apoptotic pathway for phenolic compounds of propolis are presented in Figure 3. Artepillin C restores TRAIL sensitivity in TRAIL-resistant LNCaP prostate cancer cells by upregulation of TRAIL-R2, activation of caspase-8 and caspase-3, as well as the disruption of MMP [68]. Chrysin and apigenin overcome TRAIL-resistance in MDA-MB-231 breast cancer cells, HT-29 colon cancer cells, HepG2 hepatocellular cancer cells, SK-MEL-37 melanoma cells, Capan-1 pancreatic cancer cells via increased expression of TRAIL-R2 and decreased expression of FLIP [69]. In CNE1 nasopharyngeal cancer cells chrysin promotes TRAIL-induced caspase activation (caspase-8 and -3) [70]. Apigenin augments TRAIL-induced apoptosis in Jurkat leukemia T cells, DU145 prostate cancer cells, and DLD-1 colon cancer cells through upregulation of TRAIL-R2, activation of Bid and caspase-8, -10, -9, -3 [71]. Increased expression of TRAIL-R2, induction of Bid cleavage, and loss of MMP in A549 lung cancer cells by naringenin results in significant enhancement of TRAIL-mediated apoptosis [72]. Daidzein reverses TRAIL-resistance in LNCaP prostate cancer stimulating the decrease in the MMP and in LN229 glioma cells activating caspase-9 and downregulating of bcl-2 [73–76]. Biochanin-A sensitizes LNCaP and DU145 prostate cancer cells via increased expression of TRAIL-R2 and disruption of MMP [77]. Induction of TRAIL-R1 and TRAIL-R2 expression and caspase-8, -10, -9, -3 activation in SW-480 colon cancer cells and activation of caspase-8, suppression of Akt, survivin, XIAP, and antiapoptotic mitochondrial proteins from Bcl-2 family: Bcl-2, Bcl-xL, Mcl-1 in U251 and U87 glioma cells by kaempferol are sufficient to restore TRAIL sensitivity [78, 79]. Quercetin strongly cooperates with TRAIL to trigger apoptosis in HepG2, SK-Hep, SNU-387, SNU-423, SNU-449, and SNU-475 hepatocellular cancer cells by increased expression of TRAIL-R2 and decreased FLIP expression, in HT-29, SW-620, and Caco-2 colon cancer cells by upregulation of TRAIL-R1 and TRAIL-R2, induction of Bid and caspase-3 cleavage, and release of cytochrome c to the cytosol and in U87-MG, U251, A172, and LN229 glioma cells by suppression of survivin, in LNCaP, DU145, and PC3 prostate cancer cells by upregulation of TRAIL-R2, activation of caspase-8, -9 and -3, inhibition of Akt and survivin, in H460, H2009, H1299 and A549 lung cancer cells by increase of TRAIL-R2 expression, activation of caspase-8 and -3, and also by inactivation of Akt and survivin, and in VAL, RL and SUDHL4 B-cell lymphomas cells by down-regulation of survivin and additionally by degradation of Mcl-1, an antiapoptotic mitochondrial protein [80–87]. Isoliquiritigenin upregulates TRAIL-R2 protein levels in cell surface of HT-29 colon cancer and in this way supports TRAIL-mediated apoptosis [88]. Schematic presentations of the mechanisms by which polyphenols from propolis modulate the TRAIL apoptotic signaling in cancer cell are demonstrated in Table 1 and Figure 3.
Figure 3.
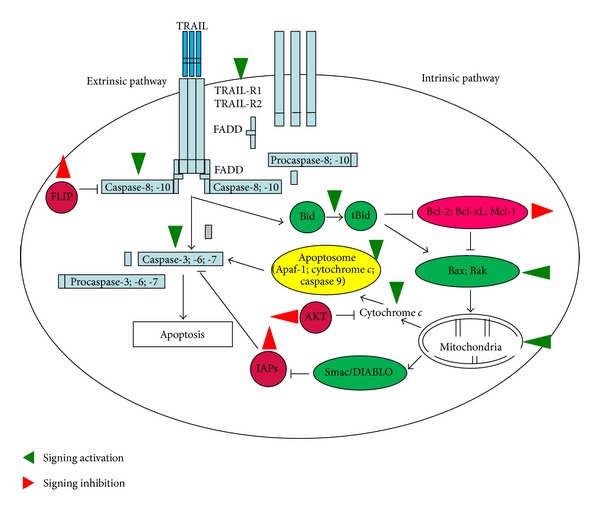
The molecular targets in TRAIL-mediated apoptotic pathways in cancer cells for polyphenols isolated from propolis. Schematic presentation of the mechanisms by which polyphenols detected in propolis modulate TRAIL apoptotic signaling in cancer cell. The green arrows signing activation and red arrows signing inhibition indicate the molecular targets for polyphenols in TRAIL-mediated apoptosis in cancer cells.
Table 1.
The mechanism by which the polyphenols identified in propolis sensitize TRAIL-resistant cancer cells.
| Compounds from propolis | Class of polyphenols | Targets | Cell lines | References |
|---|---|---|---|---|
| Artepillin C | Cinnamic acid derivative | ↑ TRAIL-R2 ↑ caspase-8 ↑ caspase-3 |
Prostate cancer LNCaP | [68] |
|
| ||||
| Chrysin | Flavone | ↑ TRAIL-R2 ↓ FLIP ↑ caspase-8 ↑ caspase-3 |
Breast cancer MDA-MB-231 Colon cancer HT-29 Hepatocellular cancer HepG2 Melanoma SK-MEL-37 Pancreatic cancer Capan-1 Nasopharyngeal cancer CNE1 |
[69, 70] |
|
| ||||
| Apigenin | Flavone | ↑ TRAIL-R2 ↓ FLIP ↑ Bid cleavage ↑ caspase-8 ↑ caspase-10 ↑ caspase-9 ↑ caspase-3 |
Breast cancer MDA-MB-231 Colon cancer HT-29 Hepatocellular cancer HepG2 Melanoma SK-MEL-37 Pancreatic cancer Capan-1 Leukemia Jurkat Prostate cancer DU145 Colon cancer DLD-1 |
[69, 71] |
|
| ||||
| Naringenin | Flavanone | ↑ TRAIL-R2 ↑ Bid cleavage ↑ loss of MMP |
Lung cancer A549 | [72] |
|
| ||||
| Daidzein | Isoflavone | ↑ loss of MMP ↑ caspase-9 ↓ Bcl-2 |
Prostate cancer LNCaP Glioma LN229 |
[73–76] |
|
| ||||
| Biochanin A | Isoflavone | ↑ TRAIL-R2 ↑ loss of MMP |
Prostate cancer LNCaP Prostate cancer DU145 |
[77] |
|
| ||||
| Kaempferol | Flavanol | ↑ TRAIL-R1 ↑ TRAIL-R2 ↑ caspase-8 ↑ caspase-10 ↑ caspase-9 ↑ caspase-3 ↓ Akt ↓ survivin ↓ XIAP ↓ Bcl-2 ↓ Bcl-xL ↓ Mcl-1 |
Colon cancer SW-480 Glioma U251 Glioma U87 |
[78, 79] |
|
| ||||
| Quercetin | Flavanol | ↑ TRAIL-R1 ↑ TRAIL-R2 ↓ FLIP ↑ Bid cleavage ↓ Mcl-1 ↑ cytochrome c release ↓ Akt ↓ survivin ↑ caspase-8 ↑ caspase-9 ↑ caspase-3 |
Hepatocellular cancer HepG2 Hepatocellular cancer SK-Hep Hepatocellular cancer SNU-387 Hepatocellular cancer SNU-423 Hepatocellular cancer SNU-449 Hepatocellular cancer SNU-475 Colon cancer HT-29 Colon cancer SW-620 Colon cancer Caco-2 Glioma U87-MG Glioma U251 Glioma A172 Glioma LN229 Prostate cancer LNCaP Prostate cancer DU145 Prostate cancer PC3 Lung cancer H460 Lung cancer H2009 Lung cancer H1299 Lung cancer A549 |
[80–87] |
| Lymphoma B VAL Lymphoma B RL Lymphoma B SUDHL4 |
||||
|
| ||||
| Isoliquiritigenin | Chalcone | ↑ TRAIL-R2 | Colon cancer HT-29 | [88] |
6. Discussion
Epidemiological and preclinical evidence suggests that polyphenols isolated from propolis possess strong cancer chemopreventive activities [7, 8, 26]. It has led to an increased emphasis on cancer prevention strategies in which propolis as the richest source of plant polyphenolics will be used as dietary supplement [10, 11, 49]. In the field of CAM, this paper focuses on the interaction between phenolic components from propolis and TRAIL on tumor cells as the example of immunomodulation through natural substances to be considered for the chemoprevention of neoplasm disease [9–11]. Polyphenols from propolis sensitize tumor cells to TRAIL-induced apoptosis. The compounds exhibit strong cytotoxic effect in combination with TRAIL on cancer cells [65–84]. The TRAIL-mediated apoptotic pathways may be a target of the chemopreventive activity of polyphenols in cancer cells.
7. Conclusion
Targeting TRAIL-induced apoptotic signaling pathway in tumor cells by propolis and its polyphenols is one of the crucial issues in cancer chemoprevention. EEP and its phenolic components sensitize TRAIL-resistant cancer cells and augment anticancer activity of TRAIL. The paper confirms that the overcoming of TRAIL-resistance by propolis and its polyphenols may be one of the mechanisms responsible for their cancer preventive effects.
Acknowledgment
This work was supported by a research Grant no. KNW-1-063/P/2/0 from the Medical University of Silesia in Katowice (Poland).
References
- 1.Holoch PA, Griffith TS. TNF-related apoptosis-inducing ligand (TRAIL): a new path to anti-cancer therapies. European Journal of Pharmacology. 2009;625(1–3):63–72. doi: 10.1016/j.ejphar.2009.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mellier G, Huang S, Shenoy K, Pervaiz S. TRAILing death in cancer. Molecular Aspects of Medicine. 2010;31(1):93–112. doi: 10.1016/j.mam.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Therapy. 2005;12(3):228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 4.Thorburn A, Behbakht K, Ford H. TRAIL receptor-targeted therapeutics: resistance mechanisms and strategies to avoid them. Drug Resistance Updates. 2008;11(1-2):17–24. doi: 10.1016/j.drup.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronikowska J, Szliszka E, Jaworska D, Czuba ZP, Krol W. The coumarin psoralidin enhances anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Molecules. 2012;17:6449–6464. doi: 10.3390/molecules17066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szliszka E, Kostrzewa-Susłow E, Bronikowska J, et al. Synthetic flavanones augment the anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Molecules. 2012;17:11693–11711. doi: 10.3390/molecules171011693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe MA, Amarante MK, Conti BJ, Sforcin JM. Cytotoxic constituents of propolis inducing anticancer effects: a review. Journal of Pharmacy and Pharmacology. 2011;63:1378–1386. doi: 10.1111/j.2042-7158.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- 8.Sawicka D, Car H, Borawska MH, Nikliński J. The anticancer activity of propolis. Folia Histochemica et Cytobiologica. 2012;50:25–37. doi: 10.2478/18693. [DOI] [PubMed] [Google Scholar]
- 9.Szliszka E, Czuba ZP, Domino M, Mazur B, Zydowicz G, Krol W. Ethanolic extract of propolis (EEP) enhances the apoptosis-inducing potential of TRAIL in cancer cells. Molecules. 2009;14(2):738–754. doi: 10.3390/molecules14020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szliszka E, Czuba ZP, Bronikowska J, Mertas A, Paradysz A, Krol W W. Ethanolic extract of propolis (EEP) augments TRAIL-induced apoptotic death in prostate cancer cells. Evidence-Based Complementary and Alternative Medicine. 2011;2011:11 pages. doi: 10.1093/ecam/nep180.535172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szliszka E, Zydowicz G, Janoszka B, Dobosz C, Kowalczyk-Ziomek G, Krol W. Ethanolic extract of Brazilian green propolis sensitizes prostate cancer cells to TRAIL-induced apoptosis. International Journal of Oncology. 2011;38(4):941–953. doi: 10.3892/ijo.2011.930. [DOI] [PubMed] [Google Scholar]
- 12.Ghisalberti EL. Propolis: a review. Bee World. 1979;60:59–84. [Google Scholar]
- 13.Bankova VS, Popov SS, Marekov NL. A study on flavonoids of propolis. Journal of Natural Products. 1983;46(4):471–474. [Google Scholar]
- 14.Gardana C, Scaglianti M, Pietta P, Simonetti P. Analysis of the polyphenolic fraction of propolis from different sources by liquid chromatography-tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2007;45(3):390–399. doi: 10.1016/j.jpba.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Krol W, Scheller S, Czuba Z, et al. Inhibition of neutrophils’ chemiluminescence by ethanol extract of propolis (EEP) and its phenolic components. Journal of Ethnopharmacology. 1996;55(1):19–25. doi: 10.1016/s0378-8741(96)01466-3. [DOI] [PubMed] [Google Scholar]
- 16.Banskota AH, Nagaoka T, Sumioka LY, et al. Antiproliferative activity of The Netherlands propolis and its active principles in cancer cell lines. Journal of Ethnopharmacology. 2002;80(1):67–73. doi: 10.1016/s0378-8741(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 17.Barbaric M, Miskovic K, Bojic M, et al. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. Journal of Ethnopharmacology. 2011;135:772–778. doi: 10.1016/j.jep.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Mavri A, Abramovic H, Polak T, et al. Chemical properties and antioxidant and antimicrobial activities of Slovenian propolis. Chemistry & Biodiversity. 2012;9:1545–1558. doi: 10.1002/cbdv.201100337. [DOI] [PubMed] [Google Scholar]
- 19.Falcao SI, Vale N, Gomes P, et al. Phenolic profiling of Portuguese propolis by LC-MS spectrometry: uncommon propolis rich in flavonoid glycosides. Phytochemical Analysis. 2012 doi: 10.1002/pca.2412. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Kapur A, Yang JX, et al. Antiproliferation of human prostate cancer cells by ethanolic extracts of Brazilian propolis and its botanical origin. International Journal of Oncology. 2007;31(3):601–606. [PubMed] [Google Scholar]
- 21.Chikaraishi Y, Izuta H, Shimazawa M, Mishima S, Hara H. Angiostatic effects of Brazilian green propolis and its chemical constituents. Molecular Nutrition and Food Research. 2010;54(4):566–575. doi: 10.1002/mnfr.200900115. [DOI] [PubMed] [Google Scholar]
- 22.Simões-Ambrosio LMC, Gregório LE, Sousa JPB, et al. The role of seasonality on the inhibitory effect of Brazilian green propolis on the oxidative metabolism of neutrophils. Fitoterapia. 2010;81(8):1102–1108. doi: 10.1016/j.fitote.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira EW, Message D, Negri G, Salatino A, Stringheta PC. Seasonal variation, chemical composition and antioxidant activity of brazilian propolis samples. Evidence-Based Complementary and Alternative Medicine. 2010;7(3):307–315. doi: 10.1093/ecam/nem177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daugsch A, Moraes CS, Fort P, Park YK. Brazilian red propolis—chemical composition and botanical origin. Evidence-Based Complementary and Alternative Medicine. 2008;5(4):435–441. doi: 10.1093/ecam/nem057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo X, Chen B, Luo L, Zhang X, Dai X, Gong S. Chemical compositions and antioxidant activities of water extracts of Chinese propolis. Journal of Agricultural and Food Chemistry. 2011;59:12610–12616. doi: 10.1021/jf202818p. [DOI] [PubMed] [Google Scholar]
- 26.Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? Journal of Ethnopharmacology. 2011;133(2):253–260. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Scheller S, Krol W, Swiacik J, Owczarek S, Gabrys J, Shani J. Antitumoral property of ethanolic extract of propolis in mice-bearing Ehrlich carcinoma, as compared to bleomycin. Zeitschrift fur Naturforschung C. 1989;44(11-12):1063–1065. doi: 10.1515/znc-1989-11-1231. [DOI] [PubMed] [Google Scholar]
- 28.Orsolic N, Saranovic AB, Basic I. Direct and indirect mechanisms of antitumour activity of propolis and its phenolic compounds. Planta Medica. 2006;72:20–27. doi: 10.1055/s-2005-873167. [DOI] [PubMed] [Google Scholar]
- 29.Bufalo MC, Candeias JM, Sforcin JM. In vitro cytotoxic effect of Brazilian green propolis on human laryngeal epidermoid carcinoma (HEP-2) cells. Evidence-Based Complementary and Alternative Medicine. 2007;22:1–5. doi: 10.1093/ecam/nem147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sforcin JM. Propolis and the immune system: a review. Journal of Ethnopharmacology. 2007;113(1):1–14. doi: 10.1016/j.jep.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Missima F, Sforcin JM. Green Brazilian propolis action on macrophages and lymphoid organs of chronically stressed mice. Evidence-Based Complementary and Alternative Medicine. 2008;5(1):71–75. doi: 10.1093/ecam/nel112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachiega TF, Orsatti CL, Pagliarone AC, Sforcin JM. The effects of propolis and its isolated compounds on cytokine production by murine macrophages. Phytotherapy Research. 2012;26:1308–1313. doi: 10.1002/ptr.3731. [DOI] [PubMed] [Google Scholar]
- 33.Franchi GC, Jr., Moraes CS, Toreti VC, Daugsch A, Nowill AE, Park YK. Comparison of effects of the ethanolic extracts of Brazilian propolis on human leukemic cells as assessed with the MTT assay. Evidence-Based Complementary and Alternative Medicine. 2012;2012:6 pages. doi: 10.1155/2012/918956.918956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takemura T, Urushisaki T, Fukuoka M, et al. 3, 4-dicaffeoylquinic acid, a major constituent of Brazilian propolis, increases TRAIL expression and extends the lifetimes of mice infected with the influenza A virus. Evidence-Based Complementary and Alternative Medicine. 2012;2012:7 pages. doi: 10.1155/2012/946867.946867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan GC, Cheung KW, Sze DM. The immunomodulatory and anticancer properties of propolis. Clinical Reviews in Allergy & Immunology. 2012 doi: 10.1007/s12016-012-8322-2. [DOI] [PubMed] [Google Scholar]
- 36.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacology and Therapeutics. 2001;90(2-3):157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 37.Khan N, Adhami VM, Mukhtar H. Apoptosis by dietary agents for prevention and treatment of cancer. Biochemical Pharmacology. 2008;76(11):1333–1339. doi: 10.1016/j.bcp.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KW, Bode AM, Dong Z. Molecular targets of phytochemicals for cancer prevention. Nature Reviews Cancer. 2011;11(3):211–218. doi: 10.1038/nrc3017. [DOI] [PubMed] [Google Scholar]
- 39.Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Research. 1976;36(7):2699–2702. [PubMed] [Google Scholar]
- 40.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. Journal of Clinical Investigation. 1999;104(2):155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsters SA, Pitti RA, Sheridan JP, Ashkenazi A. Control of apoptosis signaling by Apo2 ligand. Recent Progress in Hormone Research. 1999;54:225–234. [PubMed] [Google Scholar]
- 42.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nature Medicine. 1999;5(2):157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 43.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine and Growth Factor Reviews. 2003;14(3-4):337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 44.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 45.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. The Journal of Biological Chemistry. 1996;271(22):12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 46.Jakóbisiak M, Lasek W, Gołąb J. Natural mechanisms protecting against cancer. Immunology Letters. 2003;90:103–122. doi: 10.1016/j.imlet.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Lee JY, Huerta-Yepez S, Vega M, Baritaki S, Spandidos DA, Bonavida B. The NO TRAIL to YES TRAIL in cancer therapy (review) International Journal of Oncology. 2007;31(4):685–691. [PubMed] [Google Scholar]
- 48.Kruyt FAE. TRAIL and cancer therapy. Cancer Letters. 2008;263(1):14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Szliszka E, Krol W. The role of dietary polyphenols in tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-induced apoptosis for cancer chemoprevention. European Journal of Cancer Prevention. 2011;20(1):63–69. doi: 10.1097/CEJ.0b013e32833ecc48. [DOI] [PubMed] [Google Scholar]
- 50.Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Current Opinion in Immunology. 1998;10(5):559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 51.Szliszka E, Helewski KJ, Mizgala E, Krol W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. International Journal of Oncology. 2011;39(4):771–779. doi: 10.3892/ijo.2011.1116. [DOI] [PubMed] [Google Scholar]
- 52.Pan G, O’Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276(5309):111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 53.Szliszka E, Mazur B, Zdowicz G, Czuba ZP, Król W. TRAIL-induced apoptosis and expression of death receptor TRAIL-R1 and TRAIL-R2 in bladder cancer cells. Folia Histochemica et Cytobiologica. 2009;47(4):579–585. doi: 10.2478/v10042-009-0111-2. [DOI] [PubMed] [Google Scholar]
- 54.Szliszka E, Czuba ZP, Mazur B, Paradysz A, Krol W. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules. 2010;15(8):5336–5353. doi: 10.3390/molecules15085336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szliszka E, Czuba ZP, Jernas K, Król W. Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) International Journal of Molecular Sciences. 2008;9(1):56–64. doi: 10.3390/ijms9010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22(53):8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 57.Walczak H, Degli-Esposti MA, Johnson RS, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. The EMBO Journal. 1997;16(17):5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szliszka E, Jaworska D, Kłósek M, Czuba ZP, Krol W. Targeting death receptor TRAIL-R2 by chalcones for TRAIL-induced apoptosis in cancer cells. International Journal of Molecular Sciences. 2012;13:15343–15359. doi: 10.3390/ijms131115343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan G, Ni J, Wei YF, Yu GI, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277(5327):815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 60.Abdulghani J, El-Deiry WS. TRAIL receptor signaling and therapeutics. Expert Opinion on Therapeutic Targets. 2010;14(10):1091–1108. doi: 10.1517/14728222.2010.519701. [DOI] [PubMed] [Google Scholar]
- 61.Schneider P, Thome M, Burns K, et al. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity. 1997;7(6):831–836. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]
- 62.Bodmer JL, Holler N, Reynard S, et al. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nature Cell Biology. 2000;2(4):241–243. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 63.Szliszka E, Czuba ZP, Sȩdek Ł, Paradysz A, Król W. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia . Pharmacological Reports. 2011;63(1):139–148. doi: 10.1016/s1734-1140(11)70408-x. [DOI] [PubMed] [Google Scholar]
- 64.Holland PM. Targeting Apo2L/TRAIL receptors by soluble Apo2L/TRAIL. Cancer Letters. 2011 [Google Scholar]
- 65.Bernardi S, Secchiero P, Zauli G. State of art and recent developments of anti-cancer strategies based on TRAIL. Recent Patents of Anti-Cancer Drug Discovery. 2012;7:207–217. doi: 10.2174/157489212799972927. [DOI] [PubMed] [Google Scholar]
- 66.Voelkel-Johnson C. Combination therapy with TRAIL: recent developments and potential pitfalls. Cancer Biology and Therapy. 2009;8(1):81–83. doi: 10.4161/cbt.8.1.7519. [DOI] [PubMed] [Google Scholar]
- 67.Voelkel-Johnson C. TRAIL-mediated signaling in prostate, bladder and renal cancer. Nature Reviews Urology. 2011;8:417–427. doi: 10.1038/nrurol.2011.81. [DOI] [PubMed] [Google Scholar]
- 68.Szliszka E, Zydowicz G, Mizgala E, Krol W. Artepillin C, (3, 5-diprenyl-4-hydroxycinnamic acid) sensitizes prostate cancer LNCaP cells to TRAIL-induced apoptosis. International Journal of Oncology. 2012;41:818–828. doi: 10.3892/ijo.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding J, Polier G, Köhler R, Giaisi M, Krammer PH, Li-Weber M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. The Journal of Biological Chemistry. 2012;287:641–649. doi: 10.1074/jbc.M111.286526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Wang JN, Huang JM, et al. Chrysin promotes tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced apoptosis in human cancer cell lines. Toxicology in Vitro. 2011;25(3):630–635. doi: 10.1016/j.tiv.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 71.Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Sakai T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Molecular Cancer Therapeutics. 2006;5(4):945–951. doi: 10.1158/1535-7163.MCT-05-0431. [DOI] [PubMed] [Google Scholar]
- 72.Jin CY, Park C, Hwang HJ, et al. Naringenin up-regulates the expression of death receptor 5 and enhances TRAIL-induced apoptosis in human lung cancer A549 cells. Molecular Nutrition and Food Research. 2011;55(2):300–309. doi: 10.1002/mnfr.201000024. [DOI] [PubMed] [Google Scholar]
- 73.Szliszka E, Krol W. Soy isoflavones augment the effect of TRAIL-mediated apoptotic death in prostate cancer cells. Oncology Reports. 2011;26(3):533–541. doi: 10.3892/or.2011.1332. [DOI] [PubMed] [Google Scholar]
- 74.Szliszka E, Gebka J, Bronikowska J, Krol W. Dietary flavones enhance the effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on bladder cancer cells. Central European Journal of Urology. 2010;63(3):138–143. doi: 10.5173/ceju.2011.03.art18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bronikowska J, Szliszka E, Czuba ZP, Zwolinski D, Szmydki D, Krol W. The combination of TRAIL and isoflavones enhances apoptosis in cancer cells. Molecules. 2010;15(3):2000–2015. doi: 10.3390/molecules15032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siegelin MD, Gaiser T, Habel A, Siegelin Y. Daidzein overcomes TRAIL-resistance in malignant glioma cells by modulating the expression of the intrinsic apoptotic inhibitor, bcl-2. Neuroscience Letters. 2009;454(3):223–228. doi: 10.1016/j.neulet.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 77.Szliszka E, Czuba ZP, Mertas A, Paradysz A, Krol W. The dietary isoflavone biochanin-A sensitizes prostate cancer cells to TRAIL-induced apoptosis. Urologic Oncology. 2011 doi: 10.1016/j.urolonc.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida T, Konishi M, Horinaka M, et al. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochemical and Biophysical Research Communications. 2008;375(1):129–133. doi: 10.1016/j.bbrc.2008.07.131. [DOI] [PubMed] [Google Scholar]
- 79.Siegelin MD, Reuss DE, Habel A, Herold-Mende C, Von Deimling A. The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Molecular Cancer Therapeutics. 2008;7(11):3566–3574. doi: 10.1158/1535-7163.MCT-08-0236. [DOI] [PubMed] [Google Scholar]
- 80.Jin YK, Eun HK, Seok SP, Jun HL, Taeg KK, Kyeong SC. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. Journal of Cellular Biochemistry. 2008;105(6):1386–1398. doi: 10.1002/jcb.21958. [DOI] [PubMed] [Google Scholar]
- 81.Psahoulia FH, Drosopoulos KG, Doubravska L, Andera L, Pintzas A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Molecular Cancer Therapeutics. 2007;6(9):2591–2599. doi: 10.1158/1535-7163.MCT-07-0001. [DOI] [PubMed] [Google Scholar]
- 82.Siegelin MD, Reuss DE, Habel A, Rami A, Von Deimling A. Quercetin promotes degradation of survivin and thereby enhances death-receptor-mediated apoptosis in glioma cells. Neuro-Oncology. 2009;11(2):122–131. doi: 10.1215/15228517-2008-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim YH, Lee YJ. TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. Journal of Cellular Biochemistry. 2007;100(4):998–1009. doi: 10.1002/jcb.21098. [DOI] [PubMed] [Google Scholar]
- 84.Kim YH, Lee DH, Jeong JH, Guo ZS, Lee YJ. Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochemical Pharmacology. 2008;75(10):1946–1958. doi: 10.1016/j.bcp.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jung YH, Heo J, Lee YJ, Kwon TK, Kim YH. Quercetin enhances TRAIL-induced apoptosis in prostate cancer cells via increased protein stability of death receptor 5. Life Sciences. 2010;86(9-10):351–357. doi: 10.1016/j.lfs.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen W, Wang X, Zhuang J, Zhang L, Lin Y. Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis. 2007;28(10):2114–2121. doi: 10.1093/carcin/bgm133. [DOI] [PubMed] [Google Scholar]
- 87.Jacquemin G, Granci V, Gallouet AS, et al. Quercetin-mediated Mcl-1 and survivin downregulation restores TRAIL-induced apoptosis in non-Hodgkin's lymphoma B cells. Haematologica. 2012;97:38–46. doi: 10.3324/haematol.2011.046466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshida T, Horinaka M, Takara M, et al. Combination of isoliquiritigenin and tumor necrosis factor-related apoptosis-inducing ligand induces apoptosis in colon cancer HT29 cells. Environmental Health and Preventive Medicine. 2008;13(5):281–287. doi: 10.1007/s12199-008-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


