Abstract
Vestibular nucleus neurons have been shown to respond to stimulation of afferents innervating the limbs. However, a limitation in the potential translation of these findings is that they were obtained from decerebrate or anesthetized animals. The goal of the present study was to determine whether stimulation of hindlimb nerves similarly affects vestibular nucleus neuronal activity in conscious cats, and whether the responsiveness of neurons to the stimuli is altered following a bilateral labyrinthectomy. In labyrinth-intact animals, the firing rate of 24/59 (41%) of the neurons in the caudal vestibular nucleus complex was affected by hindlimb nerve stimulation. Most responses were excitatory; the median response latency was 20 ms, but some units had response latencies as short as 10 ms. In the first week after a bilateral labyrinthectomy, the proportion of vestibular nucleus neurons that responded to hindlimb nerve stimulation increased slightly (to 24/55 or 44% of units). However, during the subsequent postlabyrinthectomy survival period, the proportion of vestibular nucleus neurons with hindlimb inputs increased significantly (to 30/49 or 61% of units). Stimuli to hindlimb nerves needed to elicit neuronal responses was consistently over three times the threshold for eliciting an afferent volley. These data show that inputs from hindlimb afferents smaller than those innervating muscle spindles and Golgi tendon organs affect the processing of information in the vestibular nuclei, and that these inputs are enhanced following a bilateral labyrinthectomy. These findings have implications for the development of a limb neuroprosthetics device for the management of bilateral vestibular loss.
Keywords: somatosensory signals, semicircular canal, otolith organ, sensory substitution, vestibular prosthesis
in addition to processing labyrinthine signals, the vestibular nuclei receive somatosensory inputs, including those from the limbs (12). Inputs from muscle spindles in the neck are thought to play an important role in distinguishing whole body from head-on-body movements (1, 6, 21), but the role of limb inputs to the vestibular nuclei is less clear. The effects of inputs from the limbs on vestibular nucleus neuronal activity have been mainly examined in decerebrate or anesthetized animals, in studies that utilized electrical stimulation of nerves (7, 19, 37–40, 49–51). These experiments showed that electrical activation of muscle or cutaneous or mixed nerves from the limb produced excitation of vestibular nucleus neurons (7, 19, 49–51), which was punctuated by inhibition due, in part, to input from cerebellar Purkinje cells (51). Low-intensity stimulation of cutaneous nerves was often effective in altering vestibular nucleus neuronal activity. Although inputs from low-threshold afferents in muscle nerves (i.e., muscle spindle and Golgi tendon afferents) have been reported to affect vestibular nucleus neuronal activity, most cells only responded to stimulus intensities that additionally activated small-caliber muscle afferents (7, 19, 49–51).
One previous study compared the responses of vestibular nucleus neurons to limb nerve stimulation in two decerebrate cat preparations: labyrinth-intact animals, and animals that had recovered over 1 mo following a bilateral labyrinthectomy before the decerebration was performed (19). It was demonstrated that a higher fraction of vestibular nucleus neurons responded to limb nerve stimulation in the labyrinthectomized group, and that stimulation of low-threshold muscle afferents was much more effective in modulating the activity of vestibular nucleus neurons in the animals lacking labyrinthine signals (19). Marked recovery in postural stability and adjustments in blood pressure necessary during postural changes occur within a week following a bilateral labyrinthectomy (25). We speculated that recovery of responses initially lost following the removal of labyrinthine inputs might be due to amplification of limb inputs to the vestibular nuclei.
Several lines of evidence support this premise. The caudal half of the vestibular nucleus complex, which receives the heaviest concentration of inputs from the spinal cord (17, 26), mediates the responses that recover following a bilateral labyrinthectomy (25). In contrast, spatial cognition and vestibular-ocular reflexes, which are mediated by the rostral aspects of the vestibular nuclei, are permanently lost following bilateral damage to the labyrinth (25). The activity of a subset of neurons in the caudal portion of the vestibular nuclei was modulated by whole body rotations following removal of vestibular inputs, suggesting that nonlabyrinthine inputs may substitute for the lost labyrinthine signals (27, 52). In addition, the recovery of compensatory adjustments in blood pressure during postural alterations was blunted more in animals with lesions placed in the caudal vestibular nuclei than in animals with bilateral labyrinthectomy, suggesting that the vestibular nuclei participate in the functional recovery of the responses (29).
A limitation in the hypothesis that limb inputs to the vestibular nuclei participate in recovery of function following peripheral vestibular lesions is that the premise is based mainly on work in decerebrate or chloralose-anesthetized cats. Responses of brain stem neurons to sensory inputs can be amplified in such preparations, which could, therefore, lead to false positive conclusions. For example, the sensitivity to vestibular inputs of neurons in the region of the rostral ventrolateral medullary reticular formation that regulates blood pressure is far greater in decerebrate than conscious animals (10). Furthermore, decerebrate rigidity results from exaggerated responses to somatosensory inputs (44). The goal of the present study was to determine the fraction of vestibular nucleus neurons that respond to inputs from hindlimb nerves in conscious animals, and whether this fraction changes acutely or chronically following a bilateral labyrinthectomy. We tested the hypothesis that limb nerve inputs to the vestibular nuclei are amplified during the period when functional recovery of balance stability and posturally related autonomic responses occurs, approximately 1 wk after a bilateral labyrinthectomy.
METHODS
All experimental procedures conformed to the American Physiological Society's “Guiding Principles for the Care and Use of Animals,” as well as the National Research Council Guide for the Care and Use of Laboratory Animals, and were approved by the University of Pittsburgh's Institutional Animal Care and Use Committee. Data were collected from four purpose-bred adult female cats obtained from Liberty Research (Waverly, NY). Since the procedures employed in this study were similar to those discussed in recent papers (5, 10, 24, 27, 28), they are presented in abbreviated fashion below.
Surgical procedures.
Animals were acclimated for restraint and instrumented for single-unit recordings from the medulla, as described in previous papers (5, 10, 24, 27, 28). Animals were initially anesthetized using an intramuscular injection of ketamine (20 mg/kg) and acepromazine (0.2 mg/kg) and intubated, and then anesthesia was maintained using 1–2% isoflurane vaporized in O2, so that limb withdrawal reflexes were absent and heart rate was stable. Intravenous saline was provided to replace lost fluid volume, and a heating pad and heat lamp were used to maintain core temperature. A 1-cm craniotomy was made at the posterior aspect of the skull, and a David Kopf (David Kopf Instruments, Tujunga, CA) recording chamber was positioned in accordance with stereotaxic coordinates and attached to the skull adjacent to the craniotomy using Palacos bone cement (Zimmer, Warsaw, IN). The chamber was positioned to provide access to the caudal aspect of the vestibular nuclei. In addition, a fixation plate was attached to the skull and subsequently used for restraint of the head.
Platinum foil electrodes embedded in a silicone cuff (14, 33) were attached to the common peroneal and tibial nerves in both hindlimbs, and the Teflon-insulated stainless steel wire leads (Cooner Wire, Chatsworth, CA) were routed subcutaneously and soldered to a connector mounted on the skull. A small laminectomy was performed to expose a segment of the lower lumbar spinal cord, and a pair of silver ball electrodes was sutured in place, such that the tips abutted the cord dorsum to record field potentials elicited by nerve stimulation (53). In addition, electromyographic (EMG) activity was recorded bilaterally from the gastrocnemius muscles using pairs of Cooner wire, which were stripped of insulation for ∼5 mm and sutured to the muscle epimysium, together with an insulating patch of Silastic sheeting. The leads from the cord dorsum and EMG electrodes were routed subcutaneously and attached to a head-mounted connector. After surgery, animals received antibiotics (amoxicillin, two 50-mg oral doses/day) for 10 days. For 72 h after the surgery, analgesia was provided through transdermal delivery of fentanyl (25 μg/h; Janssen Pharmaceutical Products, Titusville, NJ).
After initial recordings were performed from vestibular nucleus neurons, a second surgery was conducted. During this procedure, animals were anesthetized and physiologically maintained as described above. The tympanic bulla on each side of the skull was opened using a ventrolateral approach to expose the cochlea. A drill was used to perform a labyrinthectomy centered posterolateral to the basal turn of the cochlea. The labyrinthectomy provided access to the portion of the VIIIth cranial nerve within the internal auditory canal, which was transected under microscopic observation. Thus two independent lesions affecting the vestibular system were made on both sides to ensure that vestibular inputs were eliminated. In no case did nystagmus or a tonic deviation in eye position occur after the surgery, suggesting that the peripheral lesions were complete. Antibiotics were administered for 10 days following the second surgery; in addition, 3 mg/kg of ketoprofen were injected intramuscularly every 12 h for 3 days to provide analgesia.
Recording procedures.
Animals were acclimated for head and body restraint for several weeks until they remained sedentary without vocalization for a period of 2 h. The head was tilted downward by 30° to vertically align the vertical semicircular canals. At least once per week, the minimal current strength for activating fibers in the common peroneal and tibial nerves was estimated by recording spinal cord field potentials and gastrocnemius muscle EMG activity elicited by a single 0.3-ms square-wave current pulse delivered at a variety of intensities to each nerve. Field potentials and EMG activity were amplified by a factor of 103 or 104, filtered with a band pass of 10–104 Hz, and sampled at 103 Hz using a Micro1401 mk 2 data collection system and Spike2 version 6 software (Cambridge Electronic Design, Cambridge, UK).
To perform recordings from vestibular nucleus neurons, an x-y positioner (608-B, David Kopf) was attached to the recording chamber and used to maneuver a 5-MΩ epoxy-insulated tungsten microelectrode (Frederick Haer, Bowdoin, ME), which was inserted through a 25-gauge guide tube into the cerebellum and lowered into the medulla using a hydraulic microdrive (model 650, David Kopf). Use of this system allowed electrodes to be positioned reproducibly from day to day. Neuronal activity recorded using the microelectrode was amplified by a factor of 104, filtered with a band pass of 300–10,000 Hz, and sampled at 25,000 Hz.
When a spontaneously active vestibular nucleus neuron was isolated, the “wobble” stimulus, a fixed-amplitude tilt, the direction of which moves around the animal at constant speed (43), was used to determine whether the unit responded to stimulation of the vestibular end organs. Wobble stimuli were routinely delivered at a frequency of 0.5 Hz and amplitude of 5°. The response vector orientation, or the plane of tilt that elicited the greatest change in a neuron's firing rate, was calculated from responses to clockwise (CW) and counterclockwise (CCW) wobble stimulation (43). Subsequently, rotations were delivered in the roll (longitudinal) and pitch (transverse) axes at 0.5 Hz, and the relative gains of responses to the two planes of tilts were used to confirm the response vector orientation. The response vector orientations were designated using a head-centered coordinate system, with 0° corresponding to ipsilateral ear-down roll tilt, 90° to nose-down pitch, 180° to contralateral ear-down roll, and −90° to nose-up (NU) pitch. Tilts were then delivered at or near the plane of the response vector orientation at 0.1–1 Hz to determine response dynamics.
After a neuron's responses to vertical vestibular stimulation were recorded, we tested whether the cell's firing rate was altered by single shocks delivered to the ipsilateral and contralateral tibial and common peroneal nerves. Typically, each nerve was stimulated using a current intensity 10 times greater than required to elicit responses recordable from muscle or the cord dorsum. In some cases, we also examined whether neurons responded to a five-shock train of stimuli with an interpulse interval of 3 ms at an intensity five times threshold for eliciting spinal or muscle responses. If a neuron responded to stimulation of a nerve, we systematically lowered the stimulus intensity to determine the minimal current required to elicit a change in activity. However, in some cases, the stimulus artifacts were so large that they obscured EMG activity and spinal cord field potentials, such that the threshold current intensity could not be established for a nerve. In such instances, we delivered a single pulse to each nerve at 1-mA intensity and/or a train of five pulses at 500-μA intensity to determine whether a cell's activity was altered by the inputs.
After establishing the effect of hindlimb nerve stimulation on the activity of vestibular nucleus neurons, a bilateral labyrinthectomy was performed as described above. Recordings commenced again the day following the removal of labyrinthine inputs and continued for up to 1 mo. The postlabyrinthectomy recordings focused on the region of the brain stem where we previously observed clusters of neurons that responded to whole body tilts.
Data analysis procedures.
All trials were subjected to analysis using the spike detection and sorting feature of the Spike2 software to confirm that individual units were parsed separately. Neural activity recorded during rotations was binned (500 bins/cycle) and averaged over the sinusoidal stimulus period. A least squares minimization procedure (43) conducted using MATLAB (MathWorks, Natick, MA) software fit a sine wave to responses to rotations, and the amplitude of the sine wave (response gain) and phase shift of the sine wave from the stimulus (response phase) were calculated (43). Responses were deemed to be significant if the signal-to-noise ratio exceeded 0.5, and there were no prominent components other than the first harmonic.
Poststimulus histograms were generated from neural activity recorded during electrical stimulation of nerves; ∼50 sweeps were averaged to generate each histogram. Quantitative criteria were used to determine whether a response to nerve stimulation was present, as illustrated in Fig. 1A. Poststimulus histograms were first inspected to segregate runs where there was evidence of a response (sustained change in activity continuing for ≥6 ms). We then determined the average bin counts during the response and compared these bin counts to those during the 10-ms period before the stimuli. For an excitatory response to be considered “significant”, the average bin counts during the response had to be at least 1 SD greater than the average bin counts during the baseline period, and the peak response had to be at least 3 SDs larger than mean baseline activity. It was not possible to apply these standards to inhibitory components of responses, due to a “floor effect” (mean baseline activity minus 1 SD was often < 0). However, since excitatory components were present in virtually every response, the vast majority of trials were subjected to this statistical scrutiny. Once a response was identified, we determined the response latency of the excitatory and inhibitory components, which was defined as the point when a sustained change in firing rate relative to baseline activity was initiated.
Fig. 1.
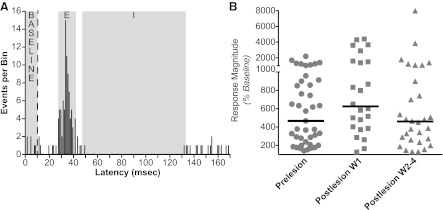
Method to determine whether responses to nerve stimulation were present. A: poststimulus histograms were first inspected to determine which responses putatively contained excitatory (E) and/or inhibitory (I) components, where activity deviated from that during the baseline period 10 ms before nerve stimulation was provided. In this example, E and I responses, as well as baseline activity, are designated on a poststimulus histogram generated from data collected 15 days following the bilateral labyrinthectomy. A vertical dashed line indicates when the stimulus was delivered; data from 61 sequential stimulus presentations are represented in the trace. The average number of events per bin during the E and I responses were determined and compared with the average number of events per bin during the baseline period. In this example, the average number of events per bin in the E, I, and baseline periods were, respectively, 5.3, 0.1, and 0.6. The SD of bin counts during the baseline period was calculated to be 0.8. The maximal bin count during the E period was 16, and the minimal bin count during the I period was 0. Two criteria were used to designate a significant E response: an average response bin count over 1 SD larger that the average bin count during the baseline period, and a peak response over 3 SDs larger than the average bin count during the baseline period. In this case, the average bin count during the baseline period (0.6) plus 1 SD (0.8) was 1.4 counts/bin. Thus the average activity during the E period (5.3 counts/bin) was over 1 SD larger than the average baseline activity, and the peak response (16 events/bin) was over 3 SDs larger than average baseline activity. Hence, the response was deemed to be significant. Although the I response is evident in the trace, we could not use the same criteria to demonstrate that it was significant, since mean baseline activity (0.6) minus 1 SD (0.8) was less than zero. B: magnitudes of responses relative to baseline activity. For E responses, response magnitudes represent: 100 × average bin count during response/average bin count during baseline period. For I responses, response magnitudes represent 100 × 1/(average bin count during response/average bin count during baseline period). When both I and E responses were present, the response magnitudes for each component were averaged to generate the value that was plotted. Horizontal lines show median values. Abbreviations: W1, first week after bilateral labyrinthectomy; W2–4, the subsequent 3 wk (weeks 2–4) after bilateral labyrinthectomy.
Data were tabulated, and statistical analyses were performed, using Prism 6 software (GraphPad Software, San Diego, CA). Pooled data are represented as means ± 1 SE.
Histological procedures.
Following recordings in each animal, two to three lesions were created in the brain stem by passing a 100- to 150-μA current through a 0.5-MΩ electrode for 60 s. Following a survival period of several days, animals were anesthetized using pentobarbital sodium and transcardially perfused with 10% formalin, as described in previous papers (5, 10, 24, 27, 28). The brain stem was cut transversely at 50-μm thickness using a freezing microtome, and tissue sections were stained using thionine. Recording sites were reconstructed on photomontages of sections with reference to the locations of electrolytic lesions, the relative positions of electrode tracks, and microelectrode depths.
RESULTS
We tested the effects of hindlimb nerve stimulation on the activity of 73 vestibular nucleus neurons before bilateral labyrinthectomies. To ensure that recordings were limited to the vestibular nuclei, we targeted neurons that responded robustly to rotations of the animal in vertical planes. However, 14 of these units were located more rostrally than the others and were shown through histological reconstructions to be positioned in the lateral vestibular nucleus. None of the rostrally located units responded to hindlimb nerve stimulation, and no units in the lateral vestibular nucleus were tested for hindlimb inputs following the bilateral labyrinthectomy. Consequently, data collected from the lateral vestibular nucleus neurons were omitted during the analysis of findings.
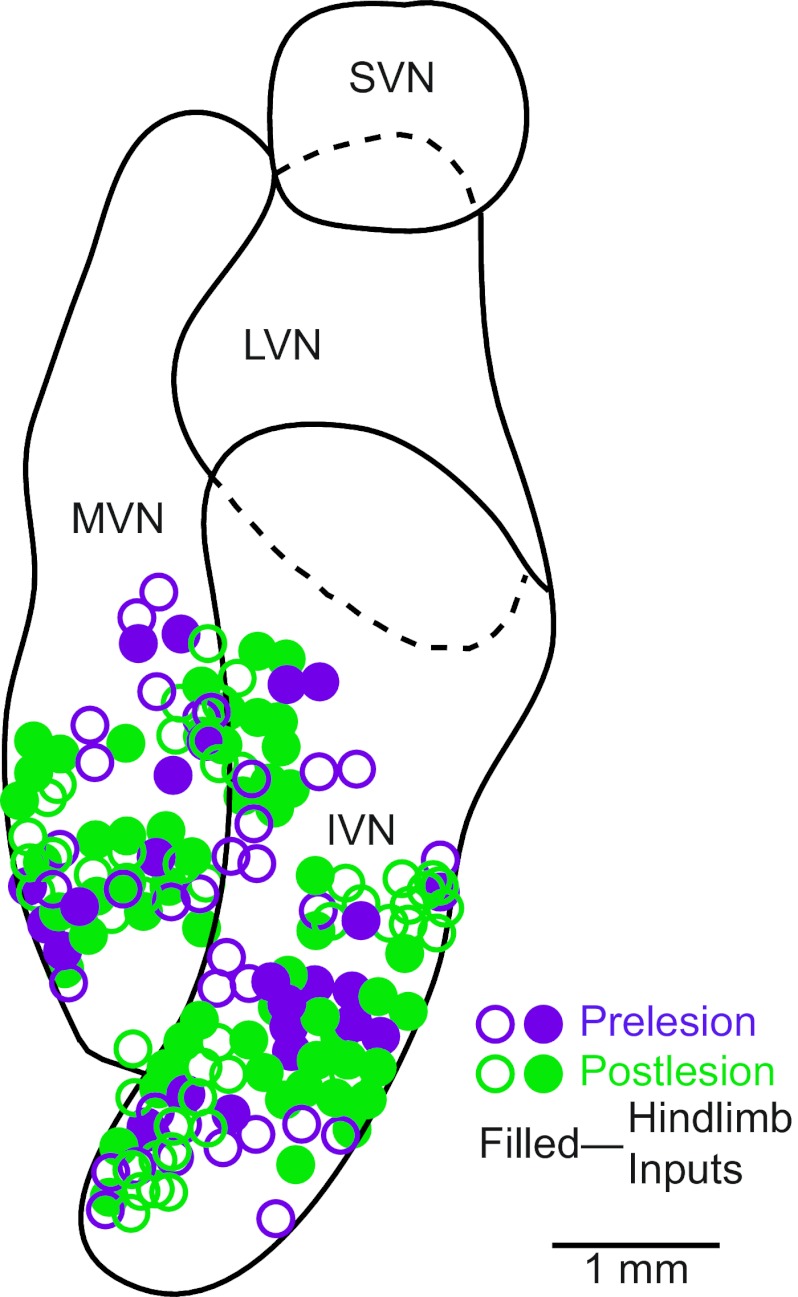
Responses to hindlimb nerve stimulation were recorded from an additional 104 vestibular nucleus neurons subsequent to a bilateral labyrinthectomy. Since we could not rely on responses to rotations to localize the vestibular nuclei after the elimination of labyrinthine inputs, we sampled units in the locations where recordings were performed before lesions. The recording sites are shown in Fig. 2 and were located in the inferior vestibular nucleus and adjacent portions of the medial vestibular nucleus.
Fig. 2.
Locations of neurons tested for responses to hindlimb nerve stimulation. The locations are plotted on a horizontal section through the vestibular nuclei. Open symbols indicate neurons that failed to respond to nerve stimulation, whereas solid symbols denote units with hindlimb inputs. IVN, inferior vestibular nucleus; LVN, lateral vestibular nucleus; MVN, medial vestibular nucleus; SVN, superior vestibular nucleus.
Responses of units to vertical vestibular stimulation.
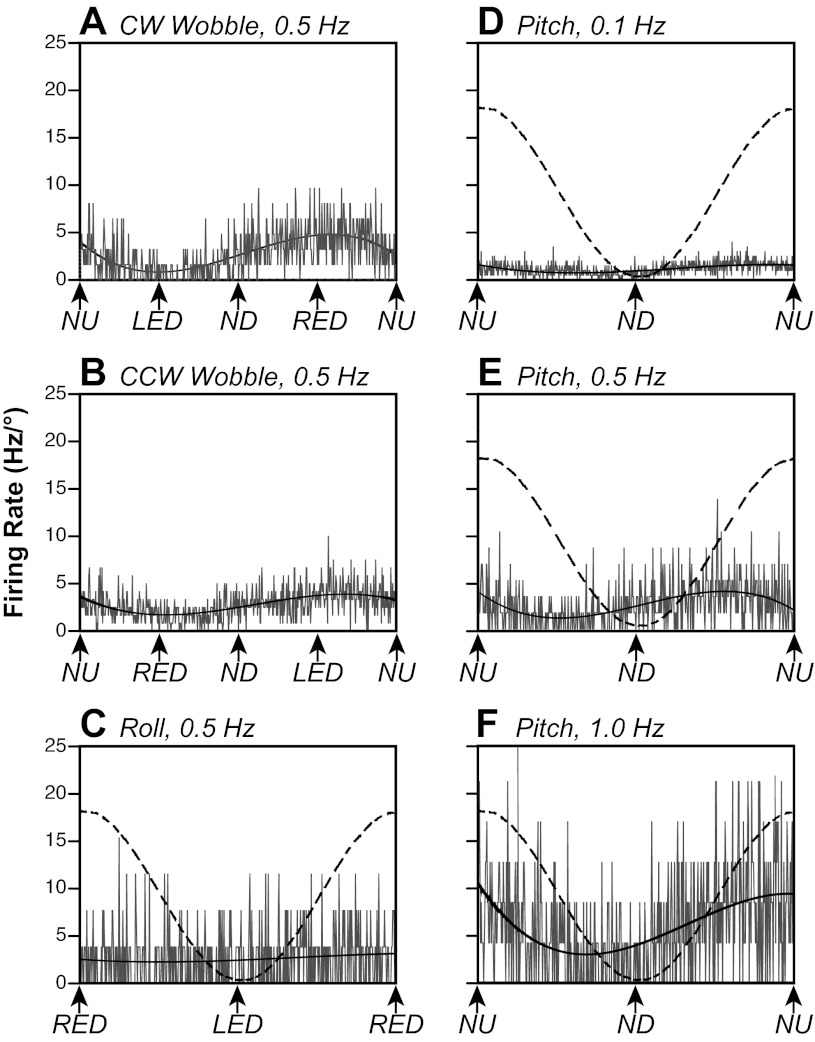
Examples of responses of a vestibular nucleus neuron to body rotations in vertical planes are shown in Fig. 3. Figure 3, A and B, show responses of the neuron to CW and CCW wobble stimulation. By considering both responses (43), we determined that the response vector orientation was six degrees from NU pitch (−96°). Consequently, roll tilts (Fig. 3C) produced little modulation of the neuron's activity, while pitch tilts (Fig. 3, D–F) elicited robust responses. The response gain increased by a factor of 14 as the stimulus frequency advanced from 0.1 (Fig. 3D) to 1 Hz (Fig. 3F), while the response phase was ∼90° advanced from stimulus position at all stimulus frequencies.
Fig. 3.
Averaged responses of a neuron to rotations in vertical planes. To facilitate comparisons of responses, firing rates are indicated as Hz/° of tilt, as smaller stimulus amplitudes were delivered at higher stimulus frequencies. A and B: responses to 0.5 Hz, 5° clockwise (CW) and counterclockwise (CCW) wobble stimuli. Solid curves superimposed on traces are sine waves fit to the responses. C: responses to 0.5 Hz, 5° sinusoidal rotations in the roll plane. A dashed line indicates table position. Roll tilt did not produce a significant modulation of the unit's activity. D–F: responses to sinusoidal rotations in the pitch plane at 0.1 Hz (10°), 0.5 Hz (5°), and 1.0 Hz (2.5°). The gain of the response to 1-Hz rotations was 14 times the gain of the response to 0.1-Hz tilts. LED, left ear-down roll; ND, nose-down pitch; NU, nose-up pitch; RED, right ear-down roll. The number of sweeps averaged to generate each trace were as follows: 31 (A), 60 (B), 13 (C), 10 (D), 29 (E), and 47 (F).
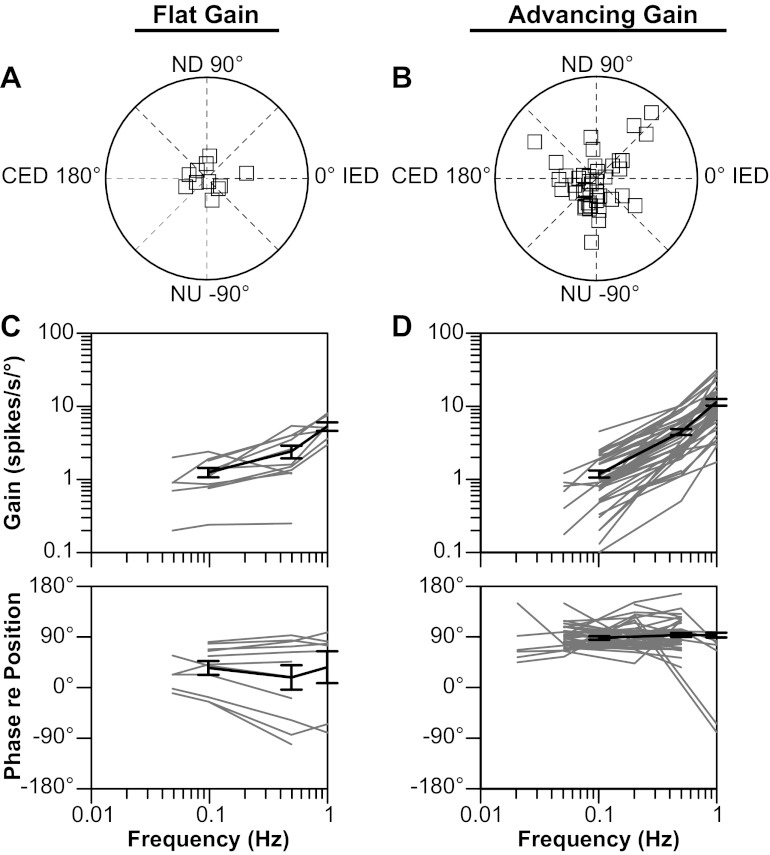
The characteristics of responses to rotations in vertical planes of vestibular nucleus neurons considered in this study are indicated in Fig. 4. As in recent studies (24, 31), we divided neurons into two categories: “flat gain” neurons whose response gains increased less than fivefold per stimulus decade (typically 0.1–1.0 Hz), and “advancing gain neurons” whose response gains increased more than fivefold per stimulus decade. Response dynamics were not ascertained for four of the vestibular nucleus neurons. These units were classified as spatiotemporal convergence neurons, as the gains of their responses to CW and CCW wobble stimuli differed by greater than a 2:1 ratio (median of 2.4) (42). Such a response pattern has been attributed to the convergence of inputs with different spatial and temporal properties (for instance, the convergence of inputs from semicircular canal afferents activated by roll rotations and otolith organ afferents activated by pitch rotations) (4, 42, 43).
Fig. 4.
Characteristics of responses of neurons to tilts in vertical planes. Data are segregated based on whether the response gain for a particular unit increased more (advancing gain) or less (flat gain) than fivefold per stimulus decade. A and B: polar plots showing response vector orientations and gains of units. Response vector orientations were determined using wobble stimuli delivered at 0.5 Hz. The maximal radius of each plot designates a response gain of 15 spikes·s−1·°−1. C and D: Bode plots illustrating the dynamic properties of responses of neurons to rotations in a fixed plane near the response vector orientation at multiple frequencies. Response gain and phase were plotted with respect to stimulus position. Thin gray lines show data for individual neurons; thick solid lines indicate average values. Error bars designate 1 SE. CED, contralateral ear-down roll; IED, ipsilateral ear-down roll tilt.
The majority of the units (44/55, 80%) for which Bode plots were constructed (Fig. 4, C and D) were classified as advancing gain neurons. The response gains of these cells increased an average of 12.0 ± 1.5 times per stimulus decade, and their response phases were typically ∼90° advanced from stimulus position (i.e., near stimulus velocity) across the range of frequencies tested (Fig. 4D). Only 11/55 units (20%) were classified as flat gain neurons, whose response gains increased an average of 3.5 ± 0.6 times per stimulus decade (Fig. 4C). One-half of the flat gain neurons had response phases either that were near stimulus position or that lagged stimulus position across the range of stimulus frequencies tested. The other one-half of this category of vestibular nucleus neurons had response phases near stimulus velocity at all frequencies (Fig. 4C). These response dynamics are similar to those previously described for neurons located in the caudal portion of the vestibular nucleus complex of conscious cats (27).
The response vector orientations for flat gain and advancing gain neurons are indicated in Fig. 4, A and B, respectively. Most (7/11) of the flat gain cells had response vector orientations closer to the roll plane than the pitch plane. In contrast, less than one-half of the advancing gain cells (20/44) had response vector orientations closer to the roll plane than the pitch plane, while 12 of these units had response vector orientations within 10° of the plane of one of the vertical semicircular canals. Most of the cells of both categories that responded better to pitch than to roll rotations (20/28, 71%) were excited by NU pitch, and 16/27 (59%) of the cells that were preferentially activated by roll tilt were excited by contralateral ear-down rotations.
Responses of units to hindlimb nerve stimulation before a bilateral labyrinthectomy.
Out of the 59 neurons tested for hindlimb nerve inputs before a bilateral labyrinthectomy, 24 (41%) responded to stimulation of one or more nerves. Of these cells, 8 responded to stimulation of 1 nerve, the firing rate of 14 was affected by stimulation of 2 nerves, 1 received inputs from 3 nerves, and 1 responded to activation of all 4 nerves implanted with stimulating electrodes. In cases where stimulation of only one nerve elicited responses, the tibial nerve was effective for one-half of the neurons, and the common peroneal nerve elicited responses in the other one-half. Stimulation on the ipsilateral side was effective for two cells, and contralateral stimulation was successful for six neurons. In most cases (13/14) where stimulation of two nerves produced responses, both the tibial and common peroneal nerves were effective, with stimulation of the contralateral nerves evoking responses two-thirds of the time.
A nearly equal fraction of advancing gain (18/44, 41%) and flat gain (5/11, 45%) neurons responded to hindlimb nerve stimulation. In addition, one spatiotemporal convergence unit received hindlimb inputs. Consequently, it appears that hindlimb inputs are targeted to vestibular nucleus neurons, regardless of their responses during whole body movements that activate labyrinthine receptors.
Of the 43 responses to stimulation of individual nerves recorded for the 24 neurons that received hindlimb inputs before bilateral labyrinthectomies, 30 were excitatory, 11 were composed of excitation then inhibition, typically followed by late excitatory components, and two included inhibition followed by excitation. The responses of a vestibular nucleus neuron to stimulation of three hindlimb nerves are shown in Fig. 5, bottom. Spinal cord field potentials elicited by the same stimulus intensities (10 times threshold for eliciting the field potentials, 250 μA) are illustrated in Fig. 5, top. The neuron's responses to stimulation of the right (contralateral) common peroneal and tibial nerves included excitation, followed by inhibition, while the unit was inhibited by stimulation of the ipsilateral tibial nerve. The minimal stimulus intensities that elicited changes in neuronal activity were 10 times the threshold for producing spinal cord field potentials for the contralateral common peroneal nerve, and four times the spinal cord field potential threshold for the ipsilateral and contralateral tibial nerves. In no case were neuronal responses observed when stimuli were delivered that were less than three times the threshold for eliciting spinal cord field potentials.
Fig. 5.
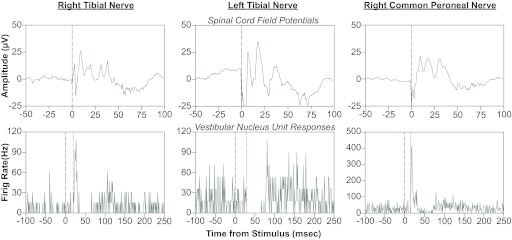
Effects of hindlimb nerve stimulation on the activity of a vestibular nucleus neuron. In each column, the bottom panel is a poststimulus histogram indicating the responses of the unit to a single shock of 250-μA magnitude, which was 10 times the threshold for producing a spinal cord field potential (shown in the top panel). A gray vertical line at time zero indicates when the stimulus was delivered, whereas a red vertical line designates the onset of each response. The poststimulus histograms were generated from the following number of sweeps: right tibial nerve, 65; left tibial nerve, 56; right common peroneal nerve, 63.
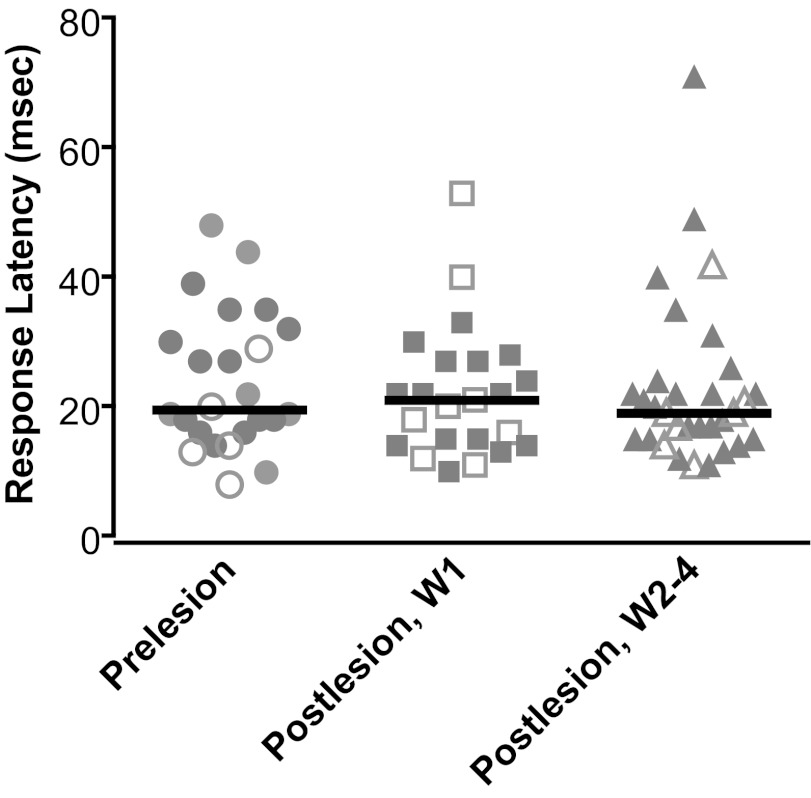
The latencies of the responses of vestibular nucleus neurons to hindlimb nerve stimulation are shown in Fig. 6. These values represent the shortest response latency of a neuron to stimulation of any nerve. Solid and open symbols, respectively, indicate the latencies of responses that were exclusively excitatory or that were composed of a combination of excitation and inhibition. Response latencies were defined as the time between the stimulus and the point when a sustained change in firing rate relative to baseline activity was initiated. To illustrate, the response latencies to hindlimb nerve stimulation are shown in Fig. 5, designated by red dashed lines. The response latencies before elimination of labyrinthine inputs ranged from 9 to 49 ms; the median was 21 ms. There was no indication that responses that included inhibition occurred at different latencies than those that were exclusively excitatory. There was also no evidence that latencies differed based on whether a response was elicited from the ipsilateral or contralateral side, or by stimulation of the tibial or common peroneal nerve.
Fig. 6.
Latencies of responses of vestibular nucleus neurons to hindlimb nerve stimulation (indicated by shaded symbols). If a neuron's activity was affected by stimulation of more than one nerve, the shortest response latency was plotted. Solid symbols indicate latencies of responses that were exclusively excitatory, whereas open signals show latencies of responses that included inhibition (such as excitation followed by inhibition). Different columns show response latencies determined before vestibular lesions (prelesion), in W1 (postlesion), and during W2–4 (postlesion). Solid lines designate median values.
To quantify the overall magnitude of responses, we compared the average bin counts (1 ms/bin) of responses observed in poststimulus histograms, relative to average bin counts in baseline activity, as indicated in Fig. 1A. We considered all early components of the responses, both excitatory and inhibitory, and when both were present we averaged the percent changes from baseline activity. Figure 1B compares the magnitudes of responses to nerve stimulation observed before (left column) and after a bilateral labyrinthectomy (middle and right columns). When stimulation of more than one nerve elicited responses, the magnitudes of each of the responses is included in Fig. 1B (such that the number of data points indicated is greater than the number of neurons whose activity was recorded).
Responses of units to hindlimb nerve stimulation following a bilateral labyrinthectomy.
Since recovery of balance and compensatory autonomic responses during postural changes occurs approximately 1 wk following a bilateral labyrinthectomy (25), we compared neuronal responses to hindlimb nerve stimulation during the first week after elimination of vestibular signals and at subsequent times. In the first week after removal of labyrinthine inputs, the responses of 55 neurons were recorded to stimulation of the ipsilateral and contralateral tibial and common peroneal nerves. Out of this sample, 24 units (44%) responded to stimulation of a single nerve, usually the contralateral common peroneal nerve (21/24 responses). Most of these responses (16) were excitatory, three were composed of excitation followed by inhibition, two were inhibitory, and another three included inhibition followed by excitation. The latencies of the responses are shown in the middle column of Fig. 6. The values ranged from 11 to 54 ms; the median was 22 ms. The magnitudes of the responses to hindlimb stimulation collected in the first week after the bilateral labyrinthectomy are indicated in the middle column of Fig. 1B.
During the subsequent 3 wk, we recorded the responses of 49 neurons to stimulation of hindlimb nerves. Out of this sample, the firing rate of 30 units (61%) was affected by hindlimb inputs. These units received inputs from only one nerve, with the contralateral common peroneal being the most effective (21/30 cells). The firing rates of the other cells were modulated by stimulation of the ipsilateral common peroneal (n = 8) and ipsilateral tibial (n = 1) nerves. A χ2 test showed that the activity of a larger fraction of units was affected by stimulation of a common peroneal nerve following vestibular lesions than when the labyrinths were intact (P < 0.01). Most of the responses were excitatory (23/30), or were composed of excitation followed by inhibition (n = 2). However, one response was inhibitory, and four others included inhibition followed by excitation.
The response latencies are indicated in the right column of Fig. 6, and ranged from 12 to 72 ms (median of 20 ms). Consequently, the response latencies were nearly identical for the three time periods relative to bilateral labyrinthectomies indicated in Fig. 6. A one-way ANOVA analysis, combined with the Kruskal-Wallis test, provided no indication that the values differed (P = 0.82).
Figure 1B compares the magnitudes of the postlesion responses to those recorded before the bilateral labyrinthectomy. The magnitudes were similar during the three time periods considered relative to the bilateral labyrinthectomy. The median values were 467% of baseline activity during the prelesion period, 625% of baseline activity during the first week after lesions, and 460% of baseline activity during the subsequent 3-wk period. A one-way ANOVA analysis combined with the Kruskal-Wallis test provided no indication that the values differed (P = 0.14).
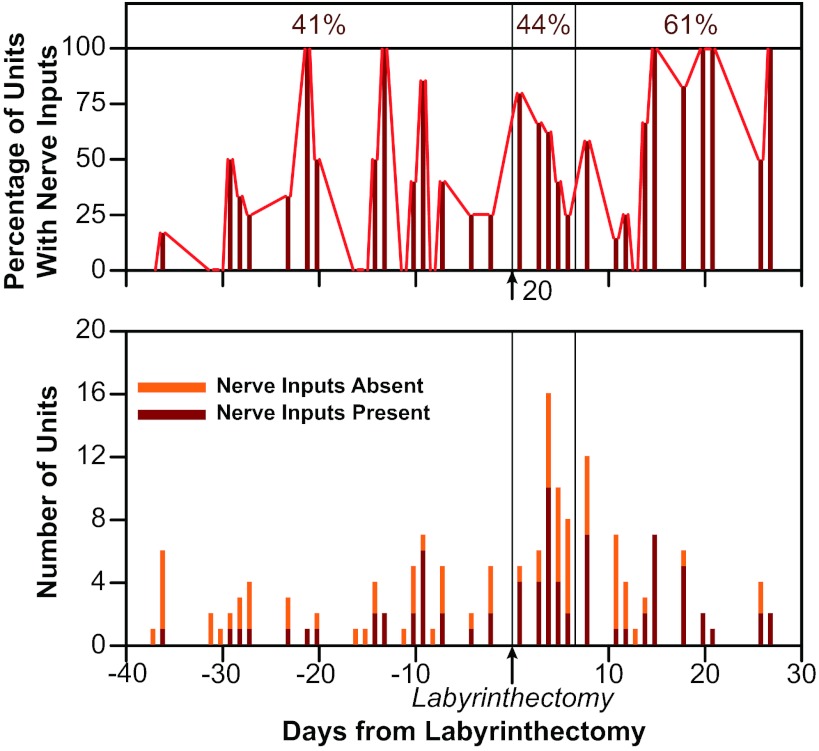
Figure 7 illustrates the fraction of vestibular nucleus neurons that responded to stimulation of at least one hindlimb nerve over time relative to the bilateral labyrinthectomy. In labyrinth-intact animals, 24/59 units (41%) responded to hindlimb nerve stimulation. The proportion increased slightly (to 24/55 or 44% of units) during the first week after removal of vestibular inputs. However, during the subsequent postlabyrinthectomy survival period, 30/49 neurons (61%) responded to stimulation of a hindlimb nerve. A χ2 test confirmed that the proportion of neurons that responded to nerve stimulation increased significantly after the first week following a bilateral labyrinthectomy (P = 0.037).
Fig. 7.
The bottom diagram shows the number of vestibular nucleus neurons tested on each day (in all animals combined) that did and did not respond to hindlimb nerve stimulation. The top diagram shows the same data, designated as percentage of units recorded on each day responsive to hindlimb inputs. Testing days are indicated relative to the date of the bilateral labyrinthectomy, which was performed on day 0, as indicated on the graph. Vertical lines separate the three time periods considered during the study: prelesion (left side of diagrams), W1 (middle section of diagrams), and W2–4 (right side of diagram). Numbers above the top panel indicate the fraction of neurons recorded during each time period that responded to hindlimb nerve stimulation.
In all cases following a bilateral labyrinthectomy, as in labyrinth-intact cats, hindlimb nerve stimulation at intensities greater than three times the threshold for producing spinal cord field potentials or EMG activity were required to produce neuronal responses. Consequently, it is unlikely that inputs from the largest hindlimb afferents (muscle spindle afferents and Golgi tendon organs) were solely responsible for producing the changes in activity of vestibular nucleus neurons.
DISCUSSION
A previous study in decerebrate cats reported that a bilateral labyrinthectomy resulted in an increase in the fraction of vestibular nucleus neurons that responded to stimulation of limb nerves (19). The present experiments provided similar observations in conscious animals, particularly after a 1-wk period of compensation following bilateral damage to the inner ear. Since improvement of balance stability (16, 46) and posturally related autonomic responses (18) occurs over the same time period, these findings support the hypothesis that augmentation of nonlabyrinthine inputs to the vestibular nuclei participates in the recovery of function following bilateral damage to the inner ear (25). This hypothesis is also supported by recent experiments conducted in nonhuman primates showing that extravestibular inputs, including those from neck proprioceptors, substitute for lost vestibular inputs to stabilize gaze at the level of single neurons in the vestibuloocular reflex premotor circuitry (41).
In conscious animals, as in decerebrate and anesthetized cats (7, 19, 35), the median response latency for vestibular nucleus neurons to hindlimb nerve stimulation was ∼20 ms, with some cells responding to nerve stimulation following a latency as short as 10–15 ms. These observations support previous anatomical studies demonstrating that, although sparse direct projections convey signals from the gray matter of the lumbar spinal cord to the vestibular nuclei, most hindlimb inputs reach vestibular nucleus neurons through multisynaptic pathways (17, 26). The effects of hindlimb nerve stimulation on vestibular nucleus neuronal activity observed in this study were primarily excitatory, as reported in experiments on decerebrate and anesthetized animals (7, 19, 35).
However, some aspects of responses to hindlimb nerve stimulation differed between decerebrate and conscious preparations. In decerebrate cats, the fraction of neurons that responded to low-threshold stimulation of muscle afferents (<2 T for producing a spinal cord field potential) increased from 16% to 63% following a bilateral labyrinthectomy (19). In contrast, stimulus intensities under three times threshold for eliciting EMG responses or spinal cord field potentials never affected the firing rate of vestibular nucleus neurons in conscious animals. These findings suggest that, while inputs from the largest muscle afferent fibers (i.e., afferents innervating muscle spindle afferents and Golgi tendon organs) affect vestibular nucleus neuronal activity in decerebrate animals, these afferent inputs are suppressed in conscious animals. However, in the conscious state, signals to the vestibular nuclei from smaller diameter hindlimb afferents are selectively amplified following a bilateral labyrinthectomy, such that a higher fraction of vestibular nucleus neurons respond to stimulation of these fibers. Joint afferents in the cat appear to be mostly type III and smaller type II fibers (15). At least one report has suggested that joint afferents could play a key role in conveying information about limb position to vestibulospinal neurons and modify vestibulospinal responses in accordance with the step cycle (11). Consequently, it is feasible that joint afferent inputs to the vestibular nuclei were selectively amplified following damage to the inner ears. This notion is supported by the observation that, after the lesions, stimulation of the common peroneal nerve was much more effective than stimulation of the tibial nerve in eliciting responses. The two nerves differ considerably in their afferent composition; the fraction of joint and cutaneous afferents in the common peroneal nerve is much higher than in the tibial nerve (8).
The mechanism responsible for the increased efficacy of hindlimb inputs in eliciting changes in vestibular nucleus neuronal activity following damage to the inner ear remains to be discerned. Since the medial cerebellar cortex and fastigial nucleus receive somatosensory and proprioceptive inputs (2, 3, 30), it is tempting to speculate that cerebellum mediates the plasticity within the vestibular nuclei that enhances responsiveness to hindlimb afferent stimulation. Additional experiments will be necessary to test this hypothesis. The latency of responses to hindlimb nerve stimulation remained constant following the bilateral labyrinthectomy, suggesting that both direct and indirect pathways became more effective in conveying hindlimb inputs to vestibular nucleus neurons. Consequently, it is feasible that plastic changes occur within the vestibular nuclei after labyrinthine damage that facilitates the transmission of signals from a variety of sources to vestibular nucleus neurons. One possibility is that the efficacy of synaptic transmission within the vestibular nuclei is enhanced as a result of the plastic changes, such that previously subthreshold excitatory inputs become effective in altering the firing rate of vestibular nucleus neurons. Such plasticity could also explain why vestibular nucleus neurons quickly regain spontaneous activity follow the loss of inputs from the inner ear (27, 36). Another possibility is that the return of spontaneous activity to the vestibular nuclei after a bilateral labyrinthectomy is due to an increase in intrinsic excitability (32), which may subsequently alter responsiveness of neurons in the vestibular nuclei to hindlimb inputs.
It is also feasible that somatosensory influences on vestibular nucleus neurons are suppressed in conscious animals, but the response suppression wanes for some inputs after bilateral damage to the inner ear, resulting in a higher fraction of neurons with responses to hindlimb nerve stimulation. It seems likely that decerebration and use of anesthesia also diminishes the response suppression, since a higher fraction of neurons has been reported to respond to stimulation of hindlimb nerves in anesthetized and decerebrate cats than observed in this study (19, 37–40, 49–51). For example, one paper reported that 57% of vestibular nucleus neurons in labyrinth-intact decerebrate animals responded to hindlimb nerve stimulation (19), as opposed to 41% noted in the present experiments. As discussed above, moderately high current intensities were required to elicit neuronal responses in this study, whereas stimulation of large-diameter muscle afferents alters vestibular nucleus neuronal activity in decerebrate cats (19). Consequently, the suppression of somatosensory inputs to the vestibular nuclei may be targeted to pathways conveying signals from the largest diameter muscle afferents. Previous studies have shown that inputs from muscle afferents to the vestibular nuclei can be selectively suppressed under some conditions. For example, Cullen and colleagues (9) showed that the responses of vestibular nucleus neurons to neck rotation, which are thought to be due to activation of neck muscle spindle afferents (22), vary tremendously in magnitude, depending on whether the neck movement is active or passive.
Curiously, none of the small number of neurons sampled in the lateral vestibular nucleus in this study responded to hindlimb nerve stimulation, whereas experiments in anesthetized and decerebrate animals reported that a substantial fraction of neurons in this nucleus receives hindlimb inputs (19, 49–51). This observation suggests that suppression of somatosensory inputs may be particularly strong in some areas of the vestibular nucleus complex.
Several caveats must be considered when interpreting the present results. Before a bilateral labyrinthectomy, we targeted neurons that responded robustly to whole body rotations to confirm that they were located in the vestibular nuclei. After lesions, we targeted all spontaneously active neurons in the same areas examined before the labyrinthectomies. Consequently, we cannot be sure whether the pre- and postlabyrinthectomy samples were identical. However, units with a variety of responses to vestibular inputs were activated by hindlimb nerve stimulation in labyrinth-intact animals, suggesting that hindlimb signals are distributed to all populations of vestibular nucleus neurons, which minimizes concerns about sampling bias. Another potential caveat is that the efficacy of nerve stimulation could have changed over time. However, the most likely possibility is that nerve stimulation gradually became less effective, due to damage to the nerve by repeated current application and accumulation of scar tissue between the electrode and nerve. As such, we may have underestimated the increase in the proportion of vestibular nucleus neurons that responded to nerve stimulation after the elimination of labyrinthine inputs. At the very least, it seems unlikely that the observed postlesion increase in the number of vestibular nucleus neurons that responded to nerve stimulation represents a false positive outcome.
Perspectives.
The main finding of this study is that electrical stimulation of hindlimb nerves is highly effective in activating vestibular nucleus neurons following a bilateral labyrinthectomy. Devices that monitor body position in space and provide somatosensory cues that indicate body movement have been used to improve balance stability in patients lacking labyrinthine inputs. For example, a vibrotactile somatosensory feedback system attached to the torso has been shown to decrease the risk of falls in patients with bilateral vestibular loss (23, 34, 47, 48). However, individuals do not ordinarily use cutaneous inputs from the trunk for spatial orientation, such that deciphering body position in space from vibrotactile stimuli likely requires cognitive processing, and thus is not automatic. In contrast, proprioceptive information from the hindlimbs is ordinarily employed to achieve balance stability (13, 20, 45), raising the prospect that artificial stimulation of hindlimb receptors could serve as a highly effective prosthesis to replace vestibular signals. It remains to be determined whether vibrotactile stimulation of the leg skin is more effective than application of the same stimuli to the torso. It also may be possible to stimulate muscle, cutaneous, or joint afferents through implanted electrodes to mimic or enhance the somatosensory inputs that indicate balance instability. Further research will be needed to determine how stimulation of hindlimb afferents should be delivered to most effectively signal perturbations in balance, and whether a prosthesis that utilizes hindlimb afferent stimulation is more beneficial than currently available treatments to improve postural stability.
GRANTS
This work was supported by Grant R01-DC00693 from the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health (NIH). J. D. Moy was supported by NIH, NIDCD Training Grant T32 DC000066.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.A.M. and B.J.Y. conception and design of research; A.A.M., J.D.M., S.R.P., W.M.D., and B.J.Y. performed experiments; A.A.M., J.D.M., S.R.P., W.M.D., and B.J.Y. analyzed data; A.A.M. and B.J.Y. interpreted results of experiments; A.A.M. and B.J.Y. prepared figures; A.A.M. and B.J.Y. drafted manuscript; A.A.M., J.D.M., and B.J.Y. edited and revised manuscript; A.A.M., J.D.M., S.R.P., W.M.D., and B.J.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Michael F. Catanzaro, Lucy A. Cotter, Daniel J. Miller, Sarah W. Ogburn, and Michael Yoder for technical assistance during the completion of these experiments, and Michel Lemay for providing detailed guidance on manufacturing the platinum cuff electrodes used during these experiments.
REFERENCES
- 1. Anastasopoulos D, Mergner T. Canal-neck interaction in vestibular nuclear neurons of the cat. Exp Brain Res 46: 269–280, 1982 [DOI] [PubMed] [Google Scholar]
- 2. Andersson G, Oscarsson O. Climbing fiber microzones in cerebellar vermis and their projection to different groups of cells in the lateral vestibular nucleus. Exp Brain Res 32: 565–579, 1978 [DOI] [PubMed] [Google Scholar]
- 3. Armstrong DM, Harvey RJ. Responses to a spino-olivo-cerebellar pathway in the cat. J Physiol 194: 147–168, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker J, Goldberg J, Hermann G, Peterson B. Spatial and temporal response properties of secondary neurons that receive convergent input in vestibular nuclei of alert cats. Brain Res 294: 138–143, 1984 [DOI] [PubMed] [Google Scholar]
- 5. Barman SM, Sugiyama Y, Suzuki T, Cotter LA, DeStefino VJ, Reighard DA, Cass SP, Yates BJ. Rhythmic activity of neurons in the rostral ventrolateral medulla of conscious cats: effect of removal of vestibular inputs. Am J Physiol Regul Integr Comp Physiol 301: R937–R946, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyle R, Pompeiano O. Responses of vestibulospinal neurons to neck and macular vestibular inputs in the presence or absence of the paleocerebellum. Ann N Y Acad Sci 374: 373–394, 1981 [DOI] [PubMed] [Google Scholar]
- 7. Bruggencate GT, Teichmann R, Weller E. Neuronal activity in the lateral vestibular nucleus of the cat. IV. Postsynaptic potentials evoked by stimulation of peripheral somatic nerves. Pflügers Arch 360: 301–320, 1975 [DOI] [PubMed] [Google Scholar]
- 8. Crouch JE. Text-Atlas of Cat Anatomy. Philadelphia, PA: Lea and Febiger, 1969 [Google Scholar]
- 9. Cullen KE, Brooks JX, Jamali M, Carriot J, Massot C. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res 210: 377–388, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Destefino VJ, Reighard DA, Sugiyama Y, Suzuki T, Cotter LA, Larson MG, Gandhi NJ, Barman SM, Yates BJ. Responses of neurons in the rostral ventrolateral medulla (RVLM) to whole-body rotations: comparisons in decerebrate and conscious cats. J Appl Physiol 110: 1699–1707, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fredrickson JM, Schwarz D, Kornhuber HH. Convergence and interaction of vestibular and deep somatic afferents upon neurons in the vestibular nuclei of the cat. Acta Otolaryngol 61: 168–188, 1966 [DOI] [PubMed] [Google Scholar]
- 12. Goldberg JM, Wilson VJ, Cullen KE, Angelaki DE, Broussard DM, Büttner-Ennever JA, Fukushima K, Minor LB. The Vestibular System. A Sixth Sense. New York: Oxford University Press, 2012, p. 541 [Google Scholar]
- 13. Hatzitaki V, Pavlou M, Bronstein AM. The integration of multiple proprioceptive information: effect of ankle tendon vibration on postural responses to platform tilt. Exp Brain Res 154: 345–354, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Haugland M. A flexible method for fabrication of nerve cuff electrodes. In: Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, edited by Boom H, Robinson C, Rutten W, Neuman M, Wijkstra H. Amsterdam: IEEE, 1996, p. 359–360 [Google Scholar]
- 15. Heppelmann B, Heuss C, Schmidt RF. Fiber size distribution of myelinated and unmyelinated axons in the medial and posterior articular nerves of the cat's knee joint. Somatosens Res 5: 273–281, 1988 [DOI] [PubMed] [Google Scholar]
- 16. Inglis JT, Macpherson JM. Bilateral labyrinthectomy in the cat: effects on the postural response to translation. J Neurophysiol 73: 1181–1191, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Jian BJ, Acernese AW, Lorenzo J, Card JP, Yates BJ. Afferent pathways to the region of the vestibular nuclei that participates in cardiovascular and respiratory control. Brain Res 1044: 241–250, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol 86: 1552–1560, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Jian BJ, Shintani T, Emanuel BA, Yates BJ. Convergence of limb, visceral, and vertical semicircular canal or otolith inputs onto vestibular nucleus neurons. Exp Brain Res 144: 247–257, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Karayannidou A, Deliagina TG, Tamarova ZA, Sirota MG, Zelenin PV, Orlovsky GN, Beloozerova IN. Influences of sensory input from the limbs on feline corticospinal neurons during postural responses. J Physiol 586: 247–263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kasper J, Schor RH, Wilson VJ. Response to vestibular neurons to head rotations in vertical planes. II. Response to neck stimulation and vestibular-neck interaction. J Neurophysiol 60: 1765–1778, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Kasper J, Wilson VJ, Yamagata Y, Yates BJ. Neck muscle spindle activity in the decerebrate, unparalyzed cat: dynamics and influence of vestibular stimulation. J Neurophysiol 62: 917–923, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Kentala E, Vivas J, Wall C., 3rd Reduction of postural sway by use of a vibrotactile balance prosthesis prototype in subjects with vestibular deficits. Ann Otol Rhinol Laryngol 112: 404–409, 2003 [DOI] [PubMed] [Google Scholar]
- 24. McCall AA, Moy JD, DeMayo WM, Puterbaugh SR, Miller DJ, Catanzaro MF, Yates BJ. Processing of vestibular inputs by the medullary lateral tegmental field of conscious cats: Implications for generation of motion sickness. Exp Brain Res. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCall AA, Yates BJ. Compensation following bilateral vestibular damage. Front Neurol 2: 88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKelvey-Briggs DK, Saint-Cyr JA, Spence SJ, Partlow GD. A reinvestigation of the spinovestibular projection in the cat using axonal transport techniques. Anat Embryol (Berl) 180: 281–291, 1989 [DOI] [PubMed] [Google Scholar]
- 27. Miller DM, Cotter LA, Gandhi NJ, Schor RH, Cass SP, Huff NO, Raj SG, Shulman JA, Yates BJ. Responses of caudal vestibular nucleus neurons of conscious cats to rotations in vertical planes, before and after a bilateral vestibular neurectomy. Exp Brain Res 188: 175–186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller DM, Cotter LA, Gandhi NJ, Schor RH, Huff NO, Raj SG, Shulman JA, Yates BJ. Responses of rostral fastigial nucleus neurons of conscious cats to rotations in vertical planes. Neuroscience 155: 317–325, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mori RL, Cotter LA, Arendt HE, Olsheski CJ, Yates BJ. Effects of bilateral vestibular nucleus lesions on cardiovascular regulation in conscious cats. J Appl Physiol 98: 526–533, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Morin F, Haddad B. Afferent projections to the cerebellum and the spinal pathways involved. Am J Physiol 172: 497–510, 1953 [DOI] [PubMed] [Google Scholar]
- 31. Moy JD, Miller DJ, Catanzaro MF, Boyle BM, Ogburn SW, Cotter LA, Yates BJ, McCall AA. Responses of neurons in the caudal medullary lateral tegmental field to visceral inputs and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol 303: R929–R940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson AB, Krispel CM, Sekirnjak C, du Lac S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron 40: 609–620, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Ollivier-Lanvin K, Krupka AJ, AuYong N, Miller K, Prilutsky BI, Lemay MA. Electrical stimulation of the sural cutaneous afferent nerve controls the amplitude and onset of the swing phase of locomotion in the spinal cat. J Neurophysiol 105: 2297–2308, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peterka RJ, Wall C, 3rd, Kentala E. Determining the effectiveness of a vibrotactile balance prosthesis. J Vestib Res 16: 45–56, 2006 [PubMed] [Google Scholar]
- 35. Pompeiano O. Spinovestibular relations: anatomical and physiological aspects. Prog Brain Res 37: 263–296, 1972 [DOI] [PubMed] [Google Scholar]
- 36. Ris L, Godaux E. Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig. J Neurophysiol 80: 2352–2367, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Rubin AM, Liedgren SC, Odkvist LM, Milne AC, Fredrickson JM. Labyrinthine and somatosensory convergence upon vestibulo-ocular units. Acta Otolaryngol (Stockh) 85: 54–62, 1978 [DOI] [PubMed] [Google Scholar]
- 38. Rubin AM, Liedgren SR, Miline AC, Young JA, Fredrickson JM. Vestibular and somatosensory interaction in the cat vestibular nuclei. Pflügers Arch 371: 155–160, 1977 [DOI] [PubMed] [Google Scholar]
- 39. Rubin AM, Liedgren SR, Odkvist LM, Larsby B, Aschan G. Limb input to the cat vestibular nuclei. Acta Otolaryngol (Stockh) 87: 113–122, 1979 [DOI] [PubMed] [Google Scholar]
- 40. Rubin AM, Liedgren SR, Odkvist LM, Milne AC, Fredrickson JM. Labyrinthine and somatosensory convergence upon vestibulospinal neurons. Acta Otolaryngol (Stockh) 86: 251–259, 1978 [DOI] [PubMed] [Google Scholar]
- 41. Sadeghi SG, Minor LB, Cullen KE. Neural correlates of sensory substitution in vestibular pathways following complete vestibular loss. J Neurosci 32: 14685–14695, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schor RH, Angelaki DE. The algebra of neural response vectors. Ann N Y Acad Sci 656: 190–204, 1992 [DOI] [PubMed] [Google Scholar]
- 43. Schor RH, Miller AD, Tomko DL. Responses to head tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol 51: 136–146, 1984 [DOI] [PubMed] [Google Scholar]
- 44. Sherrington CS. Decerebrate rigidity and reflex coordination of movements. J Physiol 22: 319–337, 1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thompson C, Belanger M, Fung J. Effects of plantar cutaneo-muscular and tendon vibration on posture and balance during quiet and perturbed stance. Hum Mov Sci 30: 153–171, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Thomson DB, Inglis JT, Schor RH, Macpherson JM. Bilateral labyrinthectomy in the cat: motor behaviour and quiet stance parameters. Exp Brain Res 85: 364–372, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Wall C, 3rd, Kentala E. Control of sway using vibrotactile feedback of body tilt in patients with moderate and severe postural control deficits. J Vestib Res 15: 313–325, 2005 [PubMed] [Google Scholar]
- 48. Wall C, 3rd, Kentala E. Effect of displacement, velocity, and combined vibrotactile tilt feedback on postural control of vestibulopathic subjects. J Vestib Res 20: 61–69, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Wilson VJ, Kato M, Peterson BW, Wylie RM. A single-unit analysis of the organization of Deiters' nucleus. J Neurophysiol 30: 603–619, 1967 [DOI] [PubMed] [Google Scholar]
- 50. Wilson VJ, Kato M, Thomas RC, Peterson BW. Excitation of lateral vestibular neurons by peripheral afferent fibers. J Neurophysiol 29: 508–529, 1966 [DOI] [PubMed] [Google Scholar]
- 51. Wylie RM, Felpel LP. The influence of the cerebellum and peripheral somatic nerves on the activity of Deiters' cells in the cat. Exp Brain Res 12: 528–546, 1971 [DOI] [PubMed] [Google Scholar]
- 52. Yates BJ, Jian BJ, Cotter LA, Cass SP. Responses of vestibular nucleus neurons to tilt following chronic bilateral removal of vestibular inputs. Exp Brain Res 130: 151–158, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Yates BJ, Thompson FJ, Mickle JP. Origin and properties of spinal cord field potentials. Neurosurgery 11: 439–450, 1982 [DOI] [PubMed] [Google Scholar]