Abstract
Cachexia, characterized by skeletal muscle mass loss, is a major contributory factor to patient morbidity and mortality during cancer. However, there are no reports on the rate of myofibrillar protein synthesis (MPS) in skeletal muscles that vary in primary metabolic phenotype during cachexia, in large part because of the small-size muscles and regional differences in larger muscles in the mouse. Here, we describe a sensitive method for measurement of MPS and its application to analysis of MPS in specific muscles of mice with (ApcMin/+) and without (C57BL/6) cancer cachexia. Mice were injected with a loading dose of deuterated phenylalanine (D5F), and myofibrillar proteins were extracted from skeletal muscles at 30 min. The relative concentrations of D5F and naturally occurring phenylalanine (F) in the myofibrillar proteins and the amino acid pool were quantified by ultra-performance liquid chromatograph (UPLC) mass spectrometry (MS). The rate of MPS was determined from D5F-to-F ratio in the protein fraction compared with the amino acid pool. The rate of MPS, measured in 2–5 mg of muscle protein, was reduced by up to 65% with cachexia in the soleus, plantaris, diaphragm, and oxidative and glycolytic regions of the gastrocnemius. The rate of MPS was significantly higher in the oxidative vs. glycolytic gastrocnemius muscle. A sufficiently sensitive UPLC MS method requiring a very small amount of muscle has been developed to measure the rate of MPS in various mouse muscles. This method should be useful for studies in other animal models for quantifying effects of cancer and anti-cancer therapies on protein synthesis in cachexia, and particularly for analysis of sequential muscle biopsies in a wide range of animal and human studies.
Keywords: cachexia, myofibrillar protein synthesis, soleus, plantaris, ultra-performance liquid chromatography mass spectrometry
skeletal muscle is a highly plastic tissue that can undergo hypertrophy or atrophy in response to a variety of stimuli including disuse, variations in nutritional and hormonal status, aging, exercise, chronic diseases, and associated metabolic syndromes (15, 21, 27). Cachexia is a chronic wasting condition associated with inflammatory diseases such as cancer and is characterized by severe loss of both muscle and adipose tissue mass (11, 20). Losses in muscle mass lead to muscle weakness and impaired muscle function, which can negatively impact quality of life and response to therapy, and can increase mortality (22, 24, 26). Cachexia affects up to 80% of patients with advanced cancers, and >20% of patients die as a result of cachexia-related complications (1, 2, 29). The rapid progressive muscle loss rooted in cachexia is the result of an imbalance between decreased protein synthesis (MPS) and increased protein degradation (28). Because of its importance as a marker of cachexia, measurement of MPS is a valuable tool for evaluating the effectiveness of strategies to combat cachexia.
Although there are several different animal models that are used to study cachexia, the genetically engineered ApcMin/+ mouse model (Min: multiple intestinal neoplasia) is commonly utilized; the slow-progressive cachexia and associated side effects exhibited by this model closely mimic human cachexia (4, 6). The ApcMin/+ mouse is heterozygous for a point mutation in the adenomatous polyposis coli (Apc) gene, which leads to development of familial adenomatous polyposis (FAP), a form of colon cancer. The mouse develops intestinal polyps at ∼4 wk of age and loses body weight gradually between 14 and 20 wk of age. At 20 wk of age, the mouse is usually severely cachectic, having lost >15% of body mass compared with its peak body mass (4, 6, 25). Muscle phenotype related to the capacity for oxidative metabolism can affect myofiber susceptibility to wasting (17), and primarily glycolytic myofibers in the ApcMin/+ mouse demonstrate more atrophy during the development of cachexia (5). The standard procedure for measuring protein synthesis in vivo involves measurement of incorporation of isotopically labeled tracers into myofibrillar protein by using liquid scintillation counting, isotope ratio mass spectrometry (IRMS), and gas chromatography/mass spectrometry (GC-MS) (16, 23, 31). Although these methods have been useful, they require relatively large amounts of tissue (typically 20–50 mg wet wt), which is not readily available except by pooling samples in some cases and, in any case, limits the availability of tissue needed for other analyses (e.g., Western blots, RT-PCR, etc.) (14, 23). To date, many researchers have been utilizing various mouse models of cachexia to understand the mechanisms responsible for muscle wasting and the role of muscle phenotype in the process (7, 9, 12, 18).
Tandem mass spectrometry (MS/MS), coupled with ultra-performance liquid chromatography (UPLC), provides high resolution, specificity, and sensitivity for measurement of amino acids in tissues (8). In this paper, we describe a method for analysis of MPS in individual mouse muscles using UPLC-MS/MS.
METHODS
Materials.
Nonafluoropentanoic acid (NFPA; 97%) and sodium hydroxide (NaOH) were purchased from Sigma (St. Louis, MO). Trifluoroacetic acid (TFA) was purchased from ThermoFisher Scientific (Waltham, MA). L-[ring-2H5]phenylalanine (D5F) was from Cambridge Isotope Laboratories (Andover, MA), and HPLC-grade water and methanol were from VWR (Radnor, PA).
Animals and tissue collection.
Male ApcMin/+ mice on a C57BL/6 background were originally purchased from Jackson Laboratories (Bar Harbor, ME) and crossed with C57BL/6 female mice at the University of South Carolina's animal resource facility. Offspring were genotyped as heterozygotes by RT-PCR for the Apc gene as described by Mehl et al. (18, 19). All mice were housed in standard cages. The room was maintained at 24°C on a 12-h light-dark cycle with the light period starting at 0700. All mice were provided with standard rodent chow (Harlan Teklad Rodent Diet, no. 8604, Madison, WI) and water ad libitum. Male C57BL/6 and ApcMin/+ mice (n = 6–7) were killed at 20 wk of age.
Thirty minutes before death, all mice received an intraperitoneal injection of 150 mM D5F in a 75 mM NaCl solution at a dose of 0.02 ml/g body wt. Subsequently, mice were given a subcutaneous injection of a ketamine-xylazine-acepromazine cocktail (1.4 ml/kg body wt). Muscles (plantaris, soleus, gastrocnemius, and diaphragm) were excised under anesthesia, rinsed in PBS, weighed, snap frozen in liquid nitrogen, and stored at −80°C until further analysis. Red and white fibers were isolated from the gastrocnemius as previously described (32). All animal experimentation was conducted in accordance with procedures approved by the University of South Carolina's Institutional Animal Care and Use Committee.
Protein extraction and isolation.
Frozen muscle tissues were processed using the method of Welle et al. (31) for measurement of myofibrillar protein synthesis by gas chromatography mass spectrometry. Tissue samples (∼5 mg) were weighed in a 1.5-ml Eppendorf plastic centrifuge tube, to which 200 μl of ice-cold homogenizing buffer (HB; 50 mM KPO4, pH 7.0, 0.25 M sucrose, 1% Triton X-100) were added, and kept on ice. Each sample was sonicated twice for 20 s on ice. The sonicate was centrifuged at 10,000 g for 10 min at 4°C, and the supernatant containing the free amino acid pool was transferred to a fresh tube and dried under vacuum (Speed Vac, Thermo Electron, Milford, MA), then reconstituted in 1% aqueous TFA (1 ml), and stored at −20°C. The pellet fraction was resuspended by sonication in 350 μl of HB and centrifuged at 10,000 g for 10 min at 4°C. The supernatant was discarded, and this washing procedure was repeated once with HB (350 μl) and twice with ice-cold deionized water (500 μl).
NaOH (0.3 M, 200 μl) was added to the final pellet, followed by centrifugation at 10,000 g for 10 min at 4°C. The supernatant, containing myofibrillar proteins, was diluted with 6 M HCl (1 ml), and the protein was hydrolyzed at 110°C for 24 h in 2-ml screw-closure polypropylene O-ring sealed vials (Sarstedt, Nümbrecht, Germany). HCl was removed under vacuum (Speed Vac), and the dried protein hydrolysate was reconstituted in 1% aqueous TFA (1 ml) and stored at −20°C. The free amino acid pool and myofibrillar protein hydrolysate were applied to a 3-ml Supelco C18 cartridge (Waters, Milford, MA) equilibrated in 1% aqueous TFA. The aromatic amino acid fraction was eluted with 1% TFA in methanol:water (20:80, vol/vol, 3 ml). The eluate was dried under vacuum and reconstituted in aqueous 5 mM NFPA (45 μl) before analysis by UPLC-MS/MS.
LC-MS conditions and instrumentation.
Samples were fractioned on a Waters (Manchester, UK) Acquity UPLC-MS/MS system using a BEH C18 column (2.1 × 50 mm; particle size, 1.7 μm), which was maintained at room temperature. Samples were eluted at a flow rate of 0.2 ml/min, eluting for 2.0 min with 100% solvent A (aqueous 5 mM NFPA), followed by linear gradient to 50% solvent B (acetonitrile) over 10 min, then washed with 80% solvent B for 15 min. The injection volume was 5 μl (equivalent to ∼3 nmol of F).
Mass spectrometry experiments and optimization of the method were performed utilizing a Micromass Quattro Premier XE Tandem Mass Spectrometer (Waters). Multiple reaction monitoring (MRM) was conducted by operating the MS in positive ion electrospray mode. Table 1 details the MRM conditions of analysis. Other MS operating conditions used were scan duration of 1 s, capillary voltage of 3 kV, cone voltage of 25 eV, source temperature of 100°C, and desolvation temperature of 350°C. Sample peaks corresponding to phenylalanine, naturally occurring [13C]phenylalanine, and D5F were integrated. Data were analyzed using MassLynx software (version 4.1) supplied by Waters. Analytes were quantified by comparing the peak area of deuterated compound: unlabeled compound. Percent protein synthesis per day was calculated using the general formula (31) by dividing the ratio of D5F/F in myofibrillar protein pellet by the ratio of D5F/F in the free amino acid pool and expressing it as a percentage of newly synthesized protein, using the following formula:
where MPS is myofibrillar protein synthesis, MPP is myofibrillar protein pellet, and FAAP is free amino acid pool.
Table 1.
MRM condition of analysis
| Compound | Parent Mass, Da | Daughter Mass, Da | Cone Voltage | Collision Energy, eV |
|---|---|---|---|---|
| D5-F | 171 | 125 | 25 | 13 |
| C13-F | 167 | 121 | 25 | 13 |
| F | 166 | 120 | 25 | 13 |
Statistical analysis.
All statistical analyses were performed using GraphPad Prism (La Jolla, CA). All comparisons were analyzed using a Student's two-tailed t-test, with significance set at P ≤ 0.05.
RESULTS
UPLC-MS conditions were optimized to resolve phenylalanine in a short chromatographic run. Addition of 5 mM NFPA, an ion-pairing agent, to the mobile phases improved signal intensity and resolution. MRM conditions were manually optimized for each compound in positive ESI ionization mode to obtain maximal response. The mass spectrum of phenylalanine is shown in Fig. 1A. The precursor ion (m/z 166) was selected for fragmentation, and the fragment corresponding to the immonium ion (m/z 120), which was the most abundant fragment in the spectrum, was recorded by the second mass analyzer. Chromatograms obtained for F, 13C-F, and D5F are shown in Fig. 1, B and C, for myofibrillar protein pellet and the amino acid pool, respectively. The natural abundance peak of 13C-F, which is 1.1% the intensity of the 12C-F peak, was used for internal standardization, since the 12C-F signal saturated the detector for some samples.
Fig. 1.
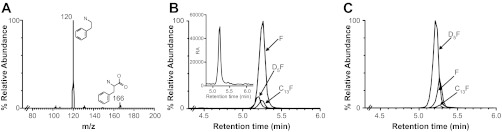
Optimization of mass spectrometric conditions for analysis of phenylalanine. Fragmentation pattern of phenylalanine ions (A), extracted ion chromatograms for phenylalanine (F), naturally occurring carbon 13 F (C13 F), and deuterated F (D5F) incorporated in the myofibrillar protein pellet (B) (D5F was magnified ×100; inset shows the well resolved chromatogram for D5F without magnification) and the supernatant (C). All peaks are normalized to the largest occurring peak either in the pellet or supernatant fraction.
All peaks were normalized to the strongest signal in the pellet and amino acid pool fractions, respectively. As expected, the myofibrillar pellet contained an abundant amount of F, followed by naturally occurring 13C-F, which accounts for ∼10% of F, and D5F. Although the peak intensity for the D5F in pellet was only ∼0.14% of F in the myofibrillar protein, this was well within the linear range of the instrument and yielded a strong signal during MRM analysis (Fig. 1B, inset).
Using the optimized MRM conditions and the formula above, myofibrillar protein synthesis was quantified in various skeletal muscles (Fig. 2). The mice chosen for the study, at 20 wk of age, were chosen from a group of severely cachectic ApcMin/+ mice (Table 2). Cachectic ApcMin/+ mice experience an average of ∼20% weight loss between weeks 12 and 19 of age (32). Not all of the weight changes are the result of loss of skeletal muscle; there are comparable losses of adipose tissue mass. Even with adjustment for the lifespan of mice compared with humans, the rate of weight loss in untreated, severely cachectic mice is extreme. In humans, pre-cachexia is generally defined as ≤5% weight loss during a 6-mo period, whereas weight loss of ≥6% during this period is described as cachexia (3).
Fig. 2.
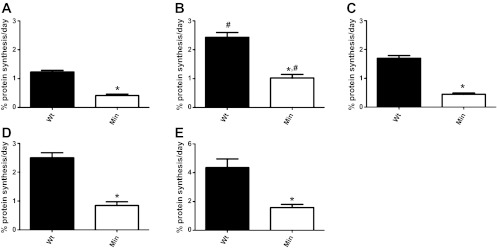
Skeletal muscle protein synthesis is decreased during cancer cachexia. Myofibrillar protein synthesis was measured at 20 wk of age in gastrocnemius (white, glycolytic portion; A) and gastrocnemius (red, oxidative portion; B), plantaris (C), soleus (D), and diaphragm (E) muscle. Values are means ± SE (n = 5). *Signifies difference from wild-type mice (P < 0.05). #Significant difference from white gastrocnemius muscle (P < 0.05).
Table 2.
Body weights and muscle weights of C57BL/6 and ApcMin/+ mice
| C57BL/6 | ApcMin/+ | |
|---|---|---|
| N | 7 | 6 |
| Body weight, g | 29.2 ± 1.3 | 18.6 ± 0.7* |
| Soleus, mg | 10.0 ± 0.6 | 6.3 ± 0.5* |
| Plantaris, mg | 18.1 ± 0.6 | 10.5 ± 0.8* |
| Gastrocnemius, mg | 134.3 ± 3.5 | 68.5 ± 6.1* |
| Diaphragm | 71.3 ± 1.6 | 30.5 ± 2.7* |
Significantly different from age-matched C57BL/6 control mice (P < 0.05).
DISCUSSION
The rate of MPS varied from ∼1.25%/day to ∼2.5%/day in white and red gastrocnemius, respectively, to ∼4.3%/day in the diaphragm muscle of control animals at 20 wk (5 mo) of age (Fig. 2), which is in reasonable agreement with the 3% estimate of MPS in the gastrocnemius of C57BL/6 mice at 6 mo of age, analyzed by the GC-MS/MS procedure (31). MPS was significantly reduced in all muscles in cachectic animals but varied significantly with the specific muscle from ∼60% in the red gastrocnemius and diaphragm muscles to ∼75% in the plantaris muscle (Fig. 2). In a previous study, using a GC-MS/MS procedure, we observed an ∼50% decrease in the rate of gastrocnemius muscle protein synthesis in a group of animals with ≥20% weight loss (33), which is reasonably consistent with the results of the UPLC-MS/MS method applied to a more severely cachectic group of mice. There is also an ∼150% increase in the rate of muscle protein degradation in these animals (33), which further contributes to the decrease in muscle mass. Percentage reductions in MPS were observed across all muscle types, including the soleus and plantaris muscles, which could not be measured (without pooling samples) by the GC-MS/MS method. We also show for the first time that the absolute rate of MPS is significantly higher in the red portion of the gastrocnemius and the soleus muscle compared with the white portion of the gastrocnemius and the plantaris muscle in the mouse. This is consistent with the observation that MPS in glycolytic muscle fibers is more susceptible to wasting compared with oxidative fibers in humans and rats (10, 13, 30). However, to the best of our knowledge, no other studies have examined the rate of MPS in different muscle phenotypes of cachectic mice due to limitations regarding tissue mass needed for the analysis.
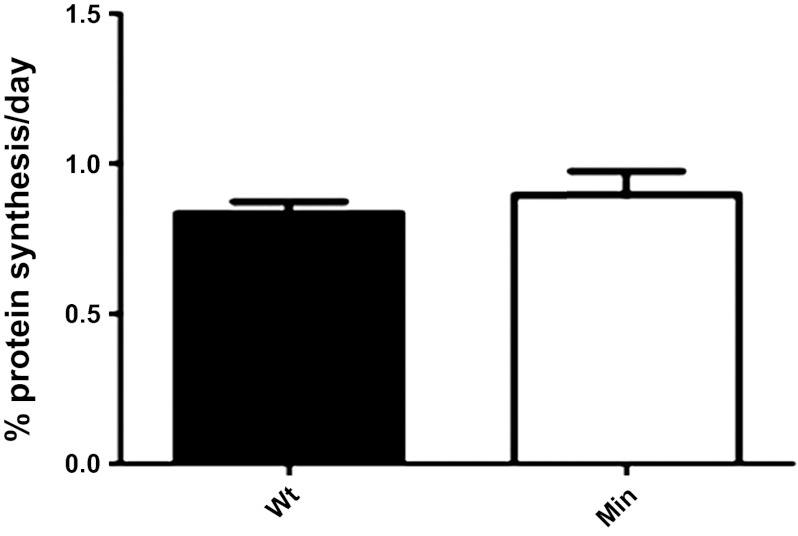
To further validate the method, MPS was measured in the younger ApcMin/+ mice group compared with the C57BL/6 control animals (aged 10 wk) (Fig. 3). These results illustrated that there was no significant difference in MPS across genotypes, which supports the observation that Apc Min/+ mice are not cachetic at this age (33), hence MPS is not decreased. Compared with the GC-MS/MS method (33), which requires ∼50 mg of tissue per analysis, the current method requires only 5 mg of tissue, which permits triplicate injections and applies more straightforward calculations. The intra-assay variance within a group of animals was <2%. Limit of detection is ∼50 fmol for F standard injected on the column. Compared with GCMS, which requires derivatization and longer analysis time, this method is far more advantageous due to its simplicity of sample preparation and short analysis time. The small sample requirement for the UPLC-MS/MS assay also allows for analysis of MPS in subcellular fractions of mouse muscles and should also be applicable to the analysis of sequential biopsy samples from animal and human studies.
Fig. 3.
Skeletal muscle protein synthesis is similar in Min mice at younger age. Wild-type and ApcMin/+ mice were killed at 10 wk of age. Myofibrillar protein synthesis was measured in whole gastrocnemius. Values are means ± SE (n = 3).
In summary, a specific, sensitive, and relatively simple method has been developed to measure MPS in mouse tissues and has been applied to analysis of MPS in control and cachectic mice. This method has been applied to analysis of small muscles (soleus and plantaris) and to analysis of MPS in different regions of the gastrocnemius muscle (red vs. white) of ApcMin/+ mice. These results show that the rate of MPS is greater in red oxidative muscles and that the rate of MPS is comparably decreased in all of these muscles, including the involuntary skeletal muscle diaphragm in ApcMin/+ mice during cachexia. Future studies on the rates of protein turnover in these tissues should provide a better understanding of the relative roles of changes in muscle protein synthesis vs. turnover in the loss of muscle mass during cachexia.
GRANTS
This work was supported by National Cancer Institute Grant R01 CA-121249.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.L., R.T.E., J.W.B., and J.A.C. conception and design of research; M.L., S.S., and R.T.E. performed experiments; M.L., S.S., R.T.E., J.W.B., and J.A.C. analyzed data; M.L., S.S., R.T.E., J.W.B., and J.A.C. interpreted results of experiments; M.L., R.T.E., and J.W.B. prepared figures; M.L., S.S., and R.T.E. drafted manuscript; M.L., S.S., R.T.E., J.W.B., and J.A.C. edited and revised manuscript; M.L., S.S., R.T.E., J.W.B., and J.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. William Cotham for technical assistance with mass spectrometry analyses and Drs. James A. White and Norma Frizzell for helpful discussions.
REFERENCES
- 1. Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJ, Fearon KC, Hollingsworth MA, Muscarella P, Burghes AH, Rafael-Fortney JA, Guttridge DC. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 8: 421–432, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Argiles JM, Busquets S, Garcia-Martinez C, Lopez-Soriano FJ. Mediators involved in the cancer anorexia-cachexia syndrome: past, present, and future. Nutrition 21: 977–985, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Argiles JM, Lopez-Soriano FJ, Toledo M, Betancourt A, Serpe R, Busquets S. The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J Cachexia Sarcopenia Muscle 2: 87–93, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 294: R393–R401, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc (Min/+) mouse. Pflügers Arch 457: 989–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baltgalvis KA, Berger FG, Pena MM, Mark Davis J, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in Apc(Min/+) mice. J Appl Physiol 109: 1155–1161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennani-Baiti N, Walsh D. Animal models of the cancer anorexia-cachexia syndrome. Support Care Cancer 19: 1451–1463, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Churchwell MI, Twaddle NC, Meeker LR, Doerge DR. Improving LC-MS sensitivity through increases in chromatographic performance: comparisons of UPLC-ES/MS/MS to HPLC-ES/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 825: 134–143, 2005 [DOI] [PubMed] [Google Scholar]
- 9. DeBoer MD. Update on melanocortin interventions for cachexia: progress toward clinical application. Nutrition 26: 146–151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dickinson JM, Lee JD, Sullivan BE, Harber MP, Trappe SW, Trappe TA. A new method to study in vivo protein synthesis in slow- and fast-twitch muscle fibers and initial measurements in humans. J Appl Physiol 108: 1410–1416, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, Steiner MS. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 62: 265–279, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Emery PW. Cachexia in experimental models. Nutrition 15: 600–603, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Garlick PJ, Maltin CA, Baillie AG, Delday MI, Grubb DA. Fiber-type composition of nine rat muscles. II. Relationship to protein turnover. Am J Physiol Endocrinol Metab 257: E828–E832, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Garlick PJ, Wernerman J, McNurlan MA, Essen P, Lobley GE, Milne E, Calder GA, Vinnars E. Measurement of the rate of protein synthesis in muscle of postabsorptive young men by injection of a ‘flooding dose’ of [1–13C]leucine. Clin Sci (Lond) 77: 329–336, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Hawley JA, Burke LM, Phillips SM, Spriet LL. Nutritional modulation of training-induced skeletal muscle adaptations. J Appl Physiol 110: 834–845, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Kubera M, Skowron-Cendrzak A, Garlicki M. Prolongation of H-Y incompatible skin grafts in mice by neonatal spleen cells. Folia Biol (Krakow) 37: 165–169, 1989 [PubMed] [Google Scholar]
- 17. Li P, Waters RE, Redfern SI, Zhang M, Mao L, Annex BH, Yan Z. Oxidative phenotype protects myofibers from pathological insults induced by chronic heart failure in mice. Am J Pathol 170: 599–608, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehl KA, Davis JM, Berger FG, Carson JA. Myofiber degeneration/regeneration is induced in the cachectic ApcMin/+ mouse. J Appl Physiol 99: 2379–2387, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol 98: 2219–2225, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr 83: 735–743, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Murton AJ, Constantin D, Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim Biophys Acta 1782: 730–743, 2008 [DOI] [PubMed] [Google Scholar]
- 22. O'Gorman P, McMillan DC, McArdle CS. Impact of weight loss, appetite, and the inflammatory response on quality of life in gastrointestinal cancer patients. Nutr Cancer 32: 76–80, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism 46: 943–948, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Persson C, Glimelius B. The relevance of weight loss for survival and quality of life in patients with advanced gastrointestinal cancer treated with palliative chemotherapy. Anticancer Res 22: 3661–3668, 2002 [PubMed] [Google Scholar]
- 25. Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochim Biophys Acta 1812: 1601–1606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scott HR, McMillan DC, Brown DJ, Forrest LM, McArdle CS, Milroy R. A prospective study of the impact of weight loss and the systemic inflammatory response on quality of life in patients with inoperable non-small cell lung cancer. Lung Cancer 40: 295–299, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Taaffe DR, Marcus R. Musculoskeletal health and the older adult. J Rehabil Res Dev 37: 245–254, 2000 [PubMed] [Google Scholar]
- 28. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 89: 381–410, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Tisdale MJ. Pathogenesis of cancer cachexia. J Support Oncol 1: 159–168, 2003 [PubMed] [Google Scholar]
- 30. Watt PW, Kelly FJ, Goldspink DF, Goldspink G. Exercise-induced morphological and biochemical changes in skeletal muscles of the rat. J Appl Physiol 53: 1144–1151, 1982 [DOI] [PubMed] [Google Scholar]
- 31. Welle S, Bhatt K, Pinkert CA. Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab 290: E409–E415, 2006 [DOI] [PubMed] [Google Scholar]
- 32. White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol 300: R201–R211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLos One 6: e24650, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]