Abstract
Nitric oxide (NO) exerts a wide range of cellular effects in the cardiovascular system. NO is short lived, but S-nitrosoglutathione (GSNO) functions as a stable intracellular bioavailable NO pool. Accordingly, increased levels can facilitate NO-mediated processes, and conversely, catabolism of GSNO by the regulatory enzyme GSNO reductase (GSNOR) can impair these processes. Because dysregulated GSNOR can interfere with processes relevant to cardiovascular health, it follows that inhibition of GSNOR may be beneficial. However, the effect of GSNOR inhibition on vascular activity is unknown. To study the effects of GSNOR inhibition on endothelial function, we treated rats with a small-molecule inhibitor of GSNOR (N6338) that has vasodilatory effects on isolated aortic rings and assessed effects on arterial flow-mediated dilation (FMD), an NO-dependent process. GSNOR inhibition with a single intravenous dose of N6338 preserved FMD (15.3 ± 5.4 vs. 14.2 ± 6.3%, P = nonsignificant) under partial NO synthase inhibition that normally reduces FMD by roughly 50% (14.1 ± 2.9 vs. 7.6 ± 4.4%, P < 0.05). In hypertensive rats, daily oral administration of N6338 for 14 days reduced blood pressure (170.0 ± 5.3/122.7 ± 6.4 vs. 203.8 ± 1.9/143.7 ± 7.5 mmHg for vehicle, P < 0.001) and vascular resistance index (1.5 ± 0.4 vs. 3.2 ± 1.0 mmHg·min·l−1 for vehicle, P < 0.001), and restored FMD from an initially impaired state (7.4 ± 1.7%, day 0) to a level (13.0 ± 3.1%, day 14, P < 0.001) similar to that observed in normotensive rats. N6338 also reversed the pathological kidney changes exhibited by the hypertensive rats. GSNOR inhibition preserves FMD under conditions of impaired NO production and protects against both microvascular and conduit artery dysfunction in a model of hypertension.
Keywords: hypertension, flow-mediated vasodilation, nitric oxide, S-nitrosoglutathione, S-nitrosoglutathione reductase
nitric oxide (no) has emerged as a key player in the cardiovascular system, mediating diverse physiological processes, including vasodilation (31, 33, 41). Many effects of NO are mediated by the transfer of an NO group to cysteine sulfhydryls on proteins via S-nitrosylation (35), a process that plays an important role in many NO-dependent cardiovascular processes (20) and causes significant protein modification within endothelial cells (43). NO is short lived, but exists physiologically in other forms, like nitrite, nitrate, and S-nitrosothiols (SNOs), which include S-nitrosylated cysteine residues in serum proteins, and S-nitrosoglutathione (GSNO) in the cytoplasm. In particular, GSNO is thought to serve as an important intracellular bioavailable NO pool, which is in equilibrium with other intracellular SNOs that facilitate transnitrosylation (NO transfer between proteins) (12, 18). Accordingly, increased levels of GSNO can facilitate NO-mediated processes, and, conversely, catabolism of GSNO by GSNO reductase (GSNOR), a regulatory enzyme that degrades GSNO and is thought to be the main regulator of GSNO levels in vivo (22), can impair these processes. This premise is supported by the observations that, in human asthma, GSNOR regulates airway SNO content and hyperresponsiveness (29, 30), and GSNOR-deficient mice are protected from airway hyperresponsiveness in an asthma model (28).
Because dysregulated GSNOR can reduce levels of GSNO and can interfere with processes relevant to cardiovascular health, it follows that GSNOR inhibition may be beneficial in certain situations. GSNOR inhibition promotes vasorelaxation of isolated aortic rings (32), and genetic deletion of GSNOR lowers systemic vascular resistance and improves postinfarction myocardial perfusion (21, 23), although complete deletion also lowers cardiac contractility (4). Together, these observations suggest that GSNOR is highly relevant to the cardiovascular system, and that inhibition under appropriate circumstances could provide therapeutic strategies for various forms of cardiovascular disease.
One effective way to assess vascular health in humans is to measure the vasodilation of arteries in response to increased blood flow, a process called flow-mediated (vaso)dilation (FMD) (6). Transient occlusion of the brachial artery with a blood pressure (BP) cuff and subsequent release result in reactive hyperemia and vasodilation, which is measured by ultrasound detection of arterial diameter before and after occlusion. The resulting FMD is primarily NO mediated (14), which is demonstrable through inhibition of NO synthase (NOS) by inhibitors such as NG-monomethyl-l-arginine (l-NMMA). This effect relies on functional endothelium to sense fluid shear stress, activating endothelial NOS (eNOS) to produce NO, which diffuses to, and relaxes, the underlying smooth muscle. FMD has become widely accepted as a biomarker of endothelial function and cardiovascular health (2, 11, 40).
We developed an approach to measure FMD in the femoral artery of living rats that is functionally analogous to brachial artery FMD measurement in humans (17). This system holds a substantial advantage over measurement of vasorelaxation of isolated aortic segments because it reflects intact physiology and is analogous to the clinically measured effect. Using this system, we show here that a novel small-molecule reversible inhibitor of GSNOR, N6338 (36), not only preserves FMD in models of endothelial dysfunction, but also lowers BP and vascular resistance in hypertensive rats.
METHODS
Animals.
Unless otherwise indicated, Sprague-Dawley and Dahl-S rats (Charles River, Wilmington, MA) were 12 or 9 wk of age, respectively, at the initiation of N6338 treatment. Sprague-Dawley rats were fed standard diet (Purina, Richmond, IA), while Dahl-S rats were fed low-salt (0.26% NaCl) or high-salt (4% NaCl) diet to induce a normotensive or hypertensive phenotype, respectively. Nine-week-old Dahl-S rats were delivered to the University of California, San Francisco (UCSF) after 3 wk on the high- or low-salt diet. Dahl-S rats are normotensive on low-salt diet and hypertensive on high-salt diet, but a minority will not become hypertensive on high salt, so screening is a traditional step. Indeed, we found that a small number of rats on low salt spontaneously became hypertensive. Therefore, an excess of Dahl-S rats were fed the appropriate diets during the initial 3-wk period. BP was subsequently measured via tail cuff, and only those on high salt with stable hypertension (n = 14) and those on low salt with stable normal BP (n = 12) were randomized into drug or control groups for further use on day 0 (D0). The diets were maintained through the end of study (D14). For another experiment, the rats were all delivered before the switch from low- to high-salt diet, so that baseline BP could be measured before switching all rats to high-salt diet. BP was read again after 3 wk, and any rats that had not converted to hypertension were removed. The remaining rats were randomized into drug (n = 10) or vehicle (n = 7) groups, with the rats serving as their own normotensive controls based on their baseline BP. Despite differences in the delivery schedule of the rats in the two BP experiments, the two experiments did not differ with respect to the switch from low to high salt and the treatment with the drug. All experiments were approved by the UCSF Institutional Animal Care and Use Committee.
GSNOR inhibition in vitro.
N6338 (N30 Pharmaceuticals, Boulder, CO) and GSNOR (Emerald BioStructures, Bainbridge Island, WA) were prepared as previously described (36). The potency of N6338 inhibition of GSNOR activity was determined in vitro via determination of the N6338 concentration that inhibited maximal GSNOR activity by 50%. Final assay conditions were 0.5 μg/ml GSNOR, 240 μM GSNO, and 300 μM NADH in 100 mM sodium phosphate buffer, pH 7.4 at 25°C. N6338 concentrations ranged from 0 to 2 μM. Reaction volumes were 300 μl in a UV transparent 96-well plate (Costar 3635). The change in absorbance at 340 nm (from oxidation of NADH to NAD+) was followed for 3 min in a SpectraMax M2 plate reader. The initial linear reaction rate (340-nm milliabsorbance units/min) was plotted against inhibitor concentration and fit to a sigmoidal dose-response model (variable slope) using GraphPad Prism 5 software.
GSNOR immunohistochemistry.
Formalin-fixed, paraffin-embedded tissues from Dahl-S study rats (normal diet vehicle control group) were stained with a rabbit polyclonal GSNOR antibody (11051-1-AP, Proteintech, Chicago, IL). Commercially available human tissues (tissue source for Fig. 1, C and D, from Premier Laboratories, Longmont, CO; for Fig. 1E from Folio Biosciences, Columbus, OH) were stained with a monoclonal anti-GSNOR antibody (N30-C3) raised in a rat immunized with full-length human GSNOR protein at ProMab Biotechnologies (Richmond, CA) for N30 Pharmaceuticals. Slides were deparaffinized in xylene and rehydrated to water through a series of alcohol gradients. The slides were pretreated in a 95°C citrate, pH 6.1, antigenic retrieval solution (Dako, Carpinteria, CA). After the slides were allowed to cool to room temperature, a 3% hydrogen peroxide solution (EMD Chemicals, Gibbstown, NJ) was added to quench endogenous peroxidase activity. Slides were preincubated with Serum Free Protein Block (Dako), followed by incubation with the GSNOR antibody or a nonspecific rat IgG control (AbD Serotec, Raleigh, NC) or rabbit Ig fraction (Dako). Human tissue slides were then incubated with rabbit anti-rat immunoglobulin (Dako), and both human and rat tissues were labeled with a goat anti-rabbit polymer (Dako). DAB+ (Dako) was used for detection, and slides were counterstained with hematoxylin (Dako).
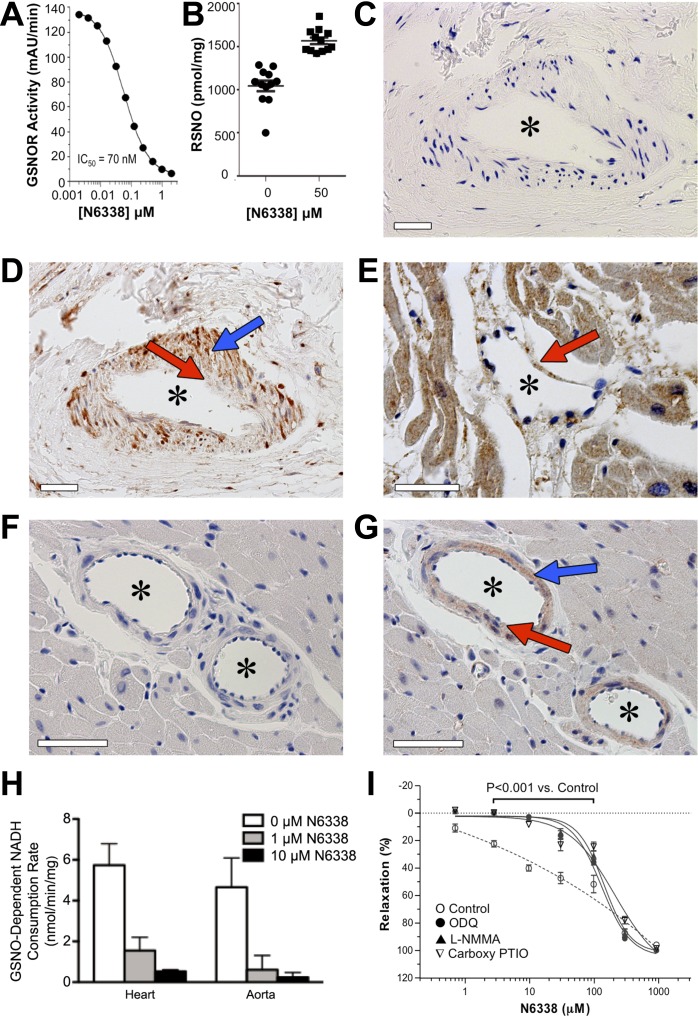
Fig. 1.
S-nitrosoglutathione (GSNO) reductase (GSNOR) inhibition, localization, and activity. A: N6338 caused dose-dependent and potent inhibition of human GSNOR activity in vitro. Graph represents one experiment using duplicate samples at each dose tested. mAU, milliabsorbance units. B: N6338 increased S-nitrosothiol (SNO) levels in mouse embryonic fibroblasts. Mean SNO levels ± SE are shown (n = 12 independent wells per group; P < 0.0001, two-tailed t-test). C–G: GSNOR protein was evident in human and rat cardiovascular tissue by immunohistochemistry: IgG negative control (C) or human-specific GSNOR antibody staining predominantly in the smooth muscle of a myocardial arteriole or small artery in human heart (D), and predominantly in the endothelium of a human myocardial venule (E); IgG-negative control (F) or rat-specific GSNOR antibody staining predominantly in the smooth muscle of an arteriole in rat heart (G). In all micrographs, scale bars = 50 μm, asterisks denote vessel lumens, blue arrows show smooth muscle, and red arrows show endothelium. H: GSNOR activity was present in rat heart and aorta and was inhibited by N6338 when this compound was added in vitro. Activity was measured in the absence (open bars) or presence of 1 μM (shaded bars) or 10 μM (solid bars) N6338. Bars are means ± SD of tissues from 4 rats. I: N6338 caused dose-dependent relaxation in rat aortic rings precontracted with phenylephrine. This relaxation by N6338 was significantly attenuated by pretreatment of rings with 10 μM 1H-[1,2,4]oxadiazolo [4,3-α]quinoxalin-1-one (ODQ), 100 μM NG-monomethyl-l-arginine (l-NMMA), or 300 μM 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide (carboxy PTIO) for 30 min before N6338 administration. Values are means ± SE of 4 rings/group.
GSNOR activity.
Hearts and aortas from male Sprague-Dawley rats (Harlan, Indianapolis, IN; 8–10 wk of age, weighing 250–300 g) were frozen in liquid nitrogen and stored at −70°C. Tissues were pulverized to powder in liquid nitrogen and then homogenized in 50 mM Tris·HCl (pH 7.4), 250 mM NaCl, 0.1 mM diethylenetriamine pentaacetic acid (DTPA), 10 mM N-ethylmaleimide (NEM), 1% Igepal CA-630, with complete, EDTA-free, protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Homogenates were clarified by centrifugation, and total protein determined by the bicinchoninic acid method. Tissue homogenates (4.4 mg/ml total protein) were diluted 10-fold into reaction buffer (100 mM sodium phosphate, pH 7.4, and 250 μM NADH). GSNO-dependent NADH depletion was determined spectrophotometrically by measuring change in absorbance at 340 nm for 3 min at 20°C in the absence and presence of 250 μM GSNO. Additions of N6338 to reactions containing GSNO were as indicated in the text.
Measurement of nitrosated species (SNOs).
Mouse embryonic fibroblast cells (a generous gift from Limin Liu, UCSF) were immortalized with SV-40 T-antigen (Applied Biological Materials, Richmond, BC, Canada). The cells were cultured in six-well plates at 1–2 × 106 cells/well and grown overnight in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. Media was replaced with fresh DMEM with 500 μM GSNO (N30 Pharmaceuticals) and either vehicle or 50 μM N6338 and incubated for 4 h. Nitrosylated species were quantitated using the triiodide-based chemiluminescence method, as described (32), with the following modifications. Cells were washed in PBS and collected into lysis buffer (50 mM NEM, 50 mM potassium phosphate, pH 7.0, 5 mM DTPA, 0.2 mM neocuproine, 20 mM ferricyanide, and 1% Igepal CA-630). Cell lysates were treated with 15% vol/vol of a sulfanilamide solution (5% wt/vol in 0.3 M HCl), and the triiodide reaction was performed at room temperature using GSNO to generate a standard curve.
Aortic ring relaxation assay.
Aortic rings from male Sprague-Dawley rats (Harlan) were mounted at isometric tension in a small vessel wire myograph (DMT 610M, DMT-USA, Atlanta, GA) in Krebs bicarbonate buffer containing 119 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 25 mM NaHCO3, 0.03 mM EDTA, and 5.5 mM glucose and continuously gassed with 95% O2/5% CO2. Rings were equilibrated at a resting tension of 2 g for 1 h and then treated with 10 μM 1H-[1,2,4]oxadiazolo [4,3-α]quinoxalin-1-one (ODQ; a guanylate cyclase inhibitor), 100 μM l-NMMA (a NOS inhibitor), or 300 μM 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide (carboxy PTIO; an NO scavenger) for 30 min. Control aortas were treated with vehicle (water). Aortas were then precontracted with 0.5 μM phenylephrine (EC85). After a plateau of phenylephrine effect was achieved, N6338 was added in half-log cumulative doses, ranging from 1 μM to 1 mM. Data were acquired and analyzed using Powerlab software (ADInstruments, Colorado Springs, CO). Additional data analyses were performed in GraphPad Prism. The amount of relaxation was reported as the percentage of maximum relaxation.
Inhibition of GSNOR and NOS in vivo.
Acute GSNOR inhibition in vivo was achieved by intravenous injection of N6338 (10 mg/kg in 0.1 ml saline/100 g). Subchronic GSNOR inhibition in vivo was achieved by daily oral administration of 10 mg·kg−1·day−1 N6338 prepared in 0.5% wt/vol carboxymethylcellulose. Inhibition of NOS was achieved by intravenous injection of l-NMMA (5 mg/kg in 0.1 ml saline/100 g).
Measurement of FMD.
FMD was measured in living rats, as described previously (17). Briefly, an arterial loop occluder was surgically positioned upstream of the femoral artery and passed through 15-cm PE-90 tubing. After a 15-min equilibration, the artery was occluded for 5 min, followed by reperfusion of the leg. Femoral artery diameter was measured with a 35-MHz ultrasound transducer (Vevo660, VisualSonics, Toronto, Ontario, Canada) over 3 min. Image analyses were performed offline from recorded loops using an automated system (Brachial Analyzer 5, Medical Imaging Applications, Coralville, IA) (17). Volumetric flow was calculated as π × (diameter/2)2 × mean flow velocity (V). V was calculated as velocity-time integral × duration of heart cycle. All diameter readings were taken at diastole, and flow velocity represents the mean angle-corrected Doppler-flow velocity. FMD was calculated as %change: (peak diameterpostischemia − diameterbaseline)/diameterbaseline × 100, and presented as both raw data and indexed to wall shear rate (WSR) by calculating FMD/WSR. WSR was calculated as 4 × velocity/diameter at peak response. We used WSR rather than wall shear stress, which also depends on blood viscosity, under the assumption that blood viscosity was constant.
BP and vascular resistance index detection.
BP was measured using a noninvasive, computerized piezoplethysmography tail-cuff system (ML125/R NIBP; ADInstruments, Colorado Springs, CO). The investigator taking BP measurements was blinded to treatment, but not blinded to diet (high vs. low salt) for technical reasons; the investigator measuring FMD and flow velocity was blinded to treatment and diet. Because tail-cuff methodology yields only approximations of diastolic BP based on systolic BP measurements, vascular resistance was expressed as an index calculated
Kidney histology.
Kidneys and hearts were wet-weighed and snap-frozen in liquid nitrogen. Superficial renal sections (10 μm) were stained with hematoxylin and eosin and examined histologically by a blinded examiner.
Molecular assays in aortic extracts.
cGMP was analyzed from homogenized aorta samples from the N6338- and vehicle-treated high-salt groups using a colorimetric Enzyme Immunoassay cGMP kit (Cayman, Ann Arbor, MI), according to the manufacturer's instructions.
Statistics.
For aortic ring relaxation, statistical analysis was performed using GraphPad Prism, and significant differences among groups were determined via two-way ANOVA with treatment and dose as factors, followed by Bonferroni's post hoc correction for multiple comparisons. Statistical analysis was performed using one-way ANOVA for weight comparisons, two-way ANOVA for aortic ring relaxation measurement, and two-way repeated-measures ANOVA for the acute FMD suppression study and for BP and FMD comparison in the chronic study with GraphPad Prism, followed by Bonferroni post hoc correction with significance adjusted for multiple comparisons, where appropriate. Single comparison between all untreated (D0) Dahl-S rats on high salt vs. low salt in the chronic study was performed by Student's t-test. For vascular resistance index, a two-way repeated-measures ANOVA was performed with main factors salt level and treatment, and planned pairwise comparisons were tested using linear contrast statements with significance determined using Bonferroni adjustment for multiple comparisons. Analyses were performed using Statistica (version 6.0, StatSoft). Statistical significance was determined after adjusting for multiple comparisons to maintain the overall α error rate of P < 0.05.
RESULTS
N6338 is a GSNOR inhibitor.
N6338 caused dose-dependent, potent inhibition of human GSNOR activity in vitro (Fig. 1A), and increased the level of SNOs in cultured mouse embryonic fibroblasts (Fig. 1B).
GSNOR expression in the vasculature.
GSNOR protein expression was detected in vascular tissue by immunohistochemistry. Antibodies against human (Fig. 1, D and E) or rat (Fig. 1G) GSNOR demonstrated the presence of GSNOR in small macrovessels in human and rat heart tissue sections. In both species, arterioles and presumably small arteries showed prominent GSNOR immunostaining in smooth muscle, but little, if any, staining in endothelium (Fig. 1, D and G). In a human venule, GSNOR staining was apparent in endothelium (Fig. 1E). GSNOR was also prominently expressed in human cardiomyocytes (Fig. 1E). Consistent with the results in human tissue, faint staining was observed in rat cardiomyocytes (Fig. 1G). No staining was evident in human (Fig. 1C) or rat (Fig. 1F) heart sections stained with the respective IgG negative control.
GSNOR activity in cardiovascular tissues.
GSNOR enzyme activity was measured in rat heart and aorta homogenates to further identify the presence of GSNOR in vascular tissue. GSNOR activity was detected in homogenates from both rat aorta and heart (Fig. 1H). N6338 inhibited this enzyme activity when added in vitro. Specifically, N6338 decreased GSNOR activity by 73.3 ± 8.5% (heart) and 85.3 ± 17.7% (aorta) at 1 μM N6338, and by 90.8 ± 1.5% (heart) and 94.5 ± 6.2% (aorta) at 10 μM N6338.
N6338 causes relaxation of preconstricted aortic rings in vitro.
To further demonstrate that GSNOR inhibition by N6338 can directly influence vascular tone, the effects of N6338 were tested in isolated aortic rings. N6338 caused a dose-dependent relaxation in rat aortic rings that had been precontracted with phenylephrine (Fig. 1I). To determine whether this relaxation was NO dependent, reagents affecting various pathways of NO-mediated relaxation were added before the phenylephrine and N6338 additions. Incubation of rings with either soluble guanylate cyclase inhibitor ODQ, NOS inhibitor l-NMMA, or NO scavenger carboxy PTIO significantly attenuated N6338 (1–100 μM) relaxation compared with vehicle-treated control rings, demonstrating the dependence of N6338's effects on the classical NO/cGMP mechanism for smooth muscle relaxation.
GSNOR inhibition prevented impairment of FMD caused by partial inhibition of eNOS.
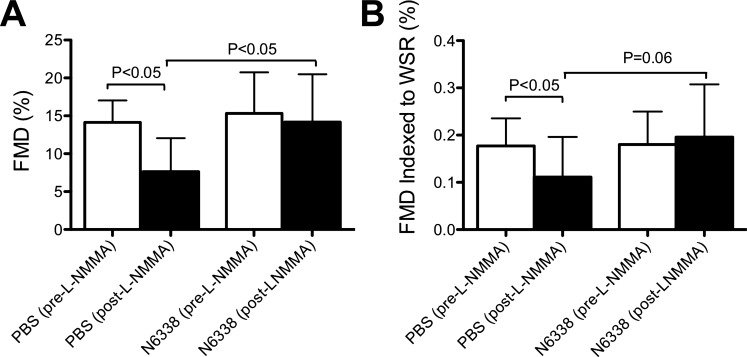
We first determined whether a single dose of GSNOR inhibitor could preserve FMD under conditions of partial NOS inhibition. Rats were injected intravenously with PBS (negative control) or N6338 (10 mg/kg in 0.1 ml saline/100 g), followed by 300 μl PBS to flush the infusion system. After 24 h, we measured FMD and then administered 5 mg/kg of the NOS inhibitor l-NMMA, a dose that we had previously determined to inhibit FMD by ∼50% (Q. Chen, unpublished observations). FMD was measured again 10 min after l-NMMA. We used a crossover design in which rats were randomly split into two groups (n = 7/group), with one group given N6338 and the other group given PBS. After a 2-wk washout period, the groups were reversed and received the opposite treatment. As shown in Fig. 2, FMD responses 24 h after treatment with PBS or N6338, but before l-NMMA, were similar, indicating that GSNOR inhibition has little or no effect on FMD in normal arteries. However, while l-NMMA significantly reduced FMD in PBS-treated rats (14.1 ± 2.9 vs. 7.6 ± 4.4%, P < 0.05), there was no significant difference in FMD before and after l-NMMA in N6338-treated rats (15.3 ± 5.4 vs. 14.2 ± 6.3%, P = nonsignificant). There was also a significant difference between post-l-NMMA FMD in the PBS-treated vs. N6338-treated rats (7.6 ± 4.4 vs. 14.2 ± 6.3%, P < 0.05). Calculation of FMD relative to WSR (FMD indexed to WSR) resulted in similar relationships. Femoral artery diameter was not significantly decreased by l-NMMA before or after occlusion in each group (data not shown). These results demonstrate that N6338 prevented the reduction in FMD that would have otherwise occurred following administration of l-NMMA.
Fig. 2.
Effect of GSNOR inhibitor on acute impairment of flow-mediated dilation (FMD). Pilot experiments showed that roughly half-maximal inhibition of FMD occurs 15 min after 5 mg/kg iv of l-NMMA. We pretreated rats with this dose of l-NMMA before eliciting FMD responses. Prior treatment (24 h, iv) with 10 mg/kg N6338 (n = 7), but not PBS (n = 7), preserved FMD in l-NMMA-treated rats in a crossover study design. FMD is shown both as raw data (A) and indexed to wall shear rate (WSR; B). Values are means ± SD.
GSNOR inhibition reduced BP in Dahl-S hypertensive rats.
We then asked if it is possible to recapitulate decreased FMD in an inflammation-based chronic hypertension model, the Dahl-S rat maintained on a high-salt diet, and whether inhibition of GSNOR could reduce BP and restore FMD in this model. N6338 in a solubilization vehicle (0.5% wt/vol carboxymethylcellulose) was delivered orally at 10 mg·kg−1·day−1 once daily, beginning at D0; control groups received only vehicle. Hypertensive and normotensive rats had been previously randomized to groups after the initial BP measurement.
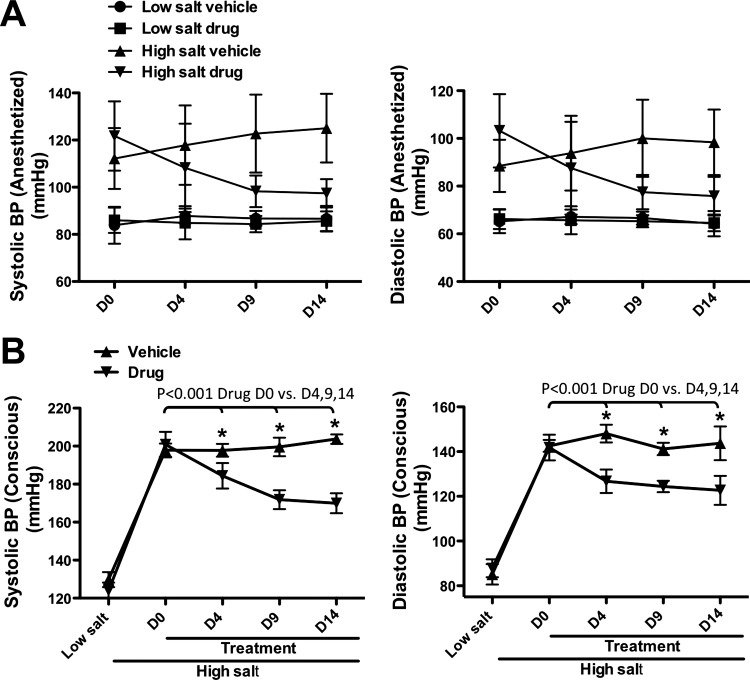
As shown in Fig. 3A and with exact values shown in Table 1 (due to the complexity of the data), at D0, D4, D9, and D14, systolic BP and diastolic BP measured by tail cuff under anesthesia did not differ between the low-salt groups treated with vehicle vs. drug, showing that the GSNOR inhibitor did not affect BP in healthy animals. (Note that diastolic BP values resulting from tail-cuff measurements are approximations based on the systolic measurements, but are presented for reference.) BP in the high-salt vehicle group was substantially higher than BP in the low-salt groups (P < 0.05 overall), confirming that this rat model of vascular disease successfully manifested a disease state similar to that of hypertensive humans.
Fig. 3.
Effect of GSNOR inhibitor on blood pressure (BP) in hypertensive rats. A: BP measurements in anesthetized rats. n = 6 for each normotensive group; n = 7 for each hypertensive group. P < 0.05 for overall effect. B: BP measurements in conscious rats. N6338 reversed an increase in BP in the hypertensive rats (n = 10). *P < 0.001, high-salt vehicle (n = 7) vs. high-salt drug (n = 10). Values are means ± SD. Note that diastolic BP measurements by tail-cuff methodology are approximations and are included here for reference, while systolic values represent true measurements.
Table 1.
Exact blood pressure measurements corresponding to Fig. 3A (isoflurane anesthesia)
| Group | D0 | D4 | D9 | D14 |
|---|---|---|---|---|
| Low-salt vehicle | 83.9 ± 7.8/65.2 ± 4.9 | 87.8 ± 3.1/67.1 ± 3.1 | 86.8 ± 3.1/66.6 ± 2.7 | 86.7 ± 5.4/64.3 ± 5.3 |
| Low-salt drug | 86.0 ± 5.4/66.2 ± 4.2 | 84.9 ± 7.0/65.7 ± 5.9 | 84.4 ± 3.5/65.3 ± 2.5 | 85.6 ± 4.2/64.6 ± 3.5 |
| High-salt vehicle | 112.2 ± 12.9/88.5 ± 10.9 | 117.9 ± 16.8/93.8 ± 15.7 | 122.8 ± 16.5/100.1 ± 16.2 | 125.0 ± 14.6/98.4 ± 13.7 |
| High-salt drug | 121.7 ± 14.7/103.2 ± 15.3 | 108.3 ± 18.7/87.6 ± 19.4 | 98.3 ± 6.7/77.5 ± 7.2 | 97.4 ± 6.0/75.8 ± 8.1 |
Values are means ± SD of systolic/diastolic blood pressure (in mmHg). D0, D4, D9, D14: treatment days 0, 4, 9, 14, respectively.
While the overall F-test was significant, specific intergroup comparisons failed to retain significance after adjusting the α error rate for multiple comparisons in the anesthetized animals. For this reason, and because anesthetized conditions result in abnormally low BP, especially as measured with tail-cuff methodology, we performed a similar experiment in which BP was measured in conscious Dahl-S rats acclimated to the procedure with exact values shown in Table 2. Notably, in the high-salt drug group, BP was initially high, but decreased significantly over time and was substantially lower by D9 and D14, demonstrating a BP-lowering effect of the GSNOR inhibitor in hypertensive, but not normotensive, Dahl-S rats (Fig. 3B). (The subsequent results presented below derive from the rats in which BP had been measured under anesthesia.)
Table 2.
Exact blood pressure measurements corresponding to Fig. 3B (conscious)
| High Salt |
|||||
|---|---|---|---|---|---|
| Group | Low Salt | D0 | D4 | D9 | D14 |
| Vehicle | 129.8 ± 4.0/85.1 ± 4.6 | 197.9 ± 3.5/142.6 ± 2.6 | 197.8 ± 3.4/148.1 ± 4.0 | 199.6 ± 4.9/141.2 ± 2.8 | 203.8 ± 1.9/143.7 ± 7.5 |
| Drug | 123.9 ± 4.2/87.8 ± 4.0 | 200.8 ± 6.6/141.8 ± 5.8 | 184.4 ± 6.7/126.8 ± 5.2 | 171.8 ± 5.0/124.4 ± 2.6 | 170.0 ± 5.3/122.7 ± 6.4 |
Values are means ± SD of systolic/diastolic blood pressure (in mmHg).
GSNOR inhibition reduced vascular resistance in Dahl-S hypertensive rats.
Measuring vascular resistance provides a more reliable indication of microvascular tone than assessing BP alone. To determine whether GSNOR inhibition protects microvascular function in Dahl-S hypertensive rats, we calculated a vascular resistance index using the systolic BP measurements and the diastolic BP approximations (see methods) both at baseline (i.e., before femoral artery occlusion) and at peak blood flow during reactive hyperemia. This provides an indication of the extent to which chronic GSNOR inhibition impacts microvascular tone at “rest” and under near-maximal dilatory conditions in a disease state with increased microvascular resistance.
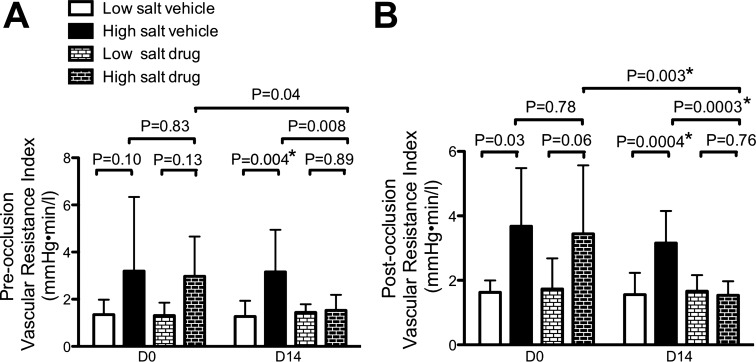
As shown in Fig. 4, in general, postocclusion vascular resistance relationships mirrored preocclusion relationships, but significance was stronger for the postocclusion numbers, and strongest at D14. At D0, postocclusion vascular resistance index trended higher in the high-salt groups than in the low-salt groups (vehicle groups at 3.7 ± 1.8 vs. 1.6 ± 0.4 mmHg·min·l−1, P = 0.03; drug groups at 3.4 ± 2.1 vs. 1.7 ± 0.9 mmHg·min·l−1, P = 0.06). Before treatment began (D0), there were no statistical differences in vascular resistance index between the high-salt vehicle and high-salt drug groups (preocclusion: 3.2 ± 3.1 vs. 3.0 ± 1.7 mmHg·min·l−1, P = 0.83; postocclusion: 3.7 ± 1.8 vs. 3.4 ± 2.1 mmHg·min·l−1, P = 0.78). In contrast, in the high-salt groups after 14 days of treatment, postocclusion vascular resistance index in the drug-treated group was reduced by roughly 50% compared with the vehicle-treated group (1.5 ± 0.4 vs. 3.2 ± 1.0 mmHg·min·l−1, P = 0.0003), with a similar reduction in preocclusion vascular resistance index (1.5 ± 0.7 vs. 3.2 ± 1.7 mmHg·min·l−1, P = 0.008). Notably, in the high-salt drug group, there was a significant decrease in postocclusion vascular resistance index from D0 to D14 (3.4 ± 2.1 vs. 1.5 ± 0.4 mmHg·min·l−1, P = 0.003), again with a similar trend in preocclusion values (3.0 ± 1.7 vs. 1.5 ± 0.7 mmHg·min·l−1, P = 0.04). Thus the reduction in BP in hypertensive rats treated with GSNOR inhibitor was accompanied by a reduction in vascular resistance.
Fig. 4.
Effect of GSNOR inhibitor on vascular resistance. A: preocclusion. B: postocclusion. Vascular resistance index was increased with high-salt diet, and N6338 reduced the vascular resistance index after 2 wk of treatment. D0, day 0; D14, day 14. Values are means ± SD. *Significant at adjusted α error rate of 0.0041 for multiple comparisons.
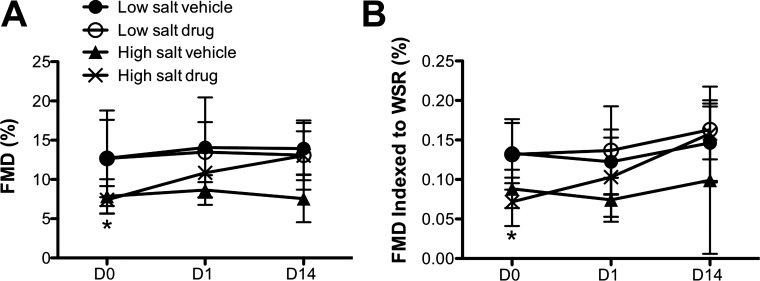
GSNOR inhibition restored FMD of Dahl-S hypertensive rats to the level of normotensive rats.
To assess the effects of GSNOR inhibition on endothelial function in the hypertensive rats, FMD was measured at D0, D1, and D14 (Fig. 5). The coefficient of variation for the low-salt vehicle group over time (calculated for individual rats over time and averaged) was 0.207. Similar to the BP results described above, FMD was normal in both low-salt groups and impaired in the high-salt vehicle group (13.9 ± 3.3 and 13.1 ± 4.4 vs. 7.6 ± 3.0%, P < 0.05). Pairwise differences between groups on any given day did not reach significance, but FMD of all high-salt rats was significantly lower than that of all low-salt rats on D0, before treatment commenced (P = 0.0027), confirming that FMD is impaired in Dahl-S rats on a high-salt diet. While GSNOR inhibitor treatment had no effect on FMD in the low-salt groups, it progressively and completely restored FMD to normal levels in the high-salt group between D0 and D14 (7.4 ± 1.7 vs. 13.0 ± 3.1%, P < 0.001). No significant difference was observed between femoral artery basal diameters in the N6338 and vehicle high-salt groups (not shown). Furthermore, there were no differences in either aorta cGMP or plasma nitrite levels between N6338 and vehicle high-salt groups (not shown). Indexing of FMD to WSR reduced the level of significance but preserved the main effect, confirming that, despite the functional defect in the microvasculature of the high-salt rats and its reversal by GSNOR inhibition, the conduit artery response to hyperemia was similarly impaired in the high-salt groups and reversed by GSNOR inhibition.
Fig. 5.
Effect of GSNOR inhibitor on FMD in hypertensive rats. FMD was substantially impaired in the hypertensive rats. N6338 fully restored FMD over the 2-wk treatment period to levels seen in normotensive control rats. FMD is shown both as raw data (A) and indexed to WSR (B). Values are means ± SD. The comparisons between individual groups did not reach significance, although significance was reached for the overall effect with P < 0.05. The difference in FMD in all high-salt rats vs. all low-salt rats at D0 was significant (P = 0.0027, t-test). The change from D0 to D14 in the high-salt drug group was significant in both analyses (*P < 0.001, D0 vs. D14).
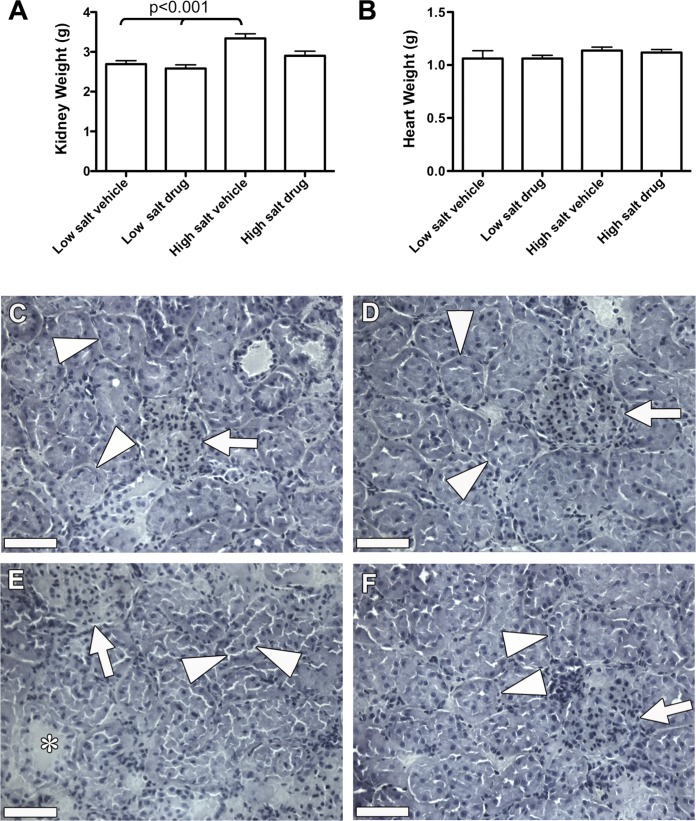
GSNOR inhibition prevented renal damage observed in hypertensive rats.
At the end of the experiment, the rats were euthanized, and the heart and kidneys from each rat were weighed. As shown in Fig. 6A, kidney weights were increased in the high-salt vehicle group (3.3 ± 0.3 g) compared with weights in both low-salt groups (2.7 ± 0.1 and 2.6 ± 0.2 g, P < 0.001). GSNOR inhibition caused a trend toward lower kidney weights (2.9 ± 0.3 g in the high-salt drug group vs. 3.3 ± 0.3 g in the high-salt vehicle group, P < 0.05). In contrast, there were no significant differences in heart weights (Fig. 6B) or body weights (not shown) among groups.
Fig. 6.
Effect of GSNOR inhibitor on the kidneys. A: at D14, kidney weights were increased in the high-salt vehicle group, while weights in the high-salt drug group trended lower. Values are means ± SD. B: there were no significant differences in heart weights among treatments. C: low-salt vehicle groups. D: low-salt drug groups. Histology of renal cortex shows normal glomerular (arrows) and tubular architecture (arrowheads) with back-to-back tubules, no interstitial edema, and intact tubular basement membrane, with no detectable difference between these low-salt groups. E: high-salt vehicle group. In contrast, histology of renal cortexes shows significant tubular swelling and dilation (asterisk), interstitial edema, loss of tubular brush borders, and basement membrane disruption (arrowheads), all of which were substantially reduced in rats treated with N6338 (F). Scale bar = 50 μm.
Histological assessment of kidneys showed that both low-salt groups had kidneys with normal glomerular and tubular architecture (Fig. 6, C and D). Kidneys of high-salt rats showed significant tubular defects, whereas the glomeruli were mostly unaffected. All of these pathological conditions were substantially reduced in rats treated with N6338 compared with vehicle (Fig. 6, E and F), demonstrating that reversal of the pathological consequences of the Dahl-S hypertensive rat model by GSNOR inhibition was not limited to the cardiovascular system.
DISCUSSION
This study shows that the GSNOR inhibitor, N6338, preserves FMD under partial NOS inhibition, reduces BP and vascular resistance in hypertensive rats, and restores FMD in hypertensive rats from a substantially impaired state to that of normotensive rats. These results suggest that N6338 protects against both microvascular and conduit artery vasoactive dysfunction in a model of vascular inflammation and hypertension (27, 46), and that GSNOR inhibition may provide a strategy to increase bioavailable NO as a therapy for a number of vascular disorders (19). As NO has typically been linked to endothelial function through eNOS activity [e.g., FMD and eNOS are both impaired by cigarette smoke exposure in humans (3), and eNOS gene therapy increases NO bioavailability and lowers BP in hypertensive rats (1, 44)], it is notable that FMD and BP can be altered by direct manipulation of NO storage pools rather than NO synthesis.
FMD measurement has been applied as a diagnostic tool in a range of physiological studies, including vascular effects of intrinsic health factors and of environmental and behavioral influences on cardiovascular health (5, 13, 15, 16). The NO dependency of FMD is demonstrable through inhibition of NOS (37). In our laboratory's recently developed rat FMD model, 8 mg/kg of l-NMMA were used to completely suppress FMD (17). In the present study, we have shown that 5 mg/kg l-NMMA induces acute partial suppression of FMD, which can be preserved if rats are pretreated with N6338.
It is not clear why acutely depressed FMD, a process that requires active synthesis of NO by eNOS, was improved by N6338, which likely increased levels of preexisting stores of NO. It is possible that, under normal conditions, some of the NO generated acutely during reactive hyperemia is immediately consumed by processes other than those directly involved with relaxation of the affected artery. The FMD normally observed, therefore, may reflect the effect of residual acutely generated NO. Because bioavailable NO is in equilibrium with both short-term NO-consuming processes and NO storage pools, including GSNO, increasing basal GSNO levels before eliciting FMD may preload the normal NO sinks that would otherwise consume NO generated acutely in response to reactive hyperemia. Hence, a higher net level of bioavailable NO may result during reactive hyperemia in the face of GSNOR inhibition than during normal GSNOR activity. The fact that N6338 did not increase FMD in the absence of l-NMMA may indicate that the NO pulse normally elicited by reactive hyperemia in our protocol elicits maximal artery dilation, and further increasing bioavailable NO via GSNOR inhibition has no effect. By contrast, the l-NMMA dose selected for our acute rat studies depressed hyperemia-induced NO below the level required to generate a maximal dilation response. Under this condition, GSNOR inhibition may increase bioavailable NO sufficiently to fully restore FMD despite coexisting partial inhibition of eNOS, as was observed. Our observation that l-NMMA inhibited the N6338-induced relaxation of preconstricted aortic rings at only the lower N6338 concentrations tested further supports the importance of the stoichiometry between acutely produced NO and the bioavailable NO pool. It is also notable that we observed substantial presence of GSNOR in the smooth muscle of arterioles and small arteries, suggesting that downstream elevation of bioavailable NO directly in the smooth muscle of arteries by GSNOR inhibition may compensate for possibly lower upstream production of NO by the endothelium.
Of perhaps greater importance is the observation that a probable chronic elevation of bioavailable NO, secondary to GSNOR inhibition, restores the magnitude of FMD in the Dahl-S hypertensive rat. This finding is consistent with reports that eNOS expression and endothelial-dependent relaxation are impaired in conduit arteries isolated from hypertensive Dahl-S rats (27, 45). Arterial β-adrenergic responsiveness is also decreased (34). Moreover, the high-salt diet may impair FMD directly, as even in healthy humans, a high-salt meal has been shown to reduce FMD in 30 min (10). Thus FMD in hypertensive Dahl-S rats may be disrupted via intrinsic defects in conduit artery endothelial signaling that would otherwise lead to smooth muscle relaxation.
Subchronic GSNOR inhibition decreased femoral artery vascular resistance in high-salt Dahl-S rats compared with resistance in vehicle control high-salt rats under baseline conditions (preocclusion) and during reactive hyperemia (postocclusion). Both of these observations have clinical relevance. Baseline vascular resistance primarily reflects basal microvascular tone, a primary determinant of BP at rest. Our preocclusion vascular resistance data are consistent with the effects of chronic GSNOR inhibition on BP, which was substantially reduced. In contrast, peak reactive hyperemia is an index of maximum vasodilatory reserve, a functional parameter of importance to the brain and heart that normally operate at high-oxygen extraction ratios. The capacity of these organs to respond to increased oxygen demand is highly dependent on increasing blood flow. Vasodilatory reserve indicates the upper limit of blood flow potentially available to these organs. Whereas reductions in vascular resistance primarily reflect the response of the femoral microvasculature to GSNOR inhibition, improvement in FMD reflects the conduit artery response to GSNOR inhibition.
The renin-angiotensin-aldosterone system and NADPH oxidase (39) within the kidney play important roles in the overall pathophysiology of renal disease through NF-κB-mediated upregulation of transforming growth factor-β (8, 46), monocyte chemotactic protein-1, and TNF-α (38, 46). These biochemical signals manifest as renal leukocyte recruitment, glomerular sclerosis, medullary fibrosis, poor renal hemodynamics, elevated BP, and proteinuria (38, 39). Pharmacological inhibition of NADPH oxidase results in decreased monocyte/macrophage infiltration and glomerular injury and improved renal hemodynamics. Notably, GSNOR inhibition has been shown to inhibit NF-κB activation (32). Inhibition of NF-κB signaling has been observed upon treatment with NO donors, such as sodium nitroprusside and GSNO, both which inhibit p65 binding to DNA promoter regions in cultured cells, resulting in downregulation of NF-κB-dependent gene expression (26). In other cell-based experiments, nitrosylation of the p50 subunit by the NO donor S-nitrosocysteine (the NO-bearing functional component of GSNO) also leads to inhibition of NF-κB DNA binding (24). GSNOR inhibitors of the same chemotype as N6338 cause both nitrosylation of the p65 subunit and decreased DNA binding activity (G. J. Rosenthal and S. C. Mutka, unpublished observations). Thus prevention of kidney weight increase and damage by N6338 may be attributable, at least partly, to anti-inflammatory activity. Notably, moderately old eNOS knockout mice suffer from hypertension and renal injury that are exacerbated by high-salt diet (9), underscoring the connection between bioavailable NO and the control of these conditions.
It has recently been reported that complete absence of GSNOR in knockout mice results in deficient DNA repair and increased propensity for tumorigenesis (25, 42). While there is a substantial difference between pharmacological inhibition of an enzyme and the life-long total absence of the protein, these results should be taken into consideration in the planning of initial clinical studies to ensure that such effects are prevented at the dosage used.
A limitation of this study is that the direct detection of N6338-induced changes in GSNOR activity in vivo is impractical because N6338 reversibly inhibits GSNOR. Therefore, disruption of tissues destroys the microenvironment within the cell and dilutes cellular contents, resulting in substantial dissociation of the inhibitor and restoration of enzymatic activity. Furthermore, direct measurement of endogenous GSNO levels is challenging and generally below levels of detection by current methods. However, GSNOR knockout mice have demonstrated increased bioavailable NO as measured by heme-bound NO (22). By addition of GSNO to a cell-based SNO assay to increase SNO levels to within detectable range, we confirmed that N6338 increases SNOs in cultured cells, implicating GSNO preservation as a primary mechanism of N6338's effects in a biological system. Taken together, the in vivo effects of treatment with GSNOR inhibitor N6338, paired with evidence of GSNOR inhibition from our in vitro experiments, strongly suggest that GSNOR inhibition led to an increase in NO-mediated signaling that cumulatively improved cardiovascular hemodynamics and renal pathology in this study.
In summary, using an approach that we developed to measure FMD in living rats, we have shown that a small-molecule inhibitor of GSNOR improves FMD and lowers BP and vascular resistance in rat models of vascular disease. GSNOR inhibition, by this or other small molecules, may, therefore, hold potential for clinical treatment of disease states characterized by vascular and renal inflammation and endothelial dysfunction.
GRANTS
This work was supported by a fellowship from the Margoes Foundation/UCSF Cardiovascular Research Institute to Q. Chen and by NHLBI Grant R01 HL-086917 and sponsored research support from N30 Pharmaceuticals to M. L. Springer.
DISCLOSURES
This study was funded in part through a research collaboration with N30 Pharmaceuticals. A. K. Patton, C. S. Delany, S. C. Mutka, J. P. Blonder, G. P. Dubé, and G. J. Rosenthal are, or have been, employed by N30.
AUTHOR CONTRIBUTIONS
Author contributions: Q.C., R.E.S., S.K., S.C.M., J.P.B., G.P.D., G.J.R., and M.L.S. conception and design of research; Q.C., R.E.S., M.V., S.K., D.J.H., A.K.P., C.S.D., and S.C.M. performed experiments; Q.C., S.K., D.J.H., A.K.P., C.S.D., S.C.M., and J.P.B. analyzed data; Q.C., S.K., S.C.M., J.P.B., G.P.D., G.J.R., and M.L.S. interpreted results of experiments; Q.C., D.J.H., and S.C.M. prepared figures; Q.C., S.K., and S.C.M. drafted manuscript; Q.C., S.C.M., J.P.B., G.P.D., G.J.R., and M.L.S. edited and revised manuscript; Q.C., R.E.S., M.V., S.K., D.J.H., A.K.P., C.S.D., S.C.M., J.P.B., G.P.D., G.J.R., and M.L.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Christian Heiss for helpful discussion, Michael Suniga and Kirsten Look for technical assistance, Limin Liu for supplying embryonic fibroblasts, and Vivian Weinberg, Xiaoyin Wang, and Kirstin Aschbacher for statistical assistance.
Present addresses: Monika Varga, Stryker Corporation, 47900 Bayside Parkway, Fremont, CA 94538-6515; Daniel J. Haddad, University of Vermont College of Medicine, Burlington, VT 05405; Gregory P. Dubé, OPK Biotech, LLC, 11 Hurley St., Cambridge, MA 02141.
REFERENCES
- 1. Alexander MY, Brosnan MJ, Hamilton CA, Fennell JP, Beattie EC, Jardine E, Heistad DD, Dominiczak AF. Gene transfer of endothelial nitric oxide synthase but not Cu/Zn superoxide dismutase restores nitric oxide availability in the SHRSP. Cardiovasc Res 47: 609–617, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG, Saha DC. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation 104: 1905–1910, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, Treuer AV, Dulce RA, Harrison RW, Saraiva RM, Premer C, Schulman IH, Stamler JS, Hare JM. Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci U S A 109: 4314–4319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 88: 2149–2155, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Chemla D, Antony I, Zamani K, Nitenberg A. Mean aortic pressure is the geometric mean of systolic and diastolic aortic pressure in resting humans. J Appl Physiol 99: 2278–2284, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Dahly AJ, Hoagland KM, Flasch AK, Jha S, Ledbetter SR, Roman RJ. Antihypertensive effects of chronic anti-TGF-beta antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 283: R757–R767, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Daumerie G, Bridges L, Yancey S, Davis W, Huang P, Loscalzo J, Pointer MA. The effect of salt on renal damage in eNOS-deficient mice. Hypertens Res 33: 170–176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dickinson KM, Clifton PM, Keogh JB. Endothelial function is impaired after a high-salt meal in healthy subjects. Am J Clin Nutr 93: 500–505, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation 126: 753–767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med 9: 160–168, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Ghiadoni L, Huang Y, Magagna A, Buralli S, Taddei S, Salvetti A. Effect of acute blood pressure reduction on endothelial function in the brachial artery of patients with essential hypertension. J Hypertens 19: 547–551, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Green D. Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1233–1234; discussion 1237–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Heiss C, Jahn S, Taylor M, Real WM, Angeli FS, Wong ML, Amabile N, Prasad M, Rassaf T, Ottaviani JI, Mihardja S, Keen CL, Springer ML, Boyle A, Grossman W, Glantz SA, Schroeter H, Yeghiazarians Y. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J Am Coll Cardiol 56: 218–224, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Heiss C, Schanz A, Amabile N, Jahn S, Chen Q, Wong ML, Rassaf T, Heinen Y, Cortese-Krott M, Grossman W, Yeghiazarians Y, Springer ML. Nitric oxide synthase expression and functional response to nitric oxide are both important modulators of circulating angiogenic cell response to angiogenic stimuli. Arterioscler Thromb Vasc Biol 30: 2212–2218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heiss C, Sievers RE, Amabile N, Momma TY, Chen Q, Natarajan S, Yeghiazarians Y, Springer ML. In vivo measurement of flow-mediated vasodilation in living rats using high-resolution ultrasound. Am J Physiol Heart Circ Physiol 294: H1086–H1093, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Jensen DE, Belka GK, Du Bois GC. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem J 331: 659–668, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leopold JA, Loscalzo J. New developments in nitrosovasodilator therapy. Vasc Med 2: 190–202, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res 106: 633–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Endogenous S-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci U S A 106: 6297–6302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410: 490–494, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell 116: 617–628, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry 40: 1688–1693, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Ozawa K, Tsumoto H, Wei W, Tang CH, Komatsubara AT, Kawafune H, Shimizu K, Liu L, Tsujimoto G. Proteomic analysis of the role of S-nitrosoglutathione reductase in lipopolysaccharide-challenged mice. Proteomics 12: 2024–2035, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paradkar PN, Roth JA. Nitric oxide transcriptionally down-regulates specific isoforms of divalent metal transporter (DMT1) via NF-kappaB. J Neurochem 96: 1768–1777, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Quaschning T, D'Uscio LV, Shaw S, Grone HJ, Ruschitzka F, Luscher TF. Vasopeptidase inhibition restores renovascular endothelial dysfunction in salt-induced hypertension. J Am Soc Nephrol 12: 2280–2287, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science 308: 1618–1621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Que LG, Yang Z, Stamler JS, Lugogo NL, Kraft M. S-nitrosoglutathione reductase: an important regulator in human asthma. Am J Respir Crit Care Med 180: 226–231, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenthal GJ, Blonder J, Damaj B, Richards J, Elia M, Scoggin C. A novel compound that inhibits S-nitrosoglutathione reductase protects against experimental asthma (Abstract). Am J Respir Crit Care Med 179: A4151, 2009 [Google Scholar]
- 31. Rudolph V, Freeman BA. Cardiovascular consequences when nitric oxide and lipid signaling converge. Circ Res 105: 511–522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanghani PC, Davis WI, Fears SL, Green SL, Zhai L, Tang Y, Martin E, Bryan NS, Sanghani SP. Kinetic and cellular characterization of novel inhibitors of S-nitrosoglutathione reductase. J Biol Chem 284: 24354–24362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sessa WC. Molecular control of blood flow and angiogenesis: role of nitric oxide. J Thromb Haemost 7, Suppl 1: 35–37, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Soltis EE, Katovich MJ. Reduction in aortic smooth muscle beta-adrenergic responsiveness results in enhanced norepinephrine responsiveness in the Dahl salt-sensitive rat. Clin Exp Hypertens A 13: 117–132, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res 106: 285–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun X, Wasley J, Qiu J, Blonder J, Stout A, Green L, Strong S, Colagiovanni D, Richards J, Mutka S, Chun L, Rosenthal G. Discovery of S-nitrosoglutathione reductase inhibitors: Potential agents for the treatment of asthma and other inflammatory diseases. ACS Med Chem Lett 2: 402–406, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker BA, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow mediated dilation (FMD) in humans: a methodological and technical guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 293: H3388–H3395, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 295: R1858–R1865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Walford G, Loscalzo J. Nitric oxide in vascular biology. J Thromb Haemost 1: 2112–2118, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Wei W, Li B, Hanes MA, Kakar S, Chen X, Liu L. S-nitrosylation from GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis. Sci Transl Med 2: 19ra13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y, Loscalzo J. S-nitrosoprotein formation and localization in endothelial cells. Proc Natl Acad Sci U S A 102: 117–122, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao CX, Xu X, Cui Y, Wang P, Wei X, Yang S, Edin ML, Zeldin DC, Wang DW. Increased endothelial nitric-oxide synthase expression reduces hypertension and hyperinsulinemia in fructose-treated rats. J Pharmacol Exp Ther 328: 610–620, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou MS, Kosaka H, Tian RX, Abe Y, Chen QH, Yoneyama H, Yamamoto A, Zhang L. l-Arginine improves endothelial function in renal artery of hypertensive Dahl rats. J Hypertens 19: 421–429, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Zhu A, Yoneda T, Demura M, Karashima S, Usukura M, Yamagishi M, Takeda Y. Effect of mineralocorticoid receptor blockade on the renal renin-angiotensin system in Dahl salt-sensitive hypertensive rats. J Hypertens 27: 800–805, 2009 [DOI] [PubMed] [Google Scholar]