Abstract
Chronic musculoskeletal pain is a significant health problem and is associated with increases in pain during acute physical activity. Regular physical activity is protective against many chronic diseases; however, it is unknown if it plays a role in development of chronic pain. The current study induced physical activity by placing running wheels in home cages of mice for 5 days or 8 wk and compared these to sedentary mice without running wheels in their home cages. Chronic muscle pain was induced by repeated intramuscular injection of pH 4.0 saline, exercise-enhanced pain was induced by combining a 2-h fatiguing exercise task with a low-dose muscle inflammation (0.03% carrageenan), and acute muscle inflammation was induced by 3% carrageenan. We tested the responses of the paw (response frequency) and muscle (withdrawal threshold) to nociceptive stimuli. Because the rostral ventromedial medulla (RVM) is involved in exercise-induced analgesia and chronic muscle pain, we tested for changes in phosphorylation of the NR1 subunit of the N-methyl-d-aspartate (NMDA) receptor in the RVM. We demonstrate that regular physical activity prevents the development of chronic muscle pain and exercise-induced muscle pain by reducing phosphorylation of the NR1 subunit of the NMDA receptor in the central nervous system. However, regular physical activity has no effect on development of acute pain. Thus physical inactivity is a risk factor for development of chronic pain and may set the nervous system to respond in an exaggerated way to low-intensity muscle insults.
Keywords: pain, NMDA, NR1, hyperalgesia, muscle
chronic pain is a significant health problem affecting over 100 million Americans—more than diabetes, cancer, and heart disease combined (21). Chronic musculoskeletal pain is the most common form of chronic pain. Physical inactivity is also recognized as a growing health concern (35) and is a risk factor for a multitude of chronic diseases including cancer, depression, and cardiovascular diseases (23, 43). As such, it has been coined the “diseasome of physical inactivity” (43). While it is clear that exercise is an effective treatment for chronic pain (2), and physical activity is reduced in people with chronic pain (18), if physical inactivity is a risk factor for development of chronic pain is unclear.
The transition from acute to chronic pain is thought to arise from neuroplastic changes in the peripheral and central nervous systems. There are numerous risk factors that are implicated in the development of chronic pain after an acute injury which includes high pain and psychosocial factors (40). In fact, better pain management in the acute postoperative (acute pain) period dramatically reduced the incidence of phantom limb pain (chronic pain) following amputation (7% vs. 75% in controls) (29). One effective treatment common for nearly all types of chronic pain, including those with musculoskeletal pain, is regular exercise (1, 2, 7, 11, 16, 20, 47, 62). Regular exercise produces analgesia in uninjured animals and reduces pain behaviors after inflammatory, noninflammatory, and neuropathic injury in animals (3, 4, 32, 34, 38, 50, 58). However, it is unclear if regular exercise can prevent the onset of chronic and widespread pain.
Exercise is believed to activate central inhibitory pathways that produce an opioid-mediated analgesia (2); the rostral ventromedial medulla (RVM) is a key central nucleus in these pathways (19). Indeed there are increases in release of endogenous opioids (met-enkephalin) in brainstem nuclei, including the RVM, during exercise, and blockade of central, but not peripheral, opioid receptors prevents the analgesia produced by exercise (58). In addition to mediating analgesia, the RVM also facilitates pain behaviors after tissue insult, including chronic muscle pain (44, 53, 55). The N-methyl-d-aspartate (NMDA) glutamate receptor in the RVM is critical for the development of hypersensitivity after muscle insult: increasing the NR1 subunit of the NMDA receptor enhances nociception, decreasing NR1 prevents chronic muscle pain (13), and blocking the NMDA receptor reverses pain sensitivity (13, 53). Further, there is increased phosphorylation of NR1 (p-NR1) in animal models of chronic muscle pain (13); these experiments were done in sedentary animals. It is unclear if similar increases in p-NR1 occur in animals that are physically active.
Acute pain is associated with tissue injury, generally involves an acute inflammatory response, and resolves once tissue healing is complete. Twenty to thirty percent of people with acute musculoskeletal pain go on to develop chronic pain that persists after normal tissue healing time and is not associated with tissue inflammation (24, 26, 30). Further, a single bout of exercise in people with existing acute inflammatory and chronic noninflammatory pain conditions enhances ongoing pain and hyperalgesia (27, 59, 61). Animal models of muscle pain have been developed to mimic acute inflammatory pain, chronic noninflammatory pain, and exercise-enhanced pain (45, 46, 54, 55, 64). These models involve assessing pain sensitivity at 1) the site of injury (muscle), termed primary hyperalgesia, which is thought to be mediated by hypersensitivity in the peripheral nervous system; and 2) outside the site of injury (paw), termed secondary hyperalgesia, and is thought to be mediated by hypersensitivity in the central nervous system. The acute inflammatory pain model, induced by 3% carrageenan, is associated with both primary and secondary hyperalgesia that is maintained primarily by acute inflammation and nociceptor activation (41). The chronic noninflammatory pain model is associated with secondary hyperalgesia and is maintained by central nervous system sensitization (54, 60). The exercise-enhanced pain model is associated with primary hyperalgesia that results from the acute inflammation and secondary hyperalgesia that results from changes in the central nervous system (53, 55). Thus the different animal models mimic different disease conditions and utilize different pain processing mechanisms.
The current study therefore hypothesized that regular physical activity would prevent the development of chronic centrally mediated muscle pain and the associated increases in p-NR1 in the RVM. Mice were made physically active by placing running wheels in their home cages, and the hyperalgesic response in models of acute inflammatory muscle pain, chronic muscle pain, and exercise-enhanced muscle pain were compared with sedentary mice. We show that regular physical activity prevents the development of chronic muscle pain and the exercise-enhanced muscle pain, and in parallel also prevents increases in p-NR1 in the RVM that normally occur in sedentary mice.
MATERIALS AND METHODS
Sedentary vs. Physically Active Mice
All experiments were approved by the Animal Care and Use Committee at the University of Iowa. Male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, http://www.jax.org/) were used for these experiments. Sedentary mice were housed in their home cage with food and water. Physically active mice had free access to running wheels in their home cages for either 5 days or 8 wk. Running wheel activity in kilometers per day was recorded. Eight weeks of physical activity was used to induce a training effect and to assess if a training effect was necessary to prevent the hyperalgesia produced by muscle insult. Five days of running wheel activity was used to test if short-duration regular physical activity produced similar effects to the long-duration effects and if the analgesic effects were independent of a training effect.
Animal Models of Pain
Noninflammatory chronic muscle pain.
This model of chronic muscle pain was induced by two injections of unbuffered pH 4.0 saline (20 μl) into one gastrocnemius muscle 5 days apart, as previously published (54). Injections were done with the mouse anesthetized with 4% isoflurane. This insult produces enhanced mechanical sensitivity of the paw and muscle without inflammation or tissue damage (12, 54). The hypersensitivity of the paw and muscle is maintained by changes in the central nervous system and involves glutamate receptors in the RVM (12). Therefore, the use of this model is testing if chronic muscle pain is prevented by regular physical activity at the site of the initial insult as well as outside the site of insult.
Exercise-induced enhancement of pain.
We have developed a model where a single bout of exercise enhances the nociceptive response to a low-dose muscle insult. A single bout of exercise was induced by placing mice in an activity wheel for 2 h at a self-selected speed prior to injection of carrageenan (55); mice typically run 2.16 ± 0.52 km during the 2 h (a speed of ∼1 km/h) (55, 64). Immediately following the exercise task, a mild muscle inflammation was produced by a single injection of 0.03% carrageenan into one gastrocnemius muscle while the mouse was anesthetized with 4% isoflurane (55). This dose of carrageenan produces a mild inflammation of the muscle with an increased mechanical sensitivity at the muscle, but not the paw (55). The exercise task is considered a mild form exercise constituting an unaccustomed physical activity in sedentary mice. The 2-h exercise task does not enhance the inflammation or produce hyperalgesia on its own, but rather produces an enhanced sensitivity at the paw or secondary mechanical hyperalgesia, and does not enhance the sensitivity at the site of inflammation in the muscle (55). Importantly the task is done prior to induction of inflammation, and the enhancement can occur if the task is given up to 2 h before the inflammation (55). We are therefore testing if spreading pain produced by exercise can be reduced by regular physical activity without affecting the acute protective pain at the site of injury.
Acute inflammatory muscle pain.
Acute muscle inflammation was induced by one injection of 3% carrageenan (20 μl) into one gastrocnemius muscle while the mouse was anesthetized with 4% isoflurane (46). This produces a robust acute inflammation associated with both muscle and paw hypersensitivity (46). Therefore, the use of this model is testing if regular exercise prevents the protective effects of acute pain in response to an acute inflammatory injury.
Behavior Testing
Muscle withdrawal thresholds of the paw.
Deep tissue sensitivity of the injected muscle was tested by examining the withdrawal threshold of the gastrocnemius muscle to a pair of calibrated forceps as previously described and validated (63). Decreased threshold is interpreted as muscle hyperalgesia.
Paw sensitivity to mechanical stimulation.
Cutaneous mechanical sensitivity of the paw was tested ipsilaterally as previously described (63). A 0.4 mN von Frey filament was applied to the hindpaw 10 times, and the number of withdrawals was assessed; this was repeated twice and averaged. Data are presented as a percent response, with 100% being 10/10 and 0% being 0/10 responses. An increased number of responses is interpreted as cutaneous hyperalgesia.
Motor Testing
Motor testing was done in separate groups of animals to eliminate the possible confounding of the injury or the motor tests. The grip force tests and the motor coordination tasks were also done in separate groups of animals. The specific motor function tests and protocols (outlined below) were done on the last day of exercise either on day 5 or week 8. Animals were brought to the laboratory in cages without running wheels and acclimated for at least 2 h prior to testing motor function.
Grip strength test.
Hindpaw and forepaw grip force was tested before and after 5 days (n = 4) or 8 wk (n = 8) of running wheel activity and compared with sedentary mice (n = 8) as previously described (6). The mice were familiarized twice per day for 2 days to the apparatus by performing the grip force task (San Diego Instruments, San Diego, CA, http://www.sandiegoinstruments.com). Mice were pulled by the tail to read grip force for either the hindpaw or the forepaw. An average of five trials was recorded for both the hindpaw and the forepaw. Grip force data were reported and analyzed as a percent of baseline to indicate the degree of change in force induced by physical activity.
Motor coordination test.
Changes in motor coordination were tested after 5 days and 8 wk of running wheel activity using a protocol previously published (8). Mice were trained four times a day for 3 days on the Roto-Rod (IITC Life Science, Woodland Hills, CA, http://www.iitcinc.com/). Day 1 training consisted of placing mice on the Roto-Rod to run at 5–24 rpm ramping in 60 s for 1 min. After 5–10 min of rest, mice were placed on the Roto-Rod again and run at the same speeds three more times. Day 2 training consisted of placing mice on the Roto-Rod to run at 10–24 rpm ramping in 30 s for 1 min. After 5–10 min of rest, the mice were placed again on Roto-Rod and run at the same speeds three more times. Day 3 training consisted of placing mice on the Roto-Rod to run at 24 rpm for 1 min. After 5–10 min of rest, mice were placed on Roto-Rod again and run at the same speeds three more times. Testing consisted of running mice on the Roto-Rod at eight different rpm speeds (5, 8, 15, 20, 24, 31, 33, and 44) two times, and the times that the mice fell off were recorded.
Immunohistochemistry
Animals were deeply anesthetized with 150 mg/kg sodium pentobarbital and transcardially perfused with heparinized saline followed by 4% paraformaldehyde. The brainstem was removed, stored in 30% sucrose overnight, blocked to include the RVM, and frozen. Sections were cut on a cryostat at 20 μm onto slides. All sections were immunohistochemically stained simultaneously using an antibody to the protein kinase A (PKA) phosphorylation site of the NR1 subunit of the NMDA receptor (pNR1) (1:1,000, Ser897, catalog no. ABN99; Millipore, Billerica, MA, http://www.millipore.com/) using standard immunofluorescent techniques as previously described (53). On day 1, sections were blocked in 3% normal goat serum (NGS), avidin, and biotin and then incubated in the primary antibody overnight at room temperature. On day 2, sections were rinsed and blocked in 3% NGS, and then incubated in biotinylated immunoglobulin G for 1 h at room temperature (1:1,000; Invitrogen, Eugene, OR, http://www.invitrogen.com/). These sections were then reacted with streptavidin conjugated to Alexa Fluor 568 (1:1,000; Invitrogen) for 1 h at room temperature. Sections were coverslipped with Vectashield (Vector Labs, Burlingame, CA, http://www.vectorlabs.com/) and stored until analysis. Brain sections for each group were stained simultaneously to avoid variability between stains across days. We previously show that the staining of p-NR1 in the RVM is significantly decreased by downregulation of the NR1 subunit of the NMDA receptor with a feline immunodeficiency virus (FIV) expressing an miRNA to NR1 or increased with upregulation of the NR1 subunit of the NMDA receptor with an FIV expressing NR1 (13).
Images of the stained sections were taken in the Central Microscopy Facility at the University of Iowa on an Olympus BX-51 light microscope equipped with a SPOT camera (RT Slider; Diagnostic Instruments, http://www.meyerinst.com/html/dgnstc/default.htm). For a resolution sufficient for counting pNR1-positive cells, images were taken with a 20× objective lens. Sections were identified at Bregma −6.00 mm from the Paxinos and Franklin Mouse Brain Atlas (42). Five sections of the nucleus raphe magnus (NRM) were digitally imaged and stored for later analysis. Cells were quantified by manually counting total numbers in with a standard area of 10,804 μm2 using ImageJ software (National Institutes of Health). For quantitative analysis, positively labeled neurons that were at least 50% darker than the average gray level of each image were counted. Cells were counted if they contained a nucleus and were positively stained for pNR1.
Protocol
Experiment 1 tested if regular physical activity could prevent the development of muscle pain. We examined muscle and paw sensitivity in three models of muscle pain in both sedentary and physically active mice. For the chronic muscle pain model (repeated pH 4.0 injections), three groups were examined: 1) sedentary (n = 18), 2) 5 days of physical activity (n = 16), and 3) 8 wk of physical activity (n = 5). For the exercise-enhanced pain model three groups were examined: 1) sedentary (n = 9), 2) 5 days of physical activity (n = 8), and 3) 8 wk of regular physical activity (n = 10). For the carrageenan-induced muscle inflammation model two groups were examined: 1) sedentary (n = 4) and 2) 8 wk of physical activity (n = 4). Mice were tested before and 24 h after induction of the model.
Experiment 2 examined the length of protective effect of physical activity using a subset of animals from Experiment 1. We tested mice before and after running wheel activity (5 days or 8 wk), and 24 h, 72 h, and 1 wk after induction of the model. For the chronic muscle pain model we tested sedentary (n = 10), 5 days of physical activity (n = 8), and 8 wk of physical activity (n = 9). For the exercise-enhanced pain model we tested sedentary (n = 8), 5 days of physical activity (n = 8), and 8 wk of physical activity (n = 5).
Experiment 3 examined the effects of physical activity on motor function in uninjured animals. Grip force was used to measure strength in the following groups: sedentary (n = 8), 5 days of physical activity (n = 4), and 8 wk of physical activity (n = 8). Rota-Rod was used to assess coordination in the following groups: sedentary (n = 8), after 5 days of physical activity (n = 7), or after 8 wk of physical activity (n = 5) in a separate group of uninjured animals.
Experiment 4 tested if regular physical activity prevents the activation of NMDA glutamate receptors in the NRM produced by muscle insult and/or acute exercise in sedentary animals by examining the phosphorylation of the NR1 subunit of the NMDA receptor using immunohistochemistry. In each situation tissues were cut and stained together and directly compared. This minimized differences related to different antibody lots, different solutions, or different days. As outlined below, there were always comparison groups stained at the same time with experimental groups. This resulted in four distinctly different sets of staining protocols: 1) sedentary mice: 24 h after induction of the exercise-enhanced pain model (0.03% carrageenan + exercise, n = 4), 24 h after acute muscle inflammation (3% carrageenan; n = 4), 24 h after control manipulations (pH 7.2 saline with 2 h exercise task, n = 4); 2) sedentary mice: 24 h after induction of chronic muscle pain model (pH 4.0 saline; n = 6) vs. 24 h after control pH 7.2 saline injections (n = 7); 3) 24 h after induction of the exercise-enhanced pain model, one group was sedentary (n = 4) and one group had access to running wheels for 5 days (n = 4); and 4) 24 h after induction of the chronic muscle pain model, one group was sedentary (n = 3) and had access to running wheels for 8 wk (n = 4).
Statistics
A repeated measures ANOVA tested for changes in withdrawal thresholds of the muscle and number of responses to repeated application of mechanical stimulation of the paw between groups and across time. This was followed by post hoc testing with a Tukey's test for differences between groups. A paired t-test tested for differences in grip force after exercise compared with before exercise. A repeated ANOVA tested for differences in Roto-Rod performance between groups. A one-way ANOVA tested for differences between groups for the changes in p-NR1. All data are means ± SE, and P < 0.05 is considered significant.
RESULTS
To test if regular physical activity prevents the development of muscle pain, mice were given free access to running wheels in their home cages for 5 days or 8 wk. The average amount of time run each day was 1.4 ± 0.34 km (range 0.30–4.5 km) over the 8-wk period and an average of 2.7 ± 0.61 km (range 0.19–5.1 km) per day over the 5-day period; the groups were not significantly different.
Experiment 1: Effects of Physical Activity in Different Pain Models
Chronic muscle pain model.
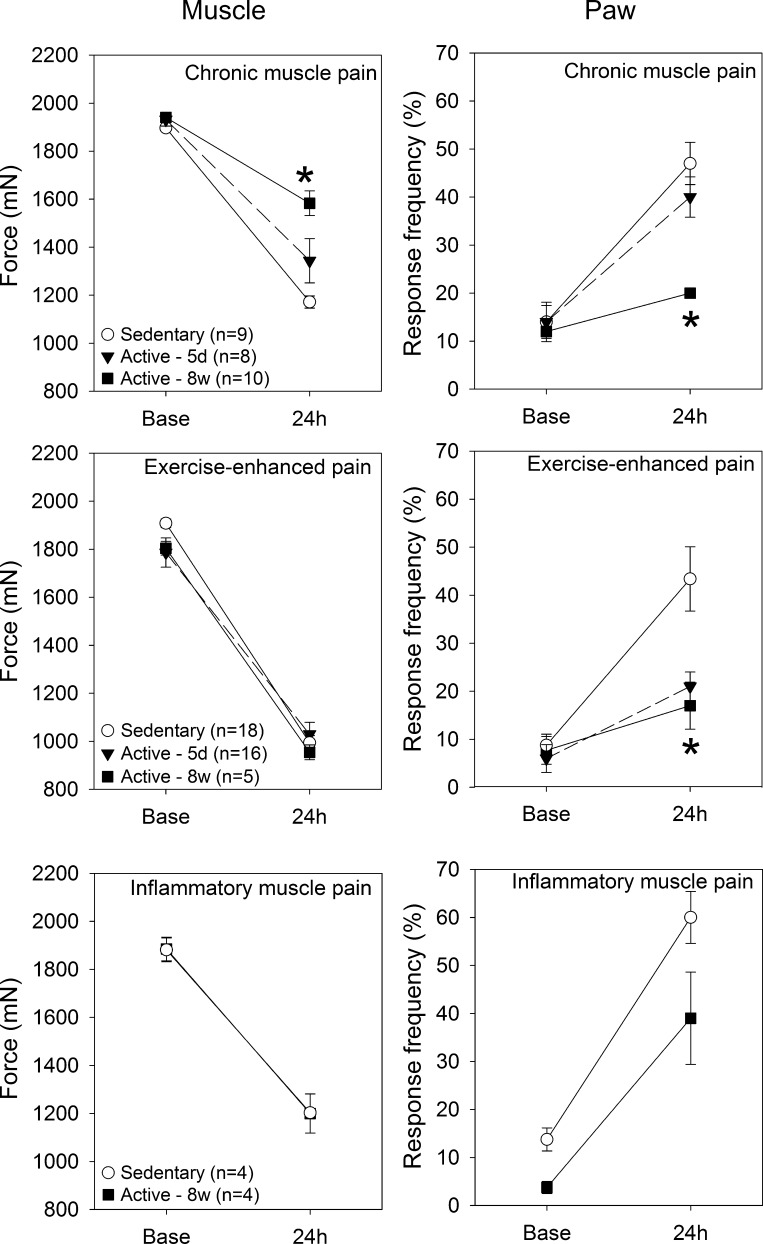
In the chronic muscle pain model, 8 wk of physical activity prevented the decrease in withdrawal thresholds of the muscle and the increased responses to mechanical stimulation of the paw 24 h after induction of the model compared with sedentary controls (Fig. 1, top). In contrast, the animals that performed 5 days of regular physical activity still showed significant decreases in withdrawal threshold of the muscle and increases in responses to repeated stimulation of the paw similar to sedentary mice.
Fig. 1.
Experiment 1. Top: in the chronic muscle pain model, 8 wk of physical activity, but not 5 days, significantly prevents the decrease in withdrawal threshold of the muscle and the increase in paw responses that normally occurs in this model compared with sedentary mice (*P < 0.05). Middle: in the exercise-enhanced pain model, 5 days or 8 wk of running wheel activity significantly prevents the increase in paw responses compared with sedentary mice (*P < 0.05). The muscle withdrawal thresholds decrease significantly in all three groups after induction of the exercise-enhanced pain model. Bottom: in the acute inflammation model, 8 wk of regular physical activity had no effect on the development of hyperalgesia of the paw or muscle. Base, after running wheel activity but before induction of model; 24 h, after induction of the model. Data are means ± SE.
Exercise-enhanced pain model.
Compared with sedentary mice, both 5 days and 8 wk of physical activity prevented the increases in responsiveness to repeated mechanical stimulation of the paw in the exercise-enhanced pain model 24 h after induction of the model compared with sedentary mice (Fig. 1, middle). However, the muscle withdrawal thresholds decrease significantly in all three groups after induction of the exercise-enhanced pain model: sedentary, 5 days of physical activity and 8 wk of physical activity (Fig. 1, middle). As a control, we tested the effects of 0.03% carrageenan without the 2-h exercise task and show a similar decrease in muscle withdrawal threshold (1,026 ± 32 mN) but no change in responses to repeated stimulation of the paw (7.5 ± 2.5%). Thus the decrease in withdrawal threshold of the muscle is related to the acute inflammation and is unaffected by regular physical activity.
Acute inflammatory muscle pain.
In mice with 8 wk of physical activity there is a significant decrease in the withdrawal thresholds of the muscle, similar to sedentary control mice after induction of acute muscle inflammation with 3% carrageenan (Fig. 1, bottom). The responses to repeated stimulation of the paw in mice with acute muscle inflammation also significantly increase in both the sedentary and the physically active mice and were not significantly different from each other.
Experiment 2: Time Course of Protective Effects of Physical Activity
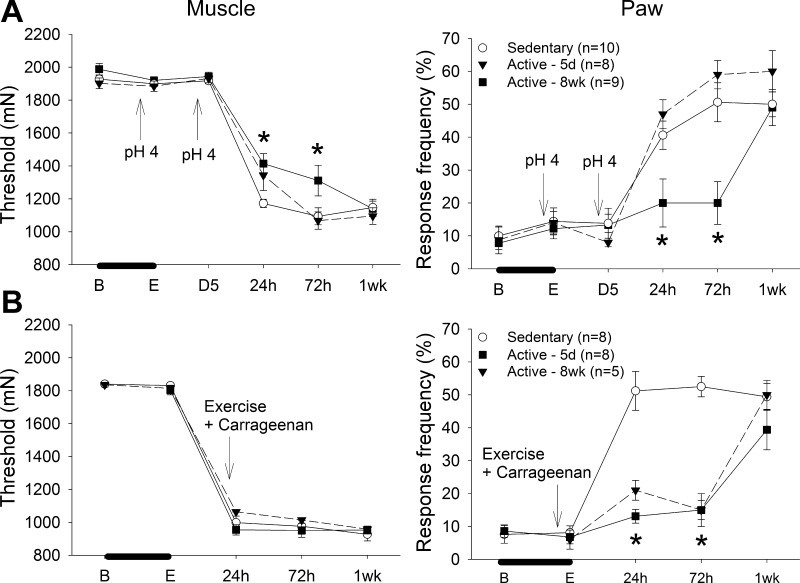
To test the length of action of the protective effects of regular physical activity, physically active mice were followed for 1 wk after induction of either the exercise-induced pain model or the chronic muscle pain model. In the chronic muscle pain model, 8 wk of running wheel activity prevented the development of hyperalgesia in the chronic muscle pain model that lasted through 72 h (8 days after stopping running wheel); the hyperalgesia returned by day 7 (13 days after stopping running wheel; Fig. 2A). Both 5 days and 8 wk of physical activity prior to induction of the exercise-enhanced pain model prevented the development of paw hypersensitivity in the exercise-enhanced pain model through 72 h—the effect was gone by 1 wk (Fig. 2B). These data suggest that the protective effects of exercise are plastic and temporary, and regular exercise must be maintained to prevent development of chronic and exercise-enhanced hyperalgesia.
Fig. 2.
Experiment 2. Time course of effects of regular physical activity on the chronic muscle pain model (A) and the exercise-enhanced pain model (B). Access to running wheels was stopped at the time of induction of the model. A: in the chronic muscle pain model, 8 wk of running wheel activity prevents the decreases in muscle withdrawal threshold and increased responses of the paw for up to 72 h after induction of the model (*P < 0.05). By 1 wk these responses are significantly increased (P < 0.05) and similar to sedentary mice. B: in the exercise-enhanced pain model, both 5 days and 8 wk of running wheel activity prevented the increases in paw responses to noxious stimuli for 72 h after induction of the model (*P < 0.05). By 1 wk these responses were significantly increased (P < 0.05). The muscle withdrawal threshold decreased in the sedentary and physically active animals to a similar extent (P < 0.05). B, before running wheel activity; E, after running wheel activity; D5, 5 days after the first acid injection and before the second acid injection; 24 h, 72 h, and 1wk refer to time after induction of the model. Data are means ± SE.
Experiment 3: Effects of Physical Activity on Motor Function
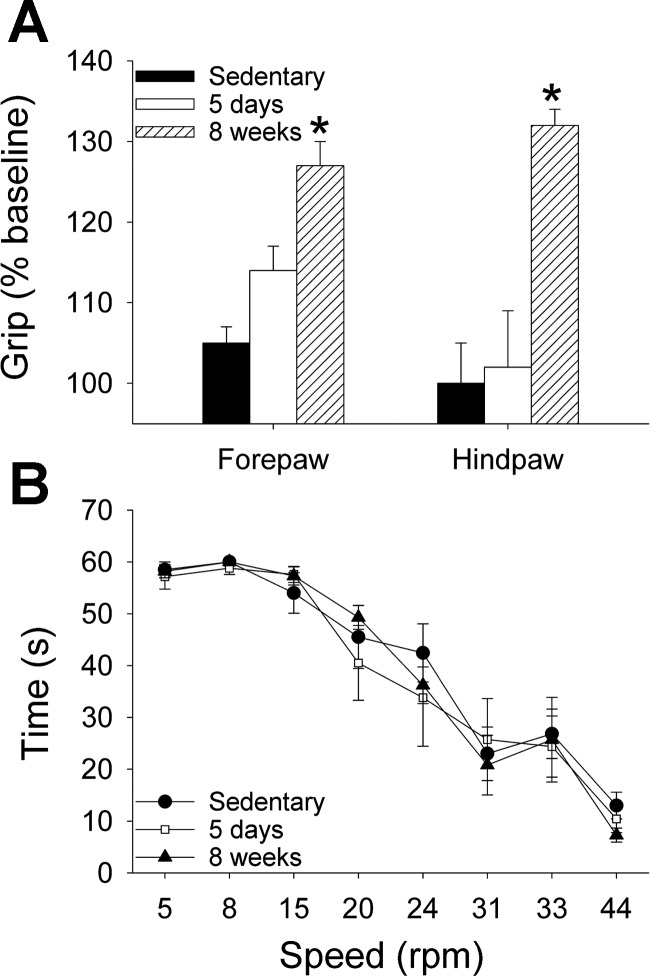
To test if neuromuscular adaptations were necessary to produce an analgesic effect, we examined changes in strength and motor coordination in separate groups of uninjured mice. Grip force strength significantly increased after 8 wk of running wheel activity, but did not change after 5 days of running wheel activity or in sedentary animals (Fig. 3A). Motor coordination, measured by Rota-Rod performance at increasing speeds, did not change after either 5 days or 8 wk of running wheel activity compared with sedentary mice (Fig. 3B). Thus because analgesic effects occur without changes in coordination, or changes in strength at 5 days, motor function alterations are not requisite for the protective effects of regular physical activity.
Fig. 3.
Experiment 3. Effects of regular physical activity on motor function in uninjured animals. A: grip force strength test shows a significant increase in strength for both the hindpaw and the forepaw after 8 wk of running wheel activity compared with sedentary animals; 5 days of running wheel activity had no effect on strength (*P < 0.05). B: Rota-rod coordination test reveals no difference in coordination in animals after 5 days or 8 wk of running wheel activity compared with sedentary mice. Data are means ± SE.
Experiment 4: Effects of Physical Activity on p-NR1 Staining in the RVM
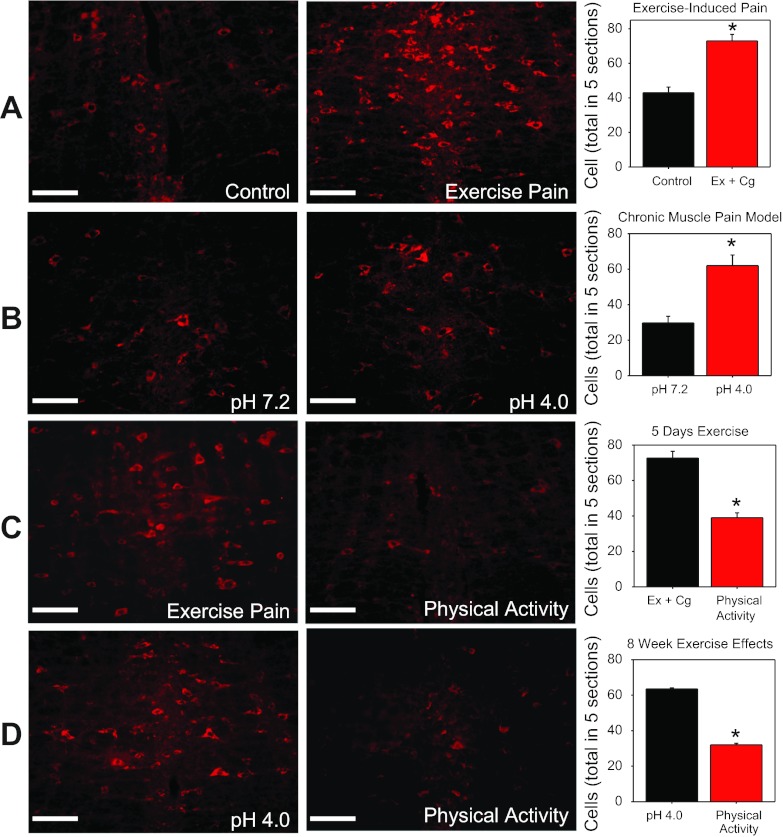
To test if the RVM is modulated by physical activity, we examined the phosphorylation of NR1 (p-NR1) in sedentary animals and physically active animals using the chronic muscle pain model and the exercise-enhanced model. Sedentary animals had significantly more neurons expressing p-NR1 after induction of chronic muscle pain, exercise-enhanced pain (Fig. 4, A and B), muscle inflammation [39 ± 4.5 vs. 14 ± 2 (control)], and after the 2-h exercise task [41 ± 0.71 vs. 14 ± 2 (control)] compared with controls. However, physically active mice with chronic muscle pain or exercise-enhanced pain showed lower numbers of neurons immunohistochemically stained for p-NR1 in the RVM compared with sedentary animals with chronic muscle pain or exercise-enhanced pain (Fig. 4, C and D). Thus regular physical activity prevented increased phosphorylation of NR1 that normally occurs in sedentary animals in response to induction of chronic muscle pain or exercise-enhanced pain.
Fig. 4.
Experiment 4. Immunohistochemical staining for p-NR1 in the rostral ventromedial medulla. A: 24 h after induction of the exercise-enhanced pain model in sedentary mice (Ex+Cg) compared with controls. B: 24 h after induction of the chronic muscle pain model (pH 4.0) in sedentary mice compared with controls (pH 7.2). C: 24 h after induction of exercise-enhanced pain (Ex+Cg) in mice with 5 days of running wheel activity compared with sedentary mice with exercise-enhanced pain. D: 24 h after induction of chronic muscle pain in mice with 8 wk of running wheel activity compared with sedentary mice with chronic muscle pain. *P < 0.05, significantly different between comparison group. Data are means ± SE.
DISCUSSION
The current data show for the first time that regular physical activity prevents the development of chronic pain, but that this effect is plastic and temporary. We further show that activation of neurons in RVM (p-NR1), a critical site in pain modulation, does not occur in animals with regular physical activity. Physical inactivity, therefore, may be an important risk factor in the development of chronic pain. This is particularly important in the light of increasing levels of physical inactivity in the general population (22) and the fact that animal studies on pain mechanisms typically use sedentary animals.
There are a number of different types of exercises that have been used in animals to produce analgesia including 1) free access to running wheels, 2) resisted exercise training, and 3) aerobic conditioning exercises using either treadmill running or swimming. These studies vary by duration of exercise, timing of exercise, animal model used, and outcome tested, making direct comparisons to the current study difficult. It should be noted that all prior studies in animal models of pain used forced exercise, which may produce analgesia through a stress response. It is well-known that stress can produce analgesia without the exercise task (4, 25, 31, 32). The current study was the first to use voluntary running wheel activity in animal models of pain, which allowed mice to choose when and if they wanted to run, thus eliminating stress as a confounder. We attempted to mimic the condition of increased physical activity rather than exercise, showing that increased physical activity levels prevents development of chronic pain. Indeed physical activity is protective against a variety of chronic pain conditions including cancer, dementia, cardiovascular diseases, and depression (23, 43).
Exercise Effects in Uninjured Animals
Some studies show exercise-induced analgesia occurs within minutes while others show that the analgesia occurs in weeks. In uninjured animals there are increases in withdrawal thresholds to noxious stimuli with a single swimming exercise task at either a low intensity or high intensity (4), 3 wk of running wheel activity (28, 36, 38), or a 12-wk resistance strengthening exercise program (39). In contrast, we show that neither 5 days nor 8 wk of running wheel activity changes withdrawal thresholds of the muscle or paw in uninjured animals. This could be due to the timing of the measurement assessed. The current study assessed withdrawal thresholds several hours after the animals had stopped their physical activity; the majority of other studies did analgesic tests immediately after the task. This suggests that the exercise tasks produce analgesia but that the immediate analgesic effect in uninjured animals is transient.
Exercise Effects in Animal Models of Pain
The current study showed that 5 days of running wheel activity prevented the spreading hyperalgesia in the exercise-enhanced pain model, but had no effect in the chronic muscle pain model. In comparison, prior studies show that 5–9 days of swimming at moderate intensity prevented spontaneous behaviors to acute intraperitoneal acetic acid injection (39) and by intraplantar formalin injection (34). It is possible that the acute inflammatory muscle hyperalgesia is more difficult to overcome since it is more robust and longer lasting than the spontaneous pain produced by acetic acid and formalin injections.
In animal models of pain, repeated aerobic exercise tasks like swimming and treadmill running reduce neuropathic pain induced by nerve injury or diabetes and chronic muscle pain induced by repeated acid injections (3, 33, 34, 50, 51, 58). Most of these studies started exercise at the time of injury or after development of hyperalgesia and show a reversal of the hyperalgesia with as little as a single treatment (3, 33, 51, 58). However, Kuphal and colleagues show that 7 days of exercise prior to induction of inflammatory pain with formalin or 2 wk of exercise prior to induction of neuropathic pain attenuated the development of pain behaviors. In fact, the second phase of the formalin test was attenuated, suggesting effects were more centrally mediated. In parallel, the current study showed a prevention of the centrally mediated secondary paw hyperalgesia in the exercise-enhanced pain model with 5 days of exercise. We also show in the current study that 8 wk of regular physical activity prevented the development of hyperalgesia in the centrally maintained chronic muscle pain model, but had no effect in the peripherally maintained acute inflammatory pain model. These data would suggest that the effects of regular physical activity primarily affects centrally mediated pain conditions and has minimal effects on peripherally mediated pain. The data further suggest that regular physical activity prevents the severity of chronic pain and enhanced pain, both of which are nonprotective. However, pain in response to an acute injury, which is protective, still occurs.
In contrast to the exercise-enhanced pain model, the current study shows that 5 days of physical activity has no effect on induction of the muscle and paw hyperalgesia in the chronic muscle pain model. Rather, only 8 wk of regular physical activity was able to delay the onset of the hyperalgesia in the chronic muscle pain model. Once the hyperalgesia develops in the chronic muscle pain model, it is independent of primary afferent input, but rather it is maintained by activation of neurons in the central nervous system—the RVM and spinal cord (15, 52, 53). Further, the second injection of acidic saline requires activation of RVM facilitation neurons (60). It may be that this model has a stronger central component to the induction and maintenance of the hyperalgesia, making it more difficult to affect with short-duration exercise. However, it should be pointed out that, once the hyperalgesia develops, short-duration forced exercise completely reverses the existing hyperalgesia (3).
Another critical finding in this study was that the effects of regular physical activity were plastic and lasted for 1–2 wk after stopping the exercise. It has become increasingly clear, and clinical guidelines recommend, that after an acute injury maintaining physical activity and avoiding bedrest is critical to recovery (11, 61). Indeed, our data are consistent with the recommendation to remain active. The current study does not determine if continued exercise after induction of the pain model will prevent the long-lasting hyperalgesia completely. A recent article by Bobinski and colleagues (5) directly answered this question by examining exercise effects if done prior to nerve injury (crush), if done prior to nerve injury and continued after nerve injury, or if done after nerve injury. In all three conditions, mechanical hyperalgesia was reduced compared with sedentary controls. However, the study, which was confirmed by a subsequent study, shows that exercise reduces local inflammatory mediators and nerve degeneration induced by the nerve injury (5, 10). Thus the decrease in mechanical hyperalgesia in the nerve injury studies could be directly related to the decrease in inflammation and injury. It remains to be determined if regular exercise that continues after the induction of a chronic pain condition will continue to have a protective effect.
Mechanisms of Exercise-Induced Analgesia
Previous studies show that aerobic exercise produces analgesia in part through activation of opioid pathways in healthy human subjects and that regular exercise in animals without tissue injury is associated with opioid receptor activation (3, 14, 28, 37, 39, 48, 50, 56–58). In animals with chronic muscle pain or diabetic neuropathy, analgesia is reduced by a single dose of systemic naloxone (3, 50), suggesting an acute release of endogenous opioid peptides. Central, but not peripheral, blockade of opioid receptors reduces the analgesia produced by exercise in animals with neuropathic pain (58), supporting a role for central opioids. Central release of endogenous opioids occurs in response to treadmill running in animals with neuropathic pain—β-endorphin increases in the periaqueductal gray and enkephalin in the RVM (58). Together these data suggest that regular exercise reduces pain by activation of opioid receptors in descending inhibitory pathways in the central nervous system.
The current study shows that increases in phosphorylation of the NR1 subunit of the NMDA receptor (PKA site) do not occur in physically active animals in the chronic muscle pain model or the exercise-enhanced pain model. Phosphorylation of NR1 would be expected to enhance neuron excitability by increasing the number of NMDA receptors transported to the cell membrane and increasing glutamate-induced currents through NMDA receptors (9, 17, 49, 65). The NMDA receptor in the RVM is critical for the development of hyperalgesia after muscle insult, with our prior studies showing blockade of NMDA receptors reduces hyperalgesia, increasing NR1 enhances nociception, and decreasing NR1 prevents chronic muscle hyperalgesia (13, 53). We propose that the normal state of the nervous system is associated with regular physical activity and that physical inactivity is the “disease state” setting up the nervous system to respond in an exaggerated way to a muscle insult and develop chronic pain (see schematic diagram Fig. 5). We further propose that phosphorylation of the NR1 subunit of the NMDA is controlled tonically by opioid receptors in the RVM during conditions of regular physical activity and that under sedentary conditions this tonic control is removed. Thus physical inactivity could be a key factor in the transition from acute to chronic pain and may provide new therapeutic directions for the prevention of chronic pain.
Fig. 5.
Schematic diagram representing a proposed pathway for the control of excitability within the rostral ventromedial medulla (RVM). A: under sedentary conditions, unaccustomed exercise and muscle insult increase the phosphorylation of the NR1 subunit (p-NR1) of the N-methyl-d-aspartate (NMDA) receptor at the protein kinase A (PKA) site as shown in the current study. Increases in p-NR1 are expected to increase excitability and produce hyperalgesia and pain. B: regular exercise and physical activity increase release of met-enkephalin in the RVM and use opioid receptors centrally to mediate analgesia (58). This would activate mu-opioid receptors on the pain facilitation neurons to inhibit activation of the cAMP-PKA pathway reducing p-NR1. Thus, in the physically active condition, there is increased activity within the opioid system that reduces cell excitability by reducing phosphorylation of the NR1 subunit to result in prevention of pain to unaccustomed exercise and chronic muscle pain. We propose that the normal state of the nervous system is under conditions of regular physical activity and that the “abnormal state” occurs under sedentary conditions. We further suggest that a sedentary lifestyle or physical inactivity is a risk factor for the development of chronic pain. NR2, NMDA receptor subunit 2.
GRANTS
This work was funded by National Institutes of Health Grants AR061371 and AR052316 to K. A. Sluka.
DISCLOSURES
No conflict of interest, financial or otherwise, is declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.A.S., J.D., and L.A.R. conception and design of research; K.A.S., J.M.O., J.D., and L.A.R. performed experiments; K.A.S., J.M.O., J.D., and L.A.R. analyzed data; K.A.S. interpreted results of experiments; K.A.S. and J.D. prepared figures; K.A.S. and L.A.R. drafted manuscript; K.A.S., J.M.O., J.D., and L.A.R. edited and revised manuscript; K.A.S., J.M.O., J.D., and L.A.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Laura Frey Law for reviewing the manuscript prior to submission.
REFERENCES
- 1. Bazelmans E, Bleijenberg G, Voeten MJ, van der Meer JW, Folgering H. Impact of a maximal exercise test on symptoms and activity in chronic fatigue syndrome. J Psychosom Res 59: 201–208, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bement MK. Exercise-induced hypoalgesia: an evidence-based review. In: Mechanisms and Management of Pain for the Physical Therapist, edited by Sluka KA. Seattle, WA: IASP Press, 2009, p. 143–166 [Google Scholar]
- 3. Bement MK, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil 86: 1736–1740, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Blustein JE, McLaughlin M, Hoffman JR. Exercise effects stress-induced analgesia and spatial learning in rats. Physiol Behav 89: 582–586, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bobinski F, Martins DF, Bratti T, Mazzardo-Martins L, Winkelmann-Duarte EC, Guglielmo LG, Santos AR. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice. Neurosci 194: 337–348, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Burnes LA, Kolker SJ, Danielson JF, Walder RY, Sluka KA. Enhanced muscle fatigue occurs in male but not female ASIC3−/− mice. Am J Physiol Regul Integr Comp Physiol 294: R1347–R1355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Busch AJ, Barber KA, Overend TJ, Peloso PM, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev CD003786, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci 19: 3248–3257, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cerne R, Rusin KI, Randic M. Enhancement of the N-methyl-D-aspartate response in spinal dorsal horn neurons by cAMP-dependent protein kinase. Neurosci Lett 161: 124–128, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Chen YW, Li YT, Chen YC, Li ZY, Hung CH. Exercise training attenuates neuropathic pain and cytokine expression after chronic constriction injury of rat sciatic nerve. Anesth Analg 114: 1330–1337, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 147: 492–504, 2007 [DOI] [PubMed] [Google Scholar]
- 12. da Silva LFS, DeSantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. Pain 11: 378–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. da Silva LFS, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulates pain behaviors. Pain 151: 155–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Oliveira MS, da Silva Fernandes MJ, Scorza FA, Persike DS, Scorza CA, da Ponte JB, de Albuquerque M, Cavalheiro EA, Arida RM. Acute and chronic exercise modulates the expression of MOR opioid receptors in the hippocampal formation of rats. Brain Res Bull 83: 278–283, 2010 [DOI] [PubMed] [Google Scholar]
- 15. DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr Pain Headache Rep 12: 338–343, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edmonds M, McGuire H, Price J. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev CD003200, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Ehlers MD, Tingley WG, Huganir RL. Regulated subcellular distribution of the NR1 subunit of the NMDA receptor. Science 269: 1734–1737, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Ellingson LD, Shields MR, Stegner AJ, Cook DB. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain 13: 195–206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: Textbook of Pain, edited by McMahon SB, Koltzenburg M. Philadelphia, PA: Elsevier, 2006, p. 125–142 [Google Scholar]
- 20. Fransen M, McConnell S, Bell M. Therapeutic exercise for people with osteoarthritis of the hip or knee. A systematic review. J Rheumatol 29: 1737–1745, 2002 [PubMed] [Google Scholar]
- 21. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 13: 715–724, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 380: 247–257, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 454: 463–469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henschke N, Maher CG, Refshauge KM, Herbert RD, Cumming RG, Bleasel J, York J, Das A, McAuley JH. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ 337: a171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hopkins E, Spinella M, Pavlovic ZW, Bodnar RJ. Alterations in swim stress-induced analgesia and hypothermia following serotonergic or NMDA antagonists in the rostral ventromedial medulla of rats. Physiol Behav 64: 219–225, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Itz CJ, Geurts JW, van Dleef M, Nelemans P. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur J Pain 17: 5–15, 2013 [DOI] [PubMed] [Google Scholar]
- 27. Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain 11: 39–47, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Kanarek RB, Gerstein AV, Wildman RP, Mathes WF, D'Anci KE. Chronic running-wheel activity decreases sensitivity to morphine-induced analgesia in male and female rats. Pharmacol Biochem Behav 61: 19–27, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Karanikolas M, Aretha D, Tsolakis I, Monantera G, Kiekkas P, Papadoulas S, Swarm RA, Filos KS. Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: a prospective, randomized, clinical trial. Anesthesiol 114: 1144–1154, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Kasch H, Qerama E, Kongsted A, Bach FW, Bendix T, Jensen TS. Deep muscle pain, tender points and recovery in acute whiplash patients: a 1-year follow-up study. Pain 140: 65–73, 2008 [DOI] [PubMed] [Google Scholar]
- 31. King CD, Devine DP, Vierck CJ, Rodgers J, Yezierski RP. Differential effects of stress on escape and reflex responses to nociceptive thermal stimuli in the rat. Brain Res 987: 214–222, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Konarzewski M, Sadowski B, Jozwik I. Metabolic correlates of selection for swim stress-induced analgesia in laboratory mice. Am J Physiol Regul Integr Comp Physiol 273: R337–R343, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Korb A, Bonetti LV, Da Silva SA, Marcuzzo S, Ilha J, Bertagnolli M, Partata WA, Faccioni-Heuser MC. Effect of treadmill exercise on serotonin immunoreactivity in medullary raphe nuclei and spinal cord following sciatic nerve transection in rats. Neurochem Res 35: 380–389, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain 8: 989–997, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Lee J, Dunlop D, Ehrlich-Jones L, Semanik P, Song J, Manheim L, Chang RW. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken) 64: 488–493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li G, Rhodes JS, Girard I, Gammie SC, Garland T., Jr Opioid-mediated pain sensitivity in mice bred for high voluntary wheel running. Physiol Behav 83: 515–524, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Mathes WF, Kanarek RB. Wheel running attenuates the antinociceptive properties of morphine and its metabolite, morphine-6-glucuronide, in rats. Physiol Behav 74: 245–251, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Mathes WF, Kanarek RB. Chronic running wheel activity attenuates the antinociceptive actions of morphine and morphine-6-glucouronide administration into the periaqueductal gray in rats. Pharmacol Biochem Behav 83: 578–584, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Mazzardo-Martins L, Martins DF, Marcon R, Dos Santos UD, Speckhann B, Gadotti VM, Sigwalt AR, Guglielmo LG, Soares Santos AR. High-intensity extended swimming exercise reduces pain-related behavior in mice: involvement of endogenous opioids and the serotonergic system. J Pain 11: 1393, 2010 [DOI] [PubMed] [Google Scholar]
- 40. McGreevy K, Bottros MM, Raja SN. Preventing chronic pain following acute pain: risk factors, preventive strategies, and their efficacy. Eur J Pain Suppl 5: 365–372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mense S. Peripheral mechanisms of muscle pain: response behaviour of muscle nociceptors and factors eliciting muscle pain. In: Muscle Pain: Understanding the Mechanisms, edited by Mense S, Gerwin RD. Heidelberg, Germany: Springer, 2010, p. 49–104 [Google Scholar]
- 42. Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York: Academic Press, 2001 [Google Scholar]
- 43. Pedersen BK. The diseasome of physical inactivity—and the role of myokines in muscle-fat cross talk. J Physiol 587: 5559–5568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci 25: 319–325, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Radhakrishnan R, Bement M, Skyba D, Kehl L, Sluka K. Models of muscle pain: carrageenan model and acidic saline model. In: Current Protocols in Pharmacology, edited by Enna SJ, Williams M, Ferkany J, Kenakin T, Porsolt R, Sullivan J. Hoboken, NJ: John Wiley & Sons, 2004, p. 1–28 [DOI] [PubMed] [Google Scholar]
- 46. Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain 104: 567–577, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schachter CL, Busch AJ, Peloso PM, Sheppard MS. Effects of short versus long bouts of aerobic exercise in sedentary women with fibromyalgia: a randomized controlled trial. Phys Ther 83: 340–358, 2003 [PubMed] [Google Scholar]
- 48. Schwarz L, Kindermann W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med 13: 25–36, 1992 [DOI] [PubMed] [Google Scholar]
- 49. Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci 21: 3063–3072, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shankarappa SA, Piedras-Renteria ES, Stubbs EB., Jr Forced-exercise delays neuropathic pain in experimental diabetes: effects on voltage-activated calcium channels. J Neurochem 118: 224–236, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Sharma NK, Ryals JM, Gajewski BJ, Wright DE. Aerobic exercise alters analgesia and neurotrophin-3 synthesis in an animal model of chronic widespread pain. Phys Ther 90: 714–725, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain 98: 69–78, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Sluka KA, Danielson J, Rasmussen L, Dasilva LF. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med Sci Sports Exerc 44: 420–427, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle & Nerve 24: 37–46, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain 148: 188–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith MA, McClean JM, Bryant PA. Sensitivity to the effects of a kappa opioid in rats with free access to exercise wheels: differential effects across behavioral measures. Pharmacol Biochem Behav 77: 49–57, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology (Berl) 168: 426–434, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip MT., Jr Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiol 114: 940–948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain 118: 176–184, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain 136: 331–339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tully MA, Bleakley CM, O'Connor SR, McDonough SM. Functional management of ankle sprains: what volume and intensity of walking is undertaken in the first week postinjury. Br J Sports Med 46: 877–882, 2012 [DOI] [PubMed] [Google Scholar]
- 62. van Tulder MW, Koes BW, Bouter LM. Conservative treatment of acute and chronic nonspecific low back pain. A systematic review of randomized controlled trials of the most common interventions. Spine 22: 2128–2156, 1997 [DOI] [PubMed] [Google Scholar]
- 63. Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J Pain 11: 210–218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle Fatigue Increases the Probability of Developing Hyperalgesia in Mice. J Pain 8: 692–699, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zou X, Lin Q, Willis WD. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neurosci 115: 775–786, 2002 [DOI] [PubMed] [Google Scholar]