Abstract
Genetically engineered mice have provided much information about gene function in the field of developmental biology. Recently, conditional gene targeting using the Cre/loxP system has been developed to control the cell type and timing of the target gene expression. The increase in number of kidney-specific Cre mice allows for the analysis of phenotypes that cannot be addressed by conventional gene targeting. The mammalian kidney is a vital organ that plays a critical homeostatic role in the regulation of body fluid composition and excretion of waste products. The interactions between epithelial and mesenchymal cells are very critical events in the field of developmental biology, especially renal development. Kidney development is a complex process, requiring inductive interactions between epithelial and mesenchymal cells that eventually lead to the growth and differentiation of multiple highly specialized stromal, vascular, and epithelial cell types. Through the use of genetically engineered mouse models, the molecular bases for many of the events in the developing kidney have been identified. Defective morphogenesis may result in clinical phenotypes that range from complete renal agenesis to diseases such as hypertension that exist in the setting of grossly normal kidneys. In this review, we focus on the growth and transcription factors that define kidney progenitor cell populations, initiate ureteric bud branching, induce nephron formation within the metanephric mesenchyme, and differentiate stromal and vascular progenitors in the metanephric mesenchyme.
Keywords: Cre/loxP system, Kidney, Development, Metanephric mesenchyme, Ureteric bud
Introduction
The development of an organism requires the precise spatiotemporal coordination of cell behavior and is therefore highly dependent on the communication among cells. The reciprocal inductive interactions between epithelial cells and adjacent mesenchymal cells are of particular importance during embryogenesis, because they result in cellular differentiation and the formation of tissues and organs. Specifically, in kidney development, the organization of polarized mesenchyme into epithelialized tubules is a characteristic feature, and is central to its physiological functions. Overall, kidney morphogenesis appears as a self-regulating process, whereby kidney function orchestrates multiple, mutually dependent cellular processes within the developing nephron [1].
In recent years, the application of new techniques to study the genes or factors involved in renal morphogenesis has represented a key advancement in nephrology, leading to better understanding of renal development during prenatal and postnatal life [2]. These techniques have also provided important insights into the mechanisms of diseases that result from alteration of the morphogenesis program [3].
In this review, we focus on molecular pathways that regulate the process of mesenchyme-to-epithelial transition (MET), the main development responsible for the origin of the nephron, the functional unit of the kidney. Progress in our understanding of the cellular and molecular mechanisms of kidney development may provide methods for the improved diagnosis of renal anomalies and targets for the prevention and treatment of kidney disease.
Kidney-specific Gene Targeting
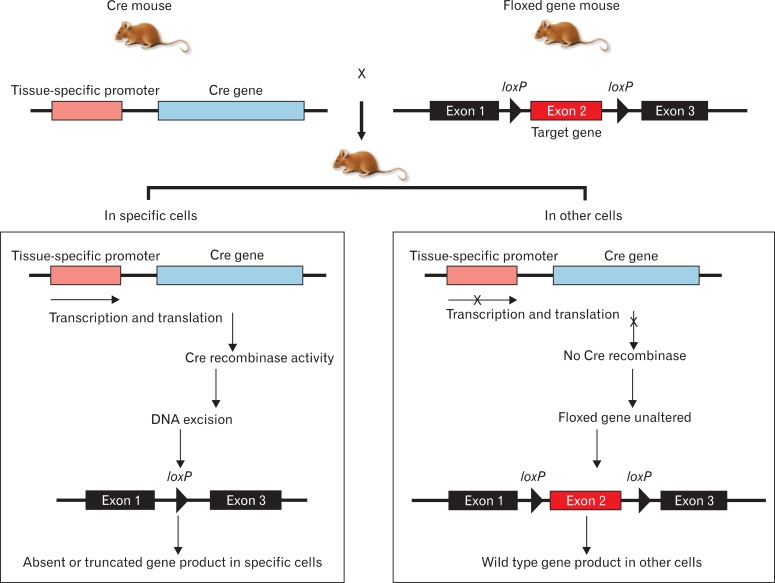
Genetic engineering in mice has proven to be a useful tool for assessing the role of specific gene products in renal health and disease. The most widely used strategy to limit gene targeting to specific tissue or cell types utilizes the Cre/loxP system [2]. Cre recombinase (cyclization recombination) is a site-specific DNA recombinase from bacteriophage P1 that mediates genetic recombination at specific 34 bp recognition sites, called loxP [4]. As illustrated in Fig. 1, any DNA sequence flanked by two loxP sites in the same orientation will be excised. A major advantage of the Cre/loxP system lies in its relative simplicity. The success of kidney-specific targeting is dependent on the availability of different Cre transgenic lines and the efficiency of the lineage-specific DNA excision. There are a number of kidney-specific Cre mouse strains currently available, which potentially allow for gene targeting in the podocyte, proximal tubule (PT), thick ascending limbs, juxtaglomerular cells, principle cells, and intercalated cells of the collecting duct [5, 6]. Much of this early work in kidney development is summarized in a classic monograph by Saxen and Sariola [7]. In recent years, new techniques have given us outlines of the genetic program that regulates organogenesis and suggested roles for several signaling molecules, growth factors, receptors, transcription factors, and extracellular matrix com ponents. We discuss the signaling molecules, growth and transcriptional factors, and cell membrane receptors that are necessary for the cascade of events to occur to form a kidney and that are targeted to date using the kidney-specific Cre/loxP strategy.
Fig. 1.
Kidney-specific Cre/loxP recombination. Cre recombinase is expressed under the control of a kidney-specific promoter (left), and two loxP sites are inserted in the introns flanking an essential exon of a gene of interest (right). In the kidney, Cre recombinase will be expressed and will excise the DNA sequence between the loxP sites, which will inactivate the gene. In all other tissues, Cre will not be expressed and the gene will remain active.
Overview of Kidney Development
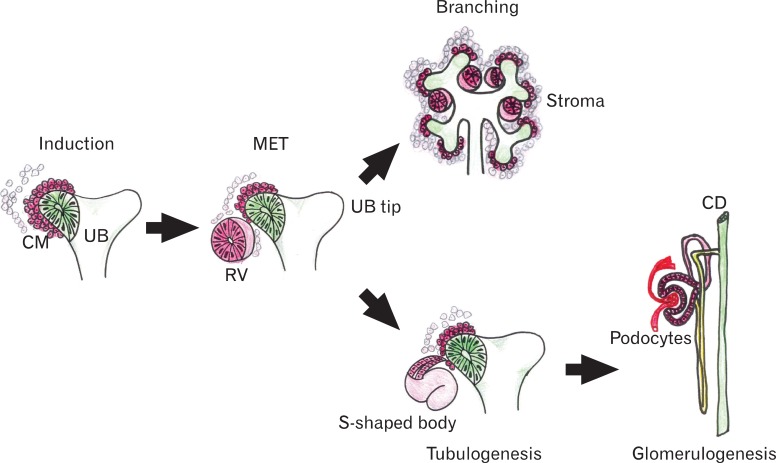
In mammals, the kidney derives from the intermediate mesoderm located between the axial mesoderm and lateral plate mesoderm. It is likely that the specification of the mesoderm involves genes participating in the control of the mediolateral and anteroposterior patterning signals [8]. Three different kidney systems are formed during intrauterine life in mammals including humans; the pronephros, the mesonephros, and the metanephros [9, 10]. The first two kidney systems lead to the formation of transient structures, and the metanephros forms the permanent kidney [11]. The metanephros begins to develop from the ureteric bud (UB), a branching epithelial tube originating from the Wolffian duct (WD) (or mesonephric duct), and the mesenchymal cells that originate from the intermediate mesoderm. Kidney organogenesis depends on a series of reciprocal inductive interactions between the UB and metanephric mesenchyme (MM). The different morphological steps of metanephric kidney development are schematically shown in Fig. 2. Signals from the MM initiate kidney development by inducing formation of the UB from the WD. The UB invades the MM and undergoes a series of repetitive branching under the influence of mesenchymal signals. In turn, the newly formed UB induces the MM that surrounds it to condensate and aggregate around its tips, transforming themselves into the cap mesenchyme (CM). The CM successively differentiates into pre-tubular aggregates, and these structures undergo MET to form renal vesicles (RVs), which proliferate to give rise to comma- and S-shaped bodies (SBs), and then nephrons (Fig. 3). At the same time, a part of the uninduced mesenchyme adopts the stromal pathway, a cell type involved in structural support and nephron maturation [12, 13]. Parallel to this differentiation process, the distal part of the SBs fuses with the collecting ducts, and the proximal parts of these structures become highly vascularized and form glomeruli.
Fig. 2.
Scheme of renal developmental processes. Kidney organogenesis depends on a series of reciprocal inductive interactions between the ureteric bud (UB) and metanephric mesenchyme (MM). Signals from the MM initiate kidney development by inducing formation of the UB from the Wolffian duct. The UB invades the MM and undergoes a series of repetitive branchings under the influence of mesenchymal signals. In turn, the newly formed UB induces the MM that surrounds it to condense around its tips. The condensed mesenchyme (CM) successively differentiates into pre-tubular aggregates, and these structures undergo a mesenchymal-to-epithelial transition (MET) to form renal vesicles (RVs), which then proliferate to give rise to comma- and S-shaped bodies, and then nephrons. Parallel to this differentiation process, the distal part of the S-shaped bodies fuses with collecting ducts, and the proximal parts of these structures become highly vascularized and form glomeruli.
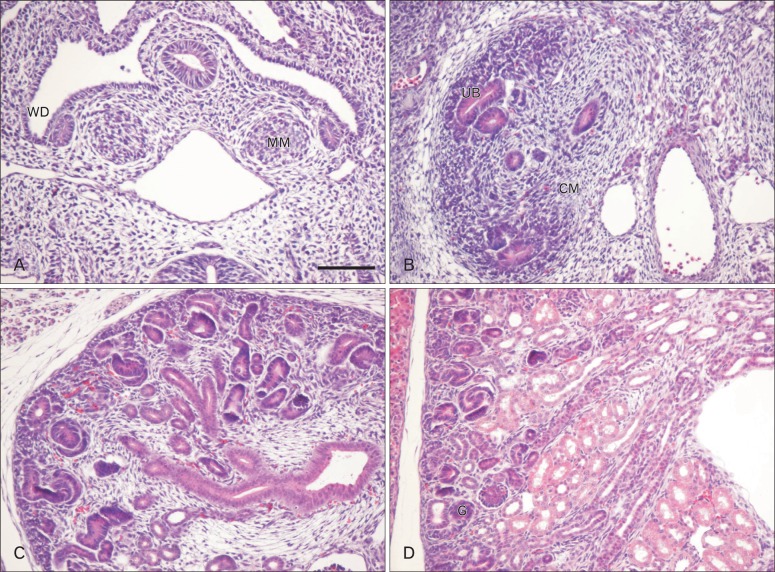
Fig. 3.
Time course of renal morphogenesis in wild-type mice. Kidneys from E10.5 (A), E12.5 (B), and E15.5 (C) embryos, and P0 (D) mouse were stained with hematoxylin and eosin. The ureteric bud (UB) is derived from the Wolffian duct (WD), and the metanephric mesenchyme (MM) is formed from the intermediate mesoderm. The condensed mesenchyme (CM) successively differentiates into the renal vesicle, which then proliferates to give rise to comma- and S-shaped bodies and glomeruli (G). Scale bar in (A)=50 µm (A.D).
It was generally believed that most of the epithelial cells of the nephron were derived from the MM, whereas the UB epithelium generates the collecting ducts and the most distal tubules (DTs). However, all the mesenchyme does not become induced and converted to epithelium, with some cells contributing to the interstitial mesenchyme or stroma, and others generating endothelial cells of the renal vasculature. Mesenchyme-derived epithelial cells become highly specialized and express markers specific for glomerular podocytes, PTs, cells of the ascending and the descending limbs of Henle's loop, and DTs.
Patterning of the Intermediate Mesoderm
In mammalian embryos, a number of marker genes are expressed in the lateral mesoderm and become restricted to the intermediate mesoderm prior to any morphological indication of urogenital development. The activation of such markers is the first indication that the lateral and subsequent intermediate mesoderms are differentiated from surrounding cells [14].
Odd skipped related1 (Odd1 or Osr1) is one of the earliest genetic markers of the kidney progenitor cell in mice [15, 16] and is first activated in these cells [17]. At E8.5-E9.5, Odd1 transcripts are found throughout the intermediate mesoderm/nephrogenic cord and in the MM, but appear in the UB only at E10.5 [16, 17]. In Odd1 null embryos, neither the UB nor a morphologically distinct population of MM can be detected, and renal agenesis occurs [17]. Odd1 mutants completely lacked the eyes absent homolog 1 (Eya1) and paired box gene 2 (Pax2) in the metanephric region at E9.5, and Odd1 expression was required for the expression of other transcription factors, including LIM-class homeodomain transcription factor Lim1 (or Lhx1), Eya1, Pax2, homeobox family members Six1, 2, and 4, Sall1, WT-1, and glial-derived neurotrophic factor (Gdnf), that lead to UB outgrowth [18-21], indicating that Odd1 acts upstream of these pathways [16]. The Lim1 gene is expressed in the visceral endoderm, and lateral and intermediate mesoderm [22]. Subsequently, Lim1 expression is restricted to the nephric duct, mesonephric tubules, and a part of the developing metanephros. Pax2 and Pax8 are expressed after Odd1 and Lim1 activation in a pattern more consistent with intermediate mesoderm specification. Several transcriptional regulators seem to regulate MM specification by complex regulation of DNA binding and transcriptional activation versus repression. In the MM, Eya-Hox-Pax [23] and Eya-Six-Pax [24] complexes are presumed to coordinate specification resulting from the coexpression of these regulators. The Eya-Hox-Pax complex acts as a direct activator of Six2 as well as Gdnf. These factors are required for nephron development and the loss of their functions in the developing MM results in either renal agenesis or hypoplasia.
Outgrowth and Branching of UB
The UB emerges as a single outgrowth from a site of the WD caudal to the hindlimb (Figs. 2, 3). Gdnf is expressed broadly throughout the nephrogenic cord at E9.5, but becomes restricted to the region of the MM by E10.5 [23]. The Gdnf/Ret pathway is a critical regulator of UB outgrowth and branching. Gdnf secreted by the MM activates a Gfra1/Ret receptor-tyrosine kinase complex and triggers outgrowth of Ret-positive cells from the nephric duct toward the Gdnf signal [18, 19]. The deficiency of Gdnf and Ret or Gfra1 in mice results in failure to form a UB, and prenatal death occurs with agenesis of the kidneys and ureters [20, 25, 26].
Other pathways, including Wnt, sonic hedgehog (Shh), bone morphogenic protein (Bmp), and fibroblast growth factor (Fgfs), that control UB branching have a central role in these signaling mechanisms within the MM and in UB branching [27, 28]. Wnt is a transmembrane Frizzled receptor ligand [29]. The interaction of Wnt with Frizzled and co-receptors lipoprotein receptor-related proteins 5/6 recruits Disheveled to the cell membrane. The activated Disheveled inhibits glycogen synthase kinase 3β that phosphorylates β-catenin and marks it for degradation. Thus, canonical signaling by Wnt results in stabilization of β-catenin, which translocates to the nucleus to initiate the transcription of downstream mediators [30]. Canonical Wnt signaling plays a role in induction of the MM and in UB bran ching [31, 32]. Loss of β-catenin in the UB lineage and nephron progenitors, or inhibition of canonical Wnt signaling by Dickkopf1 resulted in renal agenesis and hypoplasia [31-34].
Shh is an inhibitory ligand for the Patched receptor, which constitutively inhibits Smoothened [35]. Shh signaling directly controls the expression of three distinct classes of genes that are required for normal renal development, namely, early kidney inductive and patterning genes (Pax2 and Sall1), cell cycle modulators (CyclinD1 and N-myc), and the signaling effectors of the hedgehog (Hh) pathway (Gli1 and Gli2) [36]. In the absence of Hh binding, the repressor Gli3 is instead dominant. Deletion of Shh from the UB lineage resulted in hypoplastic kidneys with hydronephrosis and hydroureter [37].
Members of the transforming growth factor β (TGFβ) superfamily are Bmps, activin, and growth/differentiation factor, which act as ligands for transmembrane serine-threonine kinase receptors, the activin-like receptor kinases [38, 39]. TGFβ and activin result in phosphorylation of receptor-associated Smads (R-Smads) 2 and 3, whereas the Bmps elicit phosphorylation of R-Smads1, 5, and 8 [40].
Bmp signaling plays a key role in UB branching. Bmp2, Bmp4, and Bmp7 are expressed in the mesenchyme surrounding the tips of the UB, and stromal cells surrounding the nephric duct and the stalk of the UB outgrowth [41, 42]. Complete loss of both Bmp7 expression and inhibition of Bmp by Gremlin results in renal agenesis [43]. Deletion of the Bmp2 inhibits UB branching in vivo [44]. UB-specific deletion of Smad4 does not have an effect on UB branching, indicating that Bmps must exert their effects via a Smad4-independent pathway [39].
The family of Fgfs bind the receptor tyrosine kinases (Fgfr1 and 2), and interaction between the Fgf and receptor stimulates homodimerization and phosphorylation of the receptor, which leads to recruitment of the growth factor receptor-bound protein 2 adaptor, activation of Ras GTP proteins and extracellular signal-regulated kinase, and cell proliferation [45]. Fgfr1 and Fgfr2 in mesenchyme are critical for early MM and UB formation, and deletion of both factors in the MM results in renal aplasia with defects in MM formation and initial UB elongation and branching [46]. The deletion of Fgf7 and Fgf10, and their receptor Fgfr2-IIIb leads to smaller kidneys with fewer collecting ducts and nephrons [46]. Deletion of Fgfr2 in MM stromal cells results in formation of more than one UB, leading to duplicated collecting systems and hydroureter [47]. Deletion of Fgfr from UB results in very aberrant UB branching and fewer UB tips [48].
Recently, a role of p53 has been revealed in the regulation of metanephric development. Kidneys of p53 knockout embryos exhibited a spectrum of congenital abnormalities, including UB ectopia, double ureters/collecting systems, and hypoplastic metanephros [49].
The Cap Mesenchyme
While epithelial cords originating from the UB are branching into the MM, self-renewing progenitor cells [50] condensate and aggregate around the UB tips, transforming themselves into the cap mesenchymal cells. The CM progressively undergoes MET, which will form most of the epithelia of the nephron [13, 14].
Gene-targeting experiments with promoter-specific Cre mice have shown that Six2 and Cited1 are expressed by the nephron-committed, multipotent, self-renewing progenitor population responsible for generating all segments of the nephron, from podocyte to connecting segment [51, 52]. Six2 is expressed by the CM and functions to suppress the ectopic formation of pre-tubular aggregates on the distal side of the UB tip [52, 53]. Deletion of Six2 activity results in the premature MET of all CM to RV on the initial ingrowth of the UB. As a result, nephrogenesis ceases at an early stage [53]. Six2 is thus a critical regulator of the CM progenitor state. Six2 and Wnt regulate the self-renewal and commitment of nephron progenitors through shared gene regulatory networks [54].
Wt1 has been proposed as a master control gene that regulates the expression of a large number of genes that have a critical role in kidney development [55], as shown by the occurrence of renal agenesis in mouse embryos when it was lacking [56]. The Wt1 expression increases in the CM progenitors and promotes differentiation toward the epithelial phenotype. The Wt1 expression pattern suggests a main role in the regulation of the MET process and maturation of podocytes [57].
Among the target genes for Wt1, an important role has been assigned to Bmp7 [58], which is highly expressed during early embryonic kidney development in the renal progenitor cells [59], the UB cells, and in mature podocytes [60]. Bmp7 null embryos show poorly developed kidneys characterized by oligonephronia, due mainly to the premature loss of the progenitor population [59].
Fgf8 is expressed in the CM and may play an important role in nephron induction and maintenance of the renal progenitor population in the CM [61]. Fgf8 is also required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons [62].
Mesenchymal-to-Epithelial Transition
The CM undergoes MET to form RVs at E12.5, which then proliferate to give rise to SBs that fuse with the collecting duct epithelium (Figs. 2, 3) [63]. By E13.5, the SBs become infiltrated by endothelial precursors to form the glomerular tuft, which consists of the capillary loops, mesangium, glomerular basement membrane (GBM), and podocyte.
Six2 and Cited2 showed a decreased expression, whereas the Lim1 and Fgf8 expressions were found to increase progressively [64]. Wnt4 has the ability to induce the MET of the pre-tubular aggregation of CM to become the RV [65]. Cap mesenchymal cells respond to Wnt4 signaling by differentiating into the RV, a simple tubule undergoing extensive growth, segmentation, and differentiation that will end with the origin of the mature proximal nephron [66].
Proximal-Distal Patterning of the Resulting Tubules
The formation of an RV is only a beginning in terms of this tubulogenic event. The RV needs to elongate, bend, segment/pattern, and differentiate to generate a functional, mature nephron within the adult kidney. Ultimately, each nephron is segmented and patterned along its proximal-distal axis into a renal corpuscle (RC), followed by PTs, loop of Henle (LH), DTs, and connecting tubule (CT) segments. These are connected to the collecting ducts (CDs) to form a continuous uriniferous tubule [67, 68]. Individual tubular segments can be distinguished at the molecular, cellular, and anatomical levels, with each segment ultimately conferred a specific functional role in the regulation of electrolyte and water balance. Despite that this segmentation of the nephron is critical for normal function, and the absence of particular nephron segments can result in a variety of diseases in humans [69], the molecular programs governing nephron segmentation and proximal-distal polarity remain poorly understood.
Proximal-distal patterning along the length of the nephron begins very early in the life of a nephron. This is readily evident as early as the SB stage, which can be divided into distinct proximal, medial, and distal segments. The proximal segment is further organized into two distinct epithelial layers, the parietal (Bowman's capsule) and visceral (podocyte) layers, which will become the RC [70].
The patterning of PTs requires the Notch signaling pathway [70]. Notch1, Notch2, and the ligands Dll1 and Jag1 are all expressed within the RV [71]. The loss of Notch2 and Jag1 in mutant mice results in the loss of proximal nephron segments and also the capillaries and mesangium of RCs [72, 73]. The endocytic receptor in the PT, megalin, is important for the reabsorption of macromolecules filtered by the glomerulus. Mice with a kidney-specific megalin defect develop normally but exhibit multiple defects in vitamin and mineral handling [74, 75]. Megalin deficiency in the PT is associated with low-molecular-weight proteinuria, increased urinary loss of vitamin D-binding protein, systemic hypovitaminosis, hypocalcemia, and osteomalacia [76]. The type-II sodium phosphate co-transporter (NaPi-IIa) is located in the PT brush borders. The internalization of NaPi-IIa from the brush border membrane in response to the parathyroid hormone is also impaired in the absence of megalin [77].
Umod encodes the glycoprotein uromodulin, and its expression is widely used as a marker of the adult DT and LH segments [78]. Umod knockout mice do not display a phenotype [79], but Umod mutation in humans results in hyperuricemic nephropathy and medullary cystic kidney disease type 2 [80].
The endothelin (ET) system has been implicated in the regulation of systemic blood pressure through multiple mechanisms. Inactivation of the peptide ET1 in the CDs of mice leads to hypertension, impaired ability to excrete sodium and water loads, and enhanced vasopressin responsiveness [81]. The apical plasma membrane water channel aquaporin-2 (Aqp2), present in the CTs and the CD principal cells, is the chief target for regulating the water permeability of these segments by vasopressin. Mice lacking Aqp2 in only the CD cells survive to adulthood, but show marked polyuria and severe urinary-concentrating defect [82]. CD-specific inactivation of the nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ) in mice provided evidence that apical epithelial sodium channel (ENaC)-γ subunit transcription and ENaC-mediated sodium reabsorption are PPARγ-sensitive [37]. Inactivation of the signaling protein Shh in the murine CD epithelium results in renal hypoplasia, hydronephrosis, and hydroureter [83].
Patterning and Vascularization of the Glomerulus
Glomerular developments need to undergo sequential stages. The SBs are infiltrated by endothelial cells to generate a primitive vascular tuft within the cup-shaped glomerular precursor region. The vascular epithelial cells, podocyte precursors, contact the endothelial cells and begin to differentiate. The GBM is then formed at the boundary between podocytes and endothelial cells for normal renal function. Podocytes begin to extend primary and secondary foot processes [84], extending foot processes of podocytes interdigitate to create a unique type of tight junction, the slit diaphragm [84].
The forming podocytes express vascular endothelial growth factor-A (Vegf-A). The expression of Vegf and its receptors is temporally and spatially associated with kidney vascularization and identified angioblasts expressing Flt-1 and Flk-1 in prevascular embryonic kidneys. These data suggest that Vegf plays a critical role in renal development by promoting endothelial cell differentiation, capillary formation, and proliferation of tubular epithelia [85, 86]. No podocyte expression of Vegf-A causes severe glomerulovascular defects. Overexpression of Vegf in podocytes leads to collapsing glomerulopathy [86].
The development of the mesangium and coincident formation of the glomerular capillary network are reliant on the expressions of platelet-derived growth factor beta (Pdgfb) and Pdgf receptor β (Pdgfrβ). Genetic ablation of Pdgfb and Pdgfrβ results in defective recruitment of mesangial cells into the developing glomerulus, and the glomerular vasculature develops as one or a few dilated capillary loops [87]. The correct dosage of podocyte expression of Bmp4 during podocyte differentiation is also essential for proper glomerular tuft development. The loss of Bmp4 function in podocytes results in glomerular microaneurysms and collapsed glomerular capillary tufts. The same occurs with podocyte-specific expression of Noggin, a Bmp antagonist. Overexpression of Bmp4 in the podocytes, however, results in the absence of endothelia in the tuft [88].
The proteins Kreisler and Glepp1 are involved in podocyte differentiation and maturation. Mutations of these genes in mice lead to poorly formed podocyte foot processes, and in the case of Kreisler, proteinuria [89, 90]. Genetic mutations for the slit diaphragm proteins nephrin (Nphs1) and podocin (Nphs2) in humans show congenital nephrotic syndrome [91]. Knockout of podocin in mice leads to defects in glomerular capillary formation and mesangial cell recruitment, underlying the co-dependence of all the components of the glomerular turf for normal development [92].
Attachment of podocytes to the underlying GBM involves the transmembrane receptors integrin α3β1 and dystroglycan. These proteins bind GBM matrix molecules via their extracellular domain and are coupled to the actin cytoskeleton intracellularly. Genetic mutation of integrin α3 or podocyte-specific loss of integrinβ1 results in neonatal proteinuria and early renal failure [93, 94]. Collagen IV (Col4) and laminins are the major protein contributors to the GBM [95]. Col4a3, Col4a4, and Col4a5 are the mature collagens of the mature GBM. A mutation in either Col4a3 or Col4a4 also results in benign familial hematuria due to a thinning of the GBM [96].
Conclusion
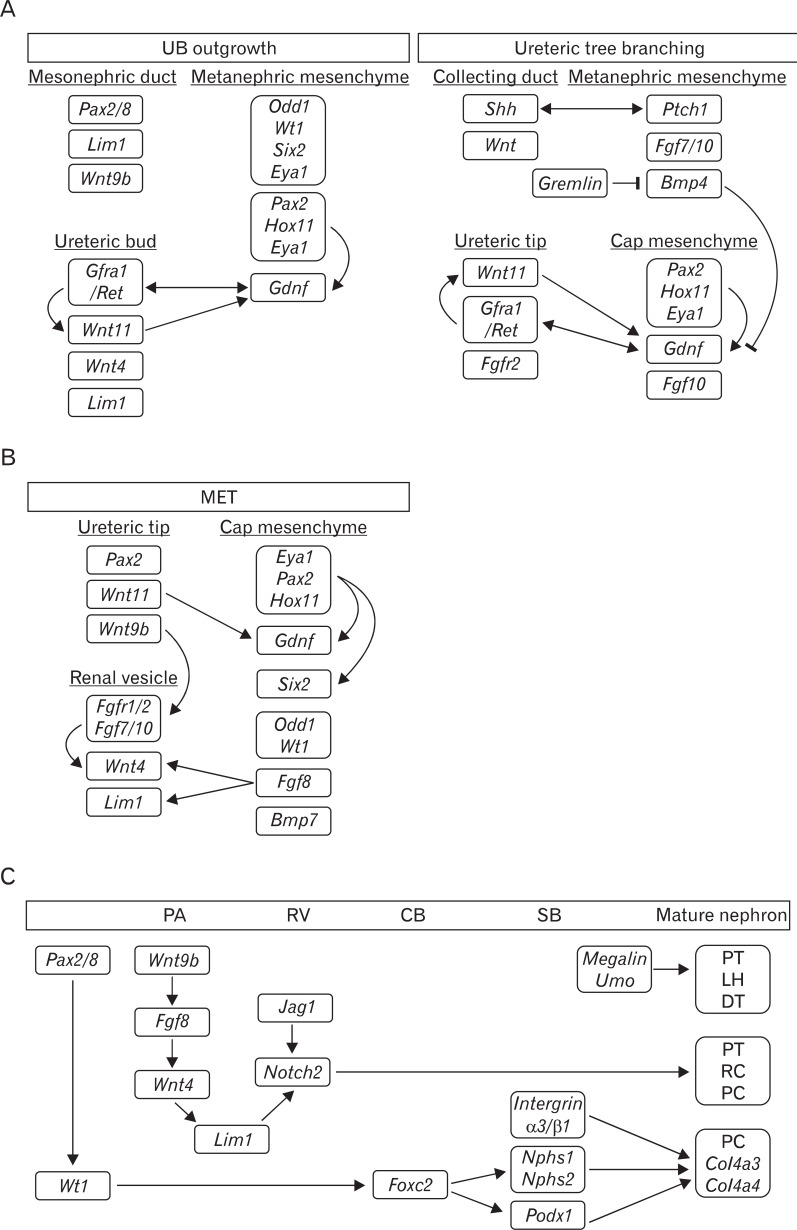
Genetic engineering in mice has provided much information about gene function in the field of developmental biology. Recently, conditional gene targeting using the Cre/loxP system has been developed to control the cell type and timing of target gene expression. The increasing number of kidney-specific Cre mice allow for the analysis of phenotypes that cannot be addressed by conventional gene targeting. In summary, this review described the molecular basis of kidney morphogenesis. As illustrated in Fig. 4, there is a multitude of molecules that are dispatched and engaged in complex interactions to drive kidney development in a concerted mode of action. Some of the factors act as repressors and others as activators, whereas some display dual effects depending on the temporal and spatial context. Genes that regulate kidney development and kidney phenotype are summarized (Table 1). Information from mouse models point to the importance of well-balanced spatiotemporally controlled molecular activities, as both deficiencies and overactivation impede development. There is no doubt that major advances from mouse models have been achieved and have contributed to a better understanding of the molecular and cellular mechanisms that regulate nephrogenesis. However, we still know relatively little about the regulation and targets of many molecules identified as playing pivotal roles during nephrogenesis.
Fig. 4.
Molecular signaling pathways involved in ureteric bud outgrowth and branching (A), mesenchymal-to-epithelial transition (MET) (B), and nephron patterning (C). (A) Molecules and growth factors (Gdnf and Fgfs) from the adjacent cells of the metanephric mesenchyme bind to a variety of receptor-tyrosine kinases (Ret and Fgfr) on the surface of the ureteric tip cell, triggering signaling cascades that regulate cell proliferation, migration, and extracellular matrix degradation. The combined actions of these signaling pathways are continued branching and elongation of the ureteric epithelium to form the collecting duct system. (B) Just as Gdnf/Ret signaling has been central to branching, Wnt signaling is key to kidney development, with both Wnt4 and Wnt9b being involved in MET. In addition to Wnt, Fgfs, Wt1, and Odd1, there are other factors required for MET. Fgf8 is ex pressed earlier than Wnt4 and is ne cessary for both Wnt4 and Lim1. A tripartite complex between Eya1, Pax2, and Hox11 positively regulates the expression of Gdnf and Six2. (C) The transcriptional hierarchy of genes governing nephron pattering is shown. Wnt9b initially activates the expression of Fgf8, Wnt4, and Pax8 in the pre-tubular aggregate (PA), and then Wnt4 maintains the expression of these genes and induces Lim1. Wt1 is restricted to the proximal S-shaped body (SB) and maintained in the podocytes throughout all stages of nephrogenesis. Notch2 is required in the patterning of the pro ximal nephron. In addition, Megalin, Umo, integrin α3/β1, and Nphs play critical roles in the patterning of the loop of Henle (LH), distal tubule (DT), and the formation of functional filters. CB, comma-shaped body; PC, podocyte cells; PT, proximal tubule; RV, renal vesicle; UB, ureteric bud.
Table 1.
Genes that regulate kidney development and kidney phenotype
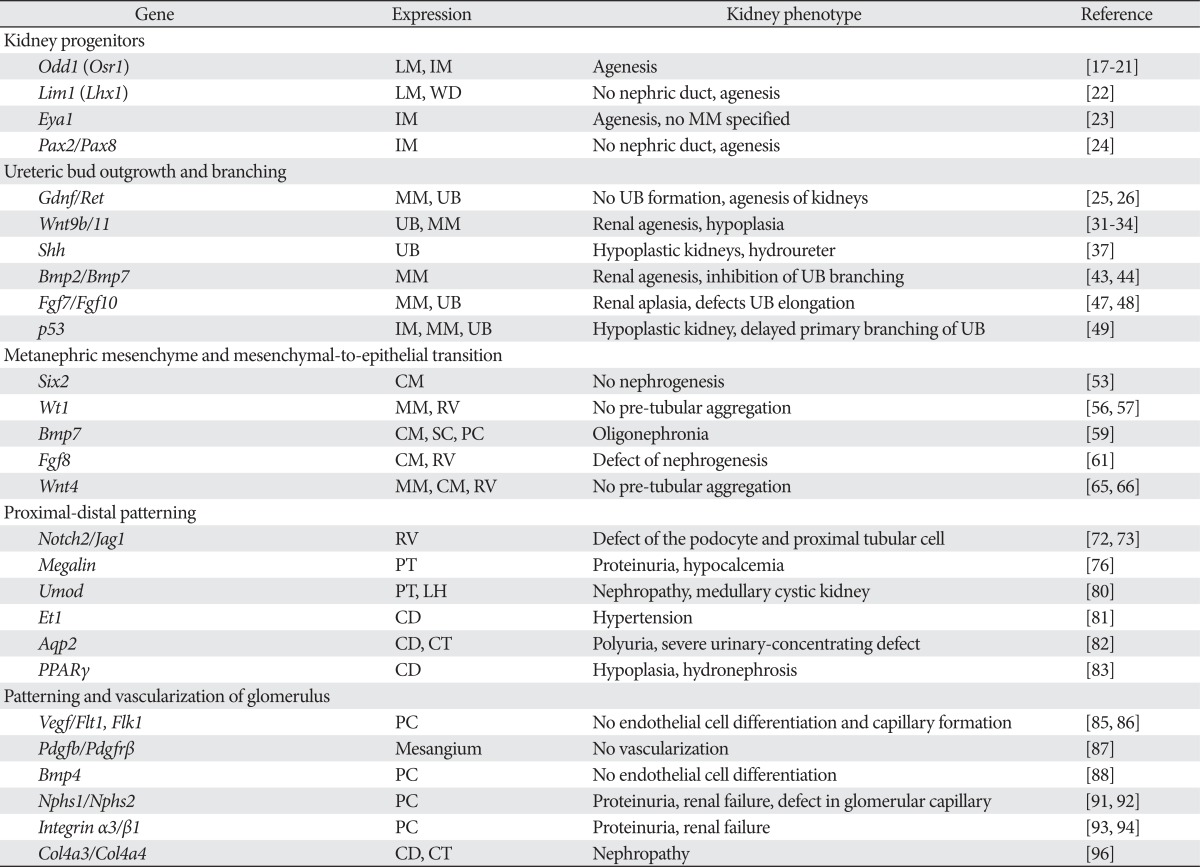
CD, collecting duct; CM, cap mesenchyme; CT, connecting tubule; IM, intermediated mesoderm; LH, loop of Henle; LM, lateral mesoderm; MM, metanephric mesenchyme; PC, podocyte cells; PT, proximal tubule; RV, renal vesicle; SC, stromal cells; UB, ureteric bud; WD, Wolffian duct.
There are so many reasons to study kidney development, but most importantly, developmental studies have provided significant insight into the realm of clinical disease. The methods and discoveries of developmental biology, and the knowledge acquired about the molecular basis of kidney morphogenesis will certainly assist in the diagnosis and treatment of renal disease as well as allow the regulation or reinitiation of nephrogenesis in the future.
Acknowledgements
This paper was supported by funding from the Biomedical Research Institute, Chonbuk National University Hospital.
References
- 1.Faa G, Gerosa C, Fanni D, Monga G, Zaffanello M, Van Eyken P, Fanos V. Morphogenesis and molecular mechanisms involved in human kidney development. J Cell Physiol. 2012;227:1257–1268. doi: 10.1002/jcp.22985. [DOI] [PubMed] [Google Scholar]
- 2.Kohan DE. Progress in gene targeting: using mutant mice to study renal function and disease. Kidney Int. 2008;74:427–437. doi: 10.1038/ki.2008.146. [DOI] [PubMed] [Google Scholar]
- 3.Little M, Georgas K, Pennisi D, Wilkinson L. Kidney development: two tales of tubulogenesis. Curr Top Dev Biol. 2010;90:193–229. doi: 10.1016/S0070-2153(10)90005-7. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981;150:467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- 5.Wu F. Conditional targeting in the kidney. Nephron Physiol. 2007;107:p10–p16. doi: 10.1159/000106483. [DOI] [PubMed] [Google Scholar]
- 6.Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD. V-ATPase B1-subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol. 2005;288:C1134–C1144. doi: 10.1152/ajpcell.00084.2004. [DOI] [PubMed] [Google Scholar]
- 7.Saxén L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- 8.Hoch M, Broadie K, Jäckle H, Skaer H. Sequential fates in a single cell are established by the neurogenic cascade in the Malpighian tubules of Drosophila. Development. 1994;120:3439–3450. doi: 10.1242/dev.120.12.3439. [DOI] [PubMed] [Google Scholar]
- 9.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012;4:pii: a008300. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reidy KJ, Rosenblum ND. Cell and molecular biology of kidney development. Semin Nephrol. 2009;29:321–337. doi: 10.1016/j.semnephrol.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuure S, Vuolteenaho R, Vainio S. Kidney morphogenesis: cellular and molecular regulation. Mech Dev. 2000;92:31–45. doi: 10.1016/s0925-4773(99)00323-8. [DOI] [PubMed] [Google Scholar]
- 12.Bouchard M. Transcriptional control of kidney development. Differentiation. 2004;72:295–306. doi: 10.1111/j.1432-0436.2004.07207001.x. [DOI] [PubMed] [Google Scholar]
- 13.Ribes D, Fischer E, Calmont A, Rossert J. Transcriptional control of epithelial differentiation during kidney development. J Am Soc Nephrol. 2003;14(Suppl 1):S9–S15. doi: 10.1097/01.asn.0000067647.05964.9f. [DOI] [PubMed] [Google Scholar]
- 14.Barak H, Rosenfelder L, Schultheiss TM, Reshef R. Cell fate specification along the anterior-posterior axis of the intermediate mesoderm. Dev Dyn. 2005;232:901–914. doi: 10.1002/dvdy.20263. [DOI] [PubMed] [Google Scholar]
- 15.Mudumana SP, Hentschel D, Liu Y, Vasilyev A, Drummond IA. Odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development. 2008;135:3355–3367. doi: 10.1242/dev.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133:2995–3004. doi: 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol. 2005;288:582–594. doi: 10.1016/j.ydbio.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sajithlal G, Zou D, Silvius D, Xu PX. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol. 2005;284:323–336. doi: 10.1016/j.ydbio.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cirio MC, Hui Z, Haldin CE, Cosentino CC, Stuckenholz C, Chen X, Hong SK, Dawid IB, Hukriede NA. Lhx1 is required for specification of the renal progenitor cell field. PLoS One. 2011;6:e18858. doi: 10.1371/journal.pone.0018858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato A, Matsumoto Y, Koide U, Kataoka Y, Yoshida N, Yokota T, Asashima M, Nishinakamura R. Zinc finger protein sall2 is not essential for embryonic and kidney development. Mol Cell Biol. 2003;23:62–69. doi: 10.1128/MCB.23.1.62-69.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodbeck S, Besenbeck B, Englert C. The transcription factor Six2 activates expression of the Gdnf gene as well as its own promoter. Mech Dev. 2004;121:1211–1222. doi: 10.1016/j.mod.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, Kania A, Jessell TM, Behringer RR, Tam PP. Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev Biol. 2000;223:77–90. doi: 10.1006/dbio.2000.9733. [DOI] [PubMed] [Google Scholar]
- 23.Gong KQ, Yallowitz AR, Sun H, Dressler GR, Wellik DM. A Hox-Eya-Pax complex regulates early kidney developmental gene expression. Mol Cell Biol. 2007;27:7661–7668. doi: 10.1128/MCB.00465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodbeck S, Englert C. Genetic determination of nephrogenesis: the Pax/Eya/Six gene network. Pediatr Nephrol. 2004;19:249–255. doi: 10.1007/s00467-003-1374-z. [DOI] [PubMed] [Google Scholar]
- 25.Davies JA, Fisher CE. Genes and proteins in renal development. Exp Nephrol. 2002;10:102–113. doi: 10.1159/000049905. [DOI] [PubMed] [Google Scholar]
- 26.Chai L. The role of HSAL (SALL) genes in proliferation and differentiation in normal hematopoiesis and leukemogenesis. Transfusion. 2011;51(Suppl 4):87S–93S. doi: 10.1111/j.1537-2995.2011.03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nystrom J, Hultenby K, Ek S, Sjölund J, Axelson H, Jirström K, Saleem MA, Nilsson K, Johansson ME. CRIM1 is localized to the podocyte filtration slit diaphragm of the adult human kidney. Nephrol Dial Transplant. 2009;24:2038–2044. doi: 10.1093/ndt/gfn743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwata T, Miyata Y, Kanda S, Nishikido M, Hayashi T, Sakai H, Kanetake H. Lymphangiogenesis and angiogenesis in conventional renal cell carcinoma: association with vascular endothelial growth factors A to D immunohistochemistry. Urology. 2008;71:749–754. doi: 10.1016/j.urology.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 29.Nikopoulos GN, Martins JF, Adams TL, Karaczyn A, Adams D, Vary C, Oxburgh L, Verdi JM. NRAGE: a potential rheostat during branching morphogenesis. Mech Dev. 2009;126:337–349. doi: 10.1016/j.mod.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marty MS, Neeper-Bradley TL, Neptun DA, Carney EW. Developmental toxicity of diethanolamine applied cutaneously to CD rats and New Zealand White rabbits. Regul Toxicol Pharmacol. 1999;30:169–181. doi: 10.1006/rtph.1999.1308. [DOI] [PubMed] [Google Scholar]
- 31.Benz K, Campean V, Cordasic N, Karpe B, Neuhuber W, Mall G, Hartner A, Hilgers KF, Amann K. Early glomerular alterations in genetically determined low nephron number. Am J Physiol Renal Physiol. 2011;300:F521–F530. doi: 10.1152/ajprenal.00490.2009. [DOI] [PubMed] [Google Scholar]
- 32.Fukushi Y, Orikasa S, Shepard T, Hakomori S. Changes of Lex and dimeric Lex haptens and their sialylated antigens during development of human kidney and kidney tumors. J Urol. 1986;135:1048–1056. doi: 10.1016/s0022-5347(17)45973-8. [DOI] [PubMed] [Google Scholar]
- 33.Jain S. The many faces of RET dysfunction in kidney. Organogenesis. 2009;5:95–108. doi: 10.4161/org.5.4.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah MM, Sakurai H, Sweeney DE, Gallegos TF, Bush KT, Esko JD, Nigam SK. Hs2st mediated kidney mesenchyme induction regulates early ureteric bud branching. Dev Biol. 2010;339:354–365. doi: 10.1016/j.ydbio.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cain JE, Rosenblum ND. Control of mammalian kidney development by the Hedgehog signaling pathway. Pediatr Nephrol. 2011;26:1365–1371. doi: 10.1007/s00467-010-1704-x. [DOI] [PubMed] [Google Scholar]
- 36.Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, Rosenblum ND. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development. 2006;133:569–578. doi: 10.1242/dev.02220. [DOI] [PubMed] [Google Scholar]
- 37.Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- 38.Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001;187:265–276. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- 39.Oxburgh L, Chu GC, Michael SK, Robertson EJ. TGFbeta superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development. 2004;131:4593–4605. doi: 10.1242/dev.01324. [DOI] [PubMed] [Google Scholar]
- 40.Bracken CM, Mizeracka K, McLaughlin KA. Patterning the embryonic kidney: BMP signaling mediates the differentiation of the pronephric tubules and duct in Xenopus laevis. Dev Dyn. 2008;237:132–144. doi: 10.1002/dvdy.21387. [DOI] [PubMed] [Google Scholar]
- 41.Godin RE, Robertson EJ, Dudley AT. Role of BMP family members during kidney development. Int J Dev Biol. 1999;43:405–411. [PubMed] [Google Scholar]
- 42.Carev D, Saraga M, Saraga-Babic M. Involvement of FGF and BMP family proteins and VEGF in early human kidney development. Histol Histopathol. 2008;23:853–862. doi: 10.14670/HH-23.853. [DOI] [PubMed] [Google Scholar]
- 43.Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131:3401–3410. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- 44.Hartwig S, Hu MC, Cella C, Piscione T, Filmus J, Rosenblum ND. Glypican-3 modulates inhibitory Bmp2-Smad signaling to control renal development in vivo. Mech Dev. 2005;122:928–938. doi: 10.1016/j.mod.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Bates CM. Role of fibroblast growth factor receptor signaling in kidney development. Am J Physiol Renal Physiol. 2011;301:F245–F251. doi: 10.1152/ajprenal.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol. 2006;291:325–339. doi: 10.1016/j.ydbio.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276:403–415. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saifudeen Z, Dipp S, Stefkova J, Yao X, Lookabaugh S, El-Dahr SS. p53 regulates metanephric development. J Am Soc Nephrol. 2009;20:2328–2337. doi: 10.1681/ASN.2008121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol. 2008;313:234–245. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park JS, Ma W, O'Brien LL, Chung E, Guo JJ, Cheng JG, Valerius MT, McMahon JA, Wong WH, McMahon AP. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell. 2012;23:637–651. doi: 10.1016/j.devcel.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreidberg JA. WT1 and kidney progenitor cells. Organogenesis. 2010;6:61–70. doi: 10.4161/org.6.2.11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 57.Fanni D, Fanos V, Monga G, Gerosa C, Locci A, Nemolato S, Van Eyken P, Faa G. Expression of WT1 during normal human kidney development. J Matern Fetal Neonatal Med. 2011;24(Suppl 2):44–47. doi: 10.3109/14767058.2011.606619. [DOI] [PubMed] [Google Scholar]
- 58.Gai Z, Zhou G, Itoh S, Morimoto Y, Tanishima H, Hatamura I, Uetani K, Ito M, Muragaki Y. Trps1 functions downstream of Bmp7 in kidney development. J Am Soc Nephrol. 2009;20:2403–2411. doi: 10.1681/ASN.2008091020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 60.Kazama I, Mahoney Z, Miner JH, Graf D, Economides AN, Kreidberg JA. Podocyte-derived BMP7 is critical for nephron development. J Am Soc Nephrol. 2008;19:2181–2191. doi: 10.1681/ASN.2007111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132:3859–3871. doi: 10.1242/dev.01945. [DOI] [PubMed] [Google Scholar]
- 62.Grieshammer U, Cebrián C, Ilagan R, Meyers E, Herzlinger D, Martin GR. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development. 2005;132:3847–3857. doi: 10.1242/dev.01944. [DOI] [PubMed] [Google Scholar]
- 63.Hendry C, Rumballe B, Moritz K, Little MH. Defining and redefining the nephron progenitor population. Pediatr Nephrol. 2011;26:1395–1406. doi: 10.1007/s00467-010-1750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell. 2008;15:781–791. doi: 10.1016/j.devcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- 66.Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- 67.Georgas K, Rumballe B, Wilkinson L, Chiu HS, Lesieur E, Gilbert T, Little MH. Use of dual section mRNA in situ hybridisation/immunohistochemistry to clarify gene expression patterns during the early stages of nephron development in the embryo and in the mature nephron of the adult mouse kidney. Histochem Cell Biol. 2008;130:927–942. doi: 10.1007/s00418-008-0454-3. [DOI] [PubMed] [Google Scholar]
- 68.Little MH, Brennan J, Georgas K, Davies JA, Davidson DR, Baldock RA, Beverdam A, Bertram JF, Capel B, Chiu HS, Clements D, Cullen-McEwen L, Fleming J, Gilbert T, Herzlinger D, Houghton D, Kaufman MH, Kleymenova E, Koopman PA, Lewis AG, McMahon AP, Mendelsohn CL, Mitchell EK, Rumballe BA, Sweeney DE, Valerius MT, Yamada G, Yang Y, Yu J. A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr Patterns. 2007;7:680–699. doi: 10.1016/j.modgep.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allanson JE, Hunter AG, Mettler GS, Jimenez C. Renal tubular dysgenesis: a not uncommon autosomal recessive syndrome: a review. Am J Med Genet. 1992;43:811–814. doi: 10.1002/ajmg.1320430512. [DOI] [PubMed] [Google Scholar]
- 70.Kopan R, Cheng HT, Surendran K. Molecular insights into segmentation along the proximal-distal axis of the nephron. J Am Soc Nephrol. 2007;18:2014–2020. doi: 10.1681/ASN.2007040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005;288:F939–F952. doi: 10.1152/ajprenal.00369.2004. [DOI] [PubMed] [Google Scholar]
- 72.McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128:491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- 73.McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- 74.Leheste JR, Melsen F, Wellner M, Jansen P, Schlichting U, Renner-Müller I, Andreassen TT, Wolf E, Bachmann S, Nykjaer A, Willnow TE. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J. 2003;17:247–249. doi: 10.1096/fj.02-0578fje. [DOI] [PubMed] [Google Scholar]
- 75.Raila J, Willnow TE, Schweigert FJ. Megalin-mediated reuptake of retinol in the kidneys of mice is essential for vitamin A homeostasis. J Nutr. 2005;135:2512–2516. doi: 10.1093/jn/135.11.2512. [DOI] [PubMed] [Google Scholar]
- 76.Bachmann S, Schlichting U, Geist B, Mutig K, Petsch T, Bacic D, Wagner CA, Kaissling B, Biber J, Murer H, Willnow TE. Kidney-specific inactivation of the megalin gene impairs trafficking of renal inorganic sodium phosphate cotransporter (NaPi-IIa) J Am Soc Nephrol. 2004;15:892–900. doi: 10.1097/01.asn.0000120389.09938.21. [DOI] [PubMed] [Google Scholar]
- 77.Bachmann S, Metzger R, Bunnemann B. Tamm-Horsfall protein-mRNA synthesis is localized to the thick ascending limb of Henle's loop in rat kidney. Histochemistry. 1990;94:517–523. doi: 10.1007/BF00272616. [DOI] [PubMed] [Google Scholar]
- 78.Raffi H, Bates JM, Laszik Z, Kumar S. Tamm-Horsfall protein knockout mice do not develop medullary cystic kidney disease. Kidney Int. 2006;69:1914–1915. doi: 10.1038/sj.ki.5000411. [DOI] [PubMed] [Google Scholar]
- 79.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ge Y, Ahn D, Stricklett PK, Hughes AK, Yanagisawa M, Verbalis JG, Kohan DE. Collecting duct-specific knockout of endothelin-1 alters vasopressin regulation of urine osmolality. Am J Physiol Renal Physiol. 2005;288:F912–F920. doi: 10.1152/ajprenal.00432.2004. [DOI] [PubMed] [Google Scholar]
- 81.Rojek A, Füchtbauer EM, Kwon TH, Frøkiaer J, Nielsen S. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci U S A. 2006;103:6037–6042. doi: 10.1073/pnas.0511324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 83.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 84.Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol. 1999;10:2125–2134. doi: 10.1681/ASN.V10102125. [DOI] [PubMed] [Google Scholar]
- 85.Tufró A. VEGF spatially directs angiogenesis during metanephric development in vitro. Dev Biol. 2000;227:558–566. doi: 10.1006/dbio.2000.9845. [DOI] [PubMed] [Google Scholar]
- 86.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- 88.Ueda H, Miyazaki Y, Matsusaka T, Utsunomiya Y, Kawamura T, Hosoya T, Ichikawa I. Bmp in podocytes is essential for normal glomerular capillary formation. J Am Soc Nephrol. 2008;19:685–694. doi: 10.1681/ASN.2006090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sadl V, Jin F, Yu J, Cui S, Holmyard D, Quaggin S, Barsh G, Cordes S. The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev Biol. 2002;249:16–29. doi: 10.1006/dbio.2002.0751. [DOI] [PubMed] [Google Scholar]
- 90.Wharram BL, Goyal M, Gillespie PJ, Wiggins JE, Kershaw DB, Holzman LB, Dysko RC, Saunders TL, Samuelson LC, Wiggins RC. Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate. J Clin Invest. 2000;106:1281–1290. doi: 10.1172/JCI7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koziell A, Grech V, Hussain S, Lee G, Lenkkeri U, Tryggvason K, Scambler P. Genotype/phenotype correlations of NPHS1 and NPHS2 mutations in nephrotic syndrome advocate a functional inter-relationship in glomerular filtration. Hum Mol Genet. 2002;11:379–388. doi: 10.1093/hmg/11.4.379. [DOI] [PubMed] [Google Scholar]
- 92.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300:1298–1300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- 93.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 94.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008;316:288–301. doi: 10.1016/j.ydbio.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miner JH. Building the glomerulus: a matricentric view. J Am Soc Nephrol. 2005;16:857–861. doi: 10.1681/ASN.2004121139. [DOI] [PubMed] [Google Scholar]
- 96.Longo I, Porcedda P, Mari F, Giachino D, Meloni I, Deplano C, Brusco A, Bosio M, Massella L, Lavoratti G, Roccatello D, Frascá G, Mazzucco G, Muda AO, Conti M, Fasciolo F, Arrondel C, Heidet L, Renieri A, De Marchi M. COL4A3/COL4A4 mutations: from familial hematuria to autosomal-dominant or recessive Alport syndrome. Kidney Int. 2002;61:1947–1956. doi: 10.1046/j.1523-1755.2002.00379.x. [DOI] [PubMed] [Google Scholar]