Abstract
Coupling of excitation to secretion, contraction, and transcription often relies upon Ca2+ computations within the nanodomain—a conceptual region extending tens of nanometers from the cytoplasmic mouth of Ca2+ channels. Theory predicts that nanodomain Ca2+ signals differ vastly from the slow submicromolar signals routinely observed in bulk cytoplasm. However, direct visualization of nanodomain Ca2+ far exceeds optical resolution of spatially distributed Ca2+ indicators. Here we couple an optical genetically encoded Ca2+ indicator (TN-XL) to the carboxyl tail of CaV2.2 Ca2+ channels, enabling nearfield imaging of the nanodomain. Under TIRF microscopy, we detect Ca2+ responses indicative of large-amplitude pulses. Single-channel electrophysiology reveals a corresponding Ca2+ influx of only 0.085 pA, and FRET measurements estimate TN-XL distance to the cytoplasmic mouth at ~55 Å. Altogether, these findings raise the possibility that Ca2+ exits the channel through the analog of molecular portals, mirroring the crystallographic images of side windows in voltage-gated K channels.
INTRODUCTION
Ca2+ signals extend pervasively across the intracellular expanse of most cells, yet these signals exhibit remarkable specificity in activating appropriate Ca2+-modulated targets. Understanding how this selectivity arises remains a foremost question in Ca2+ biology1,2. One strategy is to colocalize at molecular dimensions Ca2+ sources and targets. This co-localization is crucial to the local signaling of Ca2+ channels to nearby Ca2+-regulated ion channels and enzymes3–5, to neurotransmitter release6, as well as to excitation-contraction7 and excitation-transcription coupling8,9. Intriguingly, colocalized sensors do not always respond preferentially to local Ca2+ sources, but can require the far weaker input of remote Ca2+ sources acting through a global Ca2+ selectivity paradigm10. Critical to signaling near Ca2+ sources is the nature of Ca2+ signals within a few tens of nanometers of a Ca2+ channel (nanodomain11). Theoretical calculations12–16 postulate that nanodomain signals comprise Ca2+ pulses of equal and enormous amplitude (~100 μM), with each pulse synchronized to the millisecond stochastic openings of channels10,12. Beyond the nanodomain, [Ca2+] rapidly dissipates with diffusion. Of note, predicted nanodomain [Ca2+] amplitudes vary considerably with assumed parameters17, and elegant experimental estimates of local Ca2+ signals18,19 only indirectly probe the nanodomain itself. Thus, the critical magnitude of nanodomain Ca2+ pulses (Caspike) has eluded explicit empirical comment. This uncertainty bears on key issues, including how spatial Ca2+ decoding occurs within the nanodomain10,20–22, and the Ca2+ channel number needed to trigger neurotransmitter vesicles23–25.
Why have nanodomain Ca2+ signals remained elusive? Though fluorescent Ca2+-sensitive chemical dyes has revealed much26, visualization of nanodomain signals via freely diffusible dye molecules far exceeds the resolution limit of far-field microscopic approaches27. In this regime, fluorescence from dye molecules outside the nanodomain will overshadow that from within28. Even when imaged with total internal reflection fluorescence (TIRF) microscopy29–31, which illuminates a restricted TIRF volume within ~150 nm of the glass/cell membrane interface, freely diffusible dyes will still report Ca2+ from a region several-fold larger than a Ca2+ channel32.
One approach is to affix a Ca2+ indicator within the nanodomain, so as to support a form of near-field imaging33. The Ca2+-sensitive luminescent protein aequorin represents a traditional candidate34. Recombinant aequorins have long been targeted to various subcellular compartments by genetic means35–37, but the limited-amplitude and consumptive nature of aequorin would challenge deployment in a channel nanodomain34. Recently, promising results have been obtained using a biarsenical chemical fluorescent Ca2+ indicator (Calcium Green FlAsH, CaGF) targeted to a tetracysteine tag implanted on CaV1.2 Ca2+ channels38. CaGF features Ca2+ affinity and kinetics (Kd ~55 μM; off rate, 2 ms−1) well matched to the purported amplitudes and lifetimes of nanodomain Ca2+ pulses. Indeed, CaGF was potentially responsive to nanodomain Ca2+ fluctuations, but the observed fluorescence signals were highly heterogeneous, to an extent that precluded quantitative Ca2+ estimation. This heterogeneity was attributable to the diminutive and variable open probability of CaV1.2 channels (~0.1–0.3%), despite constitutive pharmacological manipulation by a channel opener. Indeed, the possibility of a large fraction of electrically silent CaV1.2 channels fits with their unusually large ratio of gating charge to ionic current39. Also, though the authors were careful to control for non-specific labeling of other cysteine-rich sites in the cell by CaGF, such indiscriminate attachment would remain a generic concern for approaches involving chemical targeting of sensors. A third approach would be to employ genetically encoded Ca2+ indicators (GECIs) as near-field sensors. GECIs allow genetic fusion of sensor to a Ca2+ source, offering the greatest potential selectivity in positioning sensors. Among the most popular GECIs are CFP/YFP-FRET-based molecules such as cameleons40, and GFP-intensity-based sensors like GCaMPs41. All these employ calmodulin (CaM) as their Ca2+ sensor, and convert binding of the sensor’s own Ca2+/CaM with a target peptide into altered optical readouts. When targeted to plasmalemma, cameleons often lose Ca2+ sensitivity42, potentially via target peptide binding to elevated plasmalemmal CaM43.
Accordingly, we here explore nearfield imaging of nanodomain Ca2+ using TN-XL, a GECI44 based on the Ca2+ sensor troponin C. TN-XL exploits Ca2+-driven conformational changes without a target peptide, thereby naturally resisting endogenous CaM interference42. Our specific approach is to fuse TN-XL to the C-terminus of the principle subunit of CaV2.2 Ca2+ channels, chosen for high open probability45, relative absence of silent channels46, and abundant plasmalemmal expression47. To further improve the TN-XL signal-to-noise ratio at the surface membrane, we utilize TIRF microscopy to selectively illuminate only those CaV2.2/TN-XL channels near the surface, thereby attenuating background signal from imperfectly targeted intracellular channels. Finally, to account for the high Ca2+ affinity and slow kinetics of GECIs44 (off times 100–900 ms for TN-XL) in the face of millisecond nanodomain Ca2+ pulses, we quantify the kinetics of TN-XL readouts, which our previous work suggests can distinguish between differing Caspike amplitudes48. These strategies allow us to resolve nanodomain Ca2+ activity of CaV2.2 channels undergoing native gating, without pharmacological manipulation.
RESULTS
Functional CaV2.2/TN-XL fusions at the surface membrane
Our strategy for probing nanodomain Ca2+ signals (Fig. 1) requires maintained Ca2+ responsiveness of genetically encoded sensors situated at the plasmalemma, a foremost challenge. Accordingly, we examined whether troponin-based TN-XL could respond to Ca2+ when targeted to this environment. As a baseline, Fig. 2a displays the properties of the well-studied, cytoplasmic form of TN-XL44. Confocal images of an exemplar HEK293 cell, acquired under CFP and FRET imaging modes, reveal the expected pan-cytoplasmic distribution. An epifluorescence CFP image of another cell also demonstrates this generalized expression, and switching to TIRF mode indicates no preferential TN-XL accumulation within the plasmalemmal footprint of this same cell. To gauge TN-XL responsiveness, we measured under TIRF imaging the single-cell CFP (SC, respective excitation and emission wavelengths at 442 and 485 nm) and FRET fluorescence signals (SF, excitation and emission at 442 and 545 nm), where the ratio of these signals (RF/C = SF/SC) furnishes the customary sensor readout of Ca2+ (Fig. 2b). In particular, the ratio RF/C was first determined in resting cells with cytoplasmic Ca2+ concentration (≤ 0.1 μM) well below the Kd of the TN-XL24, thus yielding Rmin. To subsequently determine Rmax, CaV2.2 channels were co-expressed as conduits of Ca2+ entry under whole-cell voltage clamp. To facilitate elevation of intracellular Ca2+, minimal internal Ca2+ buffering (1 mM EGTA) with elevated 10 mM extracellular Ca2+ was employed, such that repetitive step depolarizations (30 mV × 500 ms pulses every 15 s) readily saturated RF/C at Rmax. Thus, the ratio of Rmax and Rmin reports sensor dynamic range.
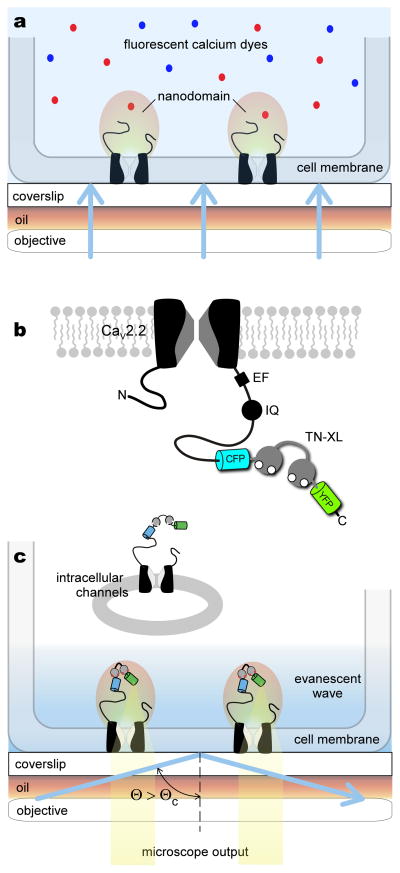
Figure 1. Approach to resolving channel nanodomain Ca2+ signals.
a, Conventional wide-field imaging using cytosolic chemical fluorescent dyes cannot resolve channel nanodomain Ca2+ signals. Blue shading denotes region of fluorescence excitation, which extends throughout the cell under wide-field imaging. b, Design of the genetically encoded Ca2+ indicator TN-XL fused to the carboxy tail of the α1B subunit of a CaV2.2 channel, yielding CaV2.2/TN-XL. For orientation, structure-function elements involved in calmodulin regulation are denoted on carboxy terminus21: EF, EF-hand region; IQ, IQ-domain for apoCaM binding. CFP denotes enhanced CFP. YFP denotes circularly permuted Citrine. c, CaV2.2/TN-XL constructs act as a ‘near-field’ sensor of nanodomain Ca2+. TIRF imaging evanescent wave illuminates only CaV2.2/TN-XL channels within ~150 nm from the glass/cell membrane interface, as indicated by the blue-shaded region. This mode of excitation potentially excludes intracellular channels from consideration. When laser illumination angle Θ exceeds a critical angle ΘC, TIRF illumination occurs.
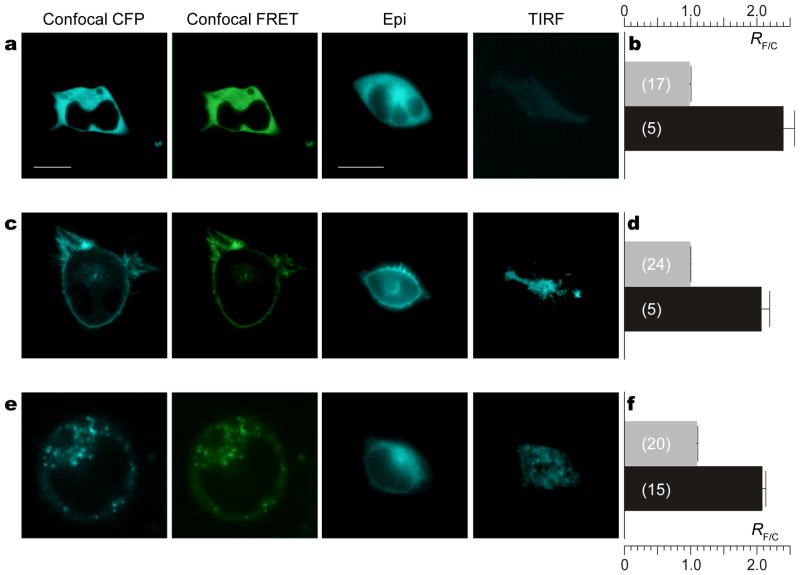
Figure 2. CaV2.2/TN-XL fusion construct preserves function of sensor.
a, Behavior of free TN-XL. Left to right: Confocal (CFP filter), confocal (FRET filter), epi-fluorescence (CFP filter), and TIRF (CFP filter) images of HEK293 cells expressing cytoplasmic TN-XL. White scale bar indicates 10 μM. Bar at far left pertains to all confocal images. Bar at middle right pertains to all epifluorescence and TIRF images. b, TN-XL ratio (RF/C=SF/SC) measured in resting cells (Rmin) (gray bars) and in cells under high Ca2+ (Rmax) (black bars). Data shown as mean ± sem with number of cells in parentheses. c and d, Behavior of TN-XL-Ras (membrane targeted TN-XL). Format as in a and b, respectively. e and f, Behavior of CaV2.2/TN-XL (N-type channel fused to TN-XL). Format as in a and b, respectively.
By contrast, for TN-XL fused to the farnesylation motif of Ras (TN-XL-Ras), sensors are sharply localized to the surface membrane in confocal and epifluorescence images (Fig. 2c), and there is marked enrichment within the TIRF footprint. Importantly, the Ca2+ dynamic range of TN-XL-Ras is nearly identical (Fig. 2d), despite residence within the plasmalemmal context. Yet more relevant are results for the CaV2.2/TN-XL fusions (Fig. 2e). Here, constructs appear only partially localized to the surface membrane, consistent with typically imperfect trafficking of recombinant Ca2+ channels to plasmalemma49. Still, there is substantial focalization of CaV2.2/TN-XL in the TIRF footprint, suggesting sufficient trafficking for nanodomain experiments (Fig. 1c). Critically, sensor dynamic range is spared when juxtaposed near CaV2.2 channels (Fig. 2f).
A second concern is the preservation of CaV2.2 channel function upon fusion to TN-XL. Reassuringly, Ca2+ currents appeared nearly identical in CaV2.2/TN-XL (Fig. 3a, black) and CaV2.2 (gray) channels. In fact, fusing TN-XL to CaV2.2 enhances opening, as seen from hyperpolarization of tail-activation curves determined with 10 mM extracellular Ca2+ as charge carrier (Fig. 3b). Even at the single-channel level, CaV2.2/TN-XL fusions exhibited robust electrophysiological function, as illustrated by the exemplar single-channel current trace shown at high-gain magnification (Fig. 3c, noisy trace). We used 90 mM Ba2+ as a charge carrier to enhance resolution of elementary events, and evoked currents via ramp depolarization to readily resolve the open-channel conduction profile (downwardly convex gray relation). Multiple single-channel sweeps (Fig. 3d), displayed at lower-gain magnification, confirm the overall reproducibility and excellent resolution of elementary events under this protocol. Accordingly, numerous sweeps from multiple patches specify a highly reliable ensemble average current (Fig. 3c, red curve), closely similar to that of CaV2.2 channels without sensor fusion45. Taking a ratio of the ensemble average current with the open-channel conduction profile (Fig. 3c, red and convex gray curves) yields a plot of open probability PO versus voltage V (Fig. 3e, gray relation). This plot matches impressively with whole-cell activation (reproduced as open circles), after shifting along the voltage axis to account for contrasting surface-charge effects of single-channel versus whole-cell solutions. As with studies of CaV2.245, the CaV2.2/TN-XL fusion exhibits a substantial PO of 0.69 at 30 mV (Fig. 3e, red arrows), a feature advantageous for probing nanodomain Ca2+.
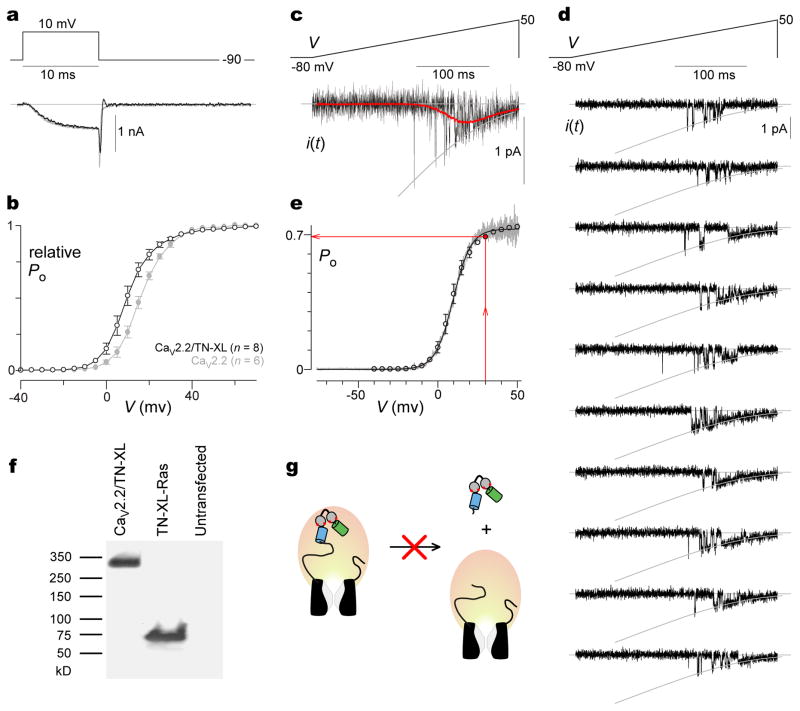
Figure 3. CaV2.2/TN-XL fusion construct preserves function of channel.
a, Exemplar whole-cell currents of HEK293 cells expressing CaV2.2 channels (gray) or CaV2.2/TN-XL channels (black), using 10 mM Ca2+ as charge carrier, and 10 mM EGTA intracellular Ca2+ buffering. b, Whole-cell tail-activation curves of CaV2.2 (gray) and CaV2.2/TN-XL (black), obtained from records as in a. Data shown as as mean ± sem with number of cells in parentheses. c, Single-channel analysis of CaV2.2/TN-XL. Top, On-cell ramp-voltage waveform delivered every 5–15 s. Bottom, Exemplar single-channel sweep shown at high-gain magnification (100–200/patch). 90 mM Ba2+ as charge carrier; 2 kHz lowpass filtering. Leak-subtracted traces were averaged, yielding red I–V curve. Unitary current relation (convex gray curve) was fitted to the open-channel current level using GHK equation10. d, Multiple exemplar single-channel sweeps from another patch, shown at lower-gain magnification to impart a sense of the overall reproducibility and excellent resolution of elementary events. Format as in c. e, Single-channel PO–V relation (gray, with fit in black, average of n = 4 patches), determined by dividing I–V curve (c, red trace) by unitary current relation (c, gray GHK fit). PO–V curve was calibrated in voltage by aligning the single-channel activation curve with the whole-cell curve reproduced from b (open circles). From calibrated PO–V curve, the Po at 30 mV is 0.69. f, Immunoblots of HEK293 cell lysates probed with anti-GFP antibody; (left) CaV2.2/TN-XL at ~320 kD; (middle) TN-XL-Ras at ~71 kD; (right) control (untransfected) cells for reference. g, Lack of lower molecular-weight species in leftmost lane of panel f excludes proteolysis of sensor from channel.
A final prerequisite is that CaV2.2/TN-XL fusions resist proteolysis. Accordingly, we utilized anti-GFP antibody to perform western immunoblots from cells expressing this construct (Fig. 3f, left lane). The other lanes correspond to cells expressing TN-XL-Ras (~71 kD), as well as untransfected cells (no signal). The absence of signal beneath the full-length ~320 kD band (Fig. 3f, left lane) substantiates maintained TN-XL fusion to channels (Fig. 3g).
Calibration of TN-XL fused to CaV2.2 channels
We next addressed more precise sensor calibration, to facilitate quantitative inferences about nanodomain Ca2+. Both kinetic and steady-state characterization would be ideal, given the millisecond kinetics of Ca2+ pulses within channel nanodomains, coupled with the comparatively slow response of free TN-XL (off rates of 1/150 to 1/850 1/ms44). Accordingly, we devised a state-mechanism approximation of sensor performance, a ‘forward transform’ that maps rapid Ca2+ transient inputs onto slower sensor outputs, as previously established for an older variant of TN-XL (TN-L15) freely expressed in myocytes48. Here, we pursued an analogous approach, but attuned to CaV2.2/TN-XL constructs resident within the TIRF volume and driven by high-amplitude dynamic Ca2+ inputs pertinent to channel nanodomains. To produce such inputs, we activated ultra-large CaV2.2 Ca2+ currents (Fig. 4a) in HEK293 cells featuring minimal exogenous intracellular Ca2+ buffering (1 mM EGTA); this configuration intentionally produced enormous spatial gradients of intracellular Ca2+ (Fig. 4b inset), far exceeding that usually encountered. To estimate these gradients, we simultaneously monitored aggregate Ca2+ concentration (Fig. 4b, noisy dark trace), as deduced by imaging the entire cell for signals emanating from 10 μM of the rapid chemical fluorescent Ca2+ indicator Fluo 4FF. The measured Ca2+ influx (Fig. 4a) and aggregate [Ca2+] signals were then used to explicitly constrain a radially symmetric Ca2+ diffusion mechanism, whose estimates of Ca2+ concentration in differing cellular regions are shown as red traces in Fig. 4b (Supplementary Figure S1 and Supplementary Methods). The estimated aggregate [Ca2+] concentration (Fig. 4b, lower red trace) closely approximates its measured analog (dark noisy trace), and the estimated [Ca2+] at the surface membrane (upper red trace) peaks at ~80 μM and later converges to the aggregate [Ca2+] (~20–25 μM). This estimate of submembranous [Ca2+] was reproducible in multiple cells exhibiting like Ca2+ influx, and was thereby taken as the relevant Ca2+ input in parallel experiments performed with CaV2.2/TN-XL fusion constructs under TIRF imaging. The green trace in Fig. 4c displays the corresponding sensor output from GECIs in the submembranous TIRF volume, averaged from multiple cells exhibiting Ca2+ influx matching that in Fig. 4a. The steady-state plateau of the response (Fig. 4c, gray dashed line at RF/C = 1.78), at a near steady Ca2+ concentration of ~20–25 μM, enabled us to calculate ~17.3 μM as the half-response point of the steady-state response curve for CaV2.2/TN-XL constructs in the TIRF volume (Supplementary Methods). This differs from the 2.5 μM half response point for free TN-XL44, consistent with prior observations that sensor performance can change with cellular/molecular environment38,42. Accordingly, steady-state response data for free TN-XL44 were shifted along the [Ca2+] axis by ×16.3/2.5 to provide an appropriate steady-state profile for CaV2.2/TN-XL constructs (Fig. 4d, green symbols). In all, these steady-state data and the dynamic sensor response (Fig. 4c, green trace) to a specified submembranous Ca2+ input (Fig. 4b, upper red trace) furnished the constraints required to deduce a forward transform for CaV2.2/TN-XL.
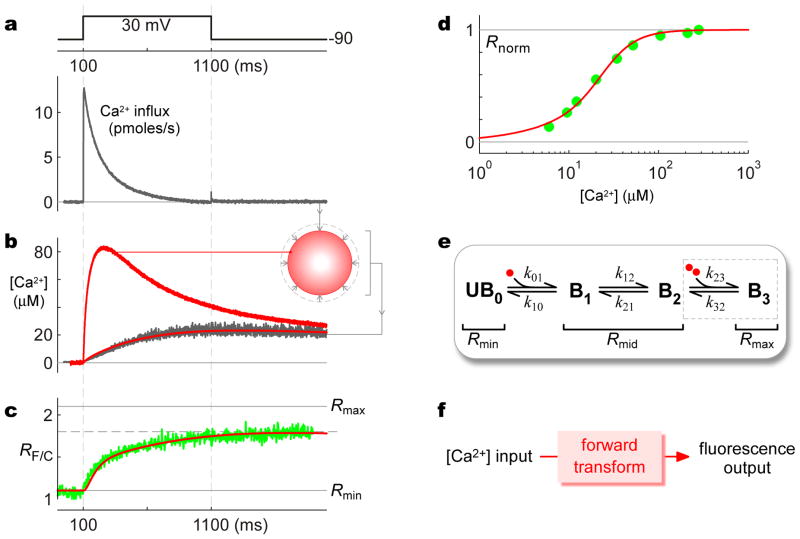
Figure 4. Calibration of CaV2.2/TN-XL fusion construct.
a, High-amplitude Ca2+ influx into HEK293 cell expressing CaV2.2 channels, observed with 10 mM external Ca2+ and minimal internal Ca2+ buffering of 1 mM EGTA. b, Simultaneous [Ca2+] measurement (dark trace) deduced from 10 μM Fluo-4FF Ca2+-indicator fluorescence from entirety of same cell (514 nm excitation, 545 nm emission). Ca2+ determination rendered ratiometric by inclusion of 2.5 μM red Alexa 568 dye (514 nm excitation, 580 nm long-pass emission). Inset, cartoon of spatial Ca2+ gradients in this cell. Red traces, projected [Ca2+] waveforms from Ca2+ diffusion mechanism constrained by Ca2+ input in a and aggregate [Ca2+] (dark trace): lower red trace, aggregate [Ca2+]; upper red trace, submembranous [Ca2+]. c, Average FRET-ratio (RF/C) response of CaV2.2/TN-XL channels in TIRF volume (green trace); same protocol as in a and b. Red trace, output of TN-XL forward transform in e, when driven by submembranous [Ca2+] input (b, upper red trace). d, Normalized steady-state FRET-ratio response (Rnorm = (RF/C − Rmin)/(Rmax − Rmin) of CaV2.2/TN-XL to [Ca2+] (experimental green symbols). Red trace, steady-state output of TN-XL forward transform in e, when driven by differing fixed [Ca2+]. e, State-diagram representation of CaV2.2/TN-XL. Red circles, Ca2+ ions. f, System of states and corresponding fluorescence/FRET properties constitutes a forward transform that predicts TN-XL sensor outputs as a function of time-varying Ca2+ inputs.
Fig. 4e displays the corresponding state-diagram approximation of CaV2.2/TN-XL responsiveness to Ca2+, based upon a mechanism for an older troponin-based GECI48 (TN-L15). State UB0 (‘unbound’) represents the Ca2+-free conformation of the sensor, characterized by a lower FRET-ratio output of Rmin. Upon binding of a single Ca2+ to the N-lobe of troponin, the sensor adopts one of two alternate ‘bound’ conformations (B1 or B2), both featuring the same elevated FRET-ratio output of Rmid. The arrangement thus far is identical to that previously established for TN-L1548. The improved performance of TN-XL arises via engineering the C-lobe of troponin to dynamically bind and unbind two signaling Ca2+ ions, thereby driving a further conformational change of the sensor44. To account for this feature, we allowed two additional Ca2+ ions to bind and induce a third bound state50,51 (B3, dashed box), exhibiting the highest FRET-ratio output of Rmax. Numerical simulations of this scheme (Supplementary Methods), coupled with error minimization via parameter variation, yielded impressive fits to the target constraints above (Figs. 4c and 4d, red curves), using the sensor parameter estimates in Table 1. Accordingly, these parameters and the scheme in Fig. 4e furnish a steady-state and dynamic representation of CaV2.2/TN-XL responsiveness to Ca2+, a ‘forward transform’ (Fig. 4f) potentially appropriate for making inferences about channel nanodomain Ca2+ fluctuations.
Table 1.
CaV2.2/TN-XL parameters for HEK293 cells under TIRF imaging
| Fluorescence Parameters1 | Value |
|---|---|
| CaV2.2/TNXL Rmin | 1.10 (n = 20 cells, Fig. 2f) |
| CaV2.2/TNXL Rmid | 1.532 |
| CaV2.2/TNXL Rmax | 2.10 (n = 15 cells, Fig. 2f) |
| CaV2.2/TNXL α0 | 2.00 (n = 8 cells) |
| CaV2.2/TNXL α1 | 1.573 |
| Kinetic Parameters4 | Value |
|---|---|
| k01 | 174.4 ms−1 M−1 |
| k10 | 0.00239 ms−1 |
| k12 | 0.00174 ms−1 |
| k21 | 0.00309 ms−1 |
| k23 | 0.126 × 106 ms−1 M−2 |
| k32 | 1/733 ms−1 |
Fluorescence parameters obtained following experimental procedures used to characterize TN-L1548, unless specified otherwise.
Set by global fitting of Fig. 4e scheme to data Figs. 4a, 4c, and 4d.
Linear interpolation on R values. Explicitly, α1 = α0 − (Rmid − Rmin)/(Rmax − Rmin).
CaV2.2/TN-XL sensors respond to nanodomain Ca2+ signals
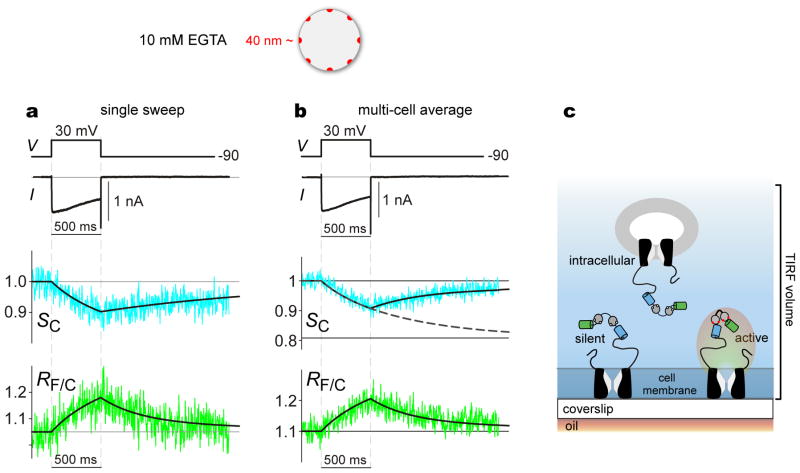
This transform could only be utilized, however, if a substantial fraction of CaV2.2/TN-XL sensors respond to channel nanodomain Ca2+ signals, as specifically produced by individual channels fluxing Ca2+ into their own nanodomain. Thus far, we had only demonstrated that CaV2.2/TN-XL sensors in the TIRF volume respond well to a generalized increase in submembranous [Ca2+], but this would occur whether the majority of channel-sensor fusion constructs are properly trafficked to the surface membrane, or still plentiful in submembranous vesicles within the TIRF volume. To address this issue, we again undertook simultaneous recordings of whole-cell Ca2+ current and TIRF imaging of CaV2.2/TN-XL sensors, but here with high intracellular Ca2+ buffering present to restrict Ca2+ elevations to the nanodomain of active channels that flux Ca2+. Specifically, we dialyzed cells with 10 mM EGTA, yielding a Ca2+ nanodomain radius of ~40 nm13 (Fig. 5), only two-fold larger than electron micrographic estimates of Ca2+ channel diameters32. Additionally, we only investigated cells with modest Ca2+ currents (~1 nA with 10 mM Ca2+ as charge carrier) and little Ca2+-dependent inactivation21 (CDI), so as to exclude Ca2+ buffer depletion. Fig. 5a summarizes the results of an exemplar cell satisfying this criterion, with the displayed data evoked by a single voltage pulse. Clearly present is a transient decrease in CFP fluorescence (SC, cyan trace), accompanied by a corresponding phasic elevation of the FRET ratio RF/C (green trace). Exponential fits (solid black curves) revealed dominant rise and fall times of 880 and 2000 ms for SC, and 880 and 400 ms for RF/C. Both the return of TN-XL waveforms towards baseline, and the modest CDI of channels under elevated Ca2+ buffering21 (Fig. 5a compared to Fig. 4a), argue against unintended depletion of Ca2+ buffer. Accordingly, these results suggest responsiveness of CaV2.2/TN-XL sensors to genuine channel nanodomain Ca2+ signals. By contrast to the exemplar, about half the cells with currents of this magnitude and limited CDI exhibited no appreciable change of TN-XL readouts, presumably due to poor trafficking of active channels. Such cells were excluded from further analysis.
Figure 5. CaV2.2/TN-XL sensors respond to channel nanodomain Ca2+ signals.
Top cartoon, Ca2+ nanodomains under elevated Ca2+ buffering, where Ca2+ elevation is restricted to nanodomains, each affiliated with individual channel. 40 nm nanodomain calculated with Equation 1, f = 1 (classical assumption), unitary current i = 0.085 pA, and conservatively requiring [Ca2+] ~60 nM (the approximate resting physiological Ca2+ concentration). a, Simultaneous records of whole-cell current (top) and TIRF fluorescence signals (middle and bottom) from HEK293 cell expressing CaV2.2/TN-XL, using 10 mM external Ca2+ and 10 mM internal EGTA Ca2+ buffering. Shown are data from a single trial, evoked by the first step depolarization in the cell. Top, Voltage and current waveforms, with moderately-sized current chosen to minimize buffer consumption. Middle, Corresponding CFP fluorescence signal after normalization to baseline level (SC, noisy trace). Black curve, approximate exponential fits (initial phase: 200 ms (10%), 880 ms (90%); recovery phase: 2000 ms (100%)). Vertical ticks, 0.1 increment. Bottom, Corresponding FRET-ratio signal (RF/C, noisy trace). Black curve, approximate exponential fits (initial phase: 200 ms (10%), 880 ms (90%); recovery phase: 400 msec (70%), 2000 ms (30%)). Vertical ticks, 0.1 increment. b, Average CaV2.2/TN-XL responses to nanodomain Ca2+ signals, as isolated with 10 mM internal EGTA Ca2+ buffering. Protocol and format as in panel a. n = 4 cells, with records taken from the first step depolarization delivered in cells. CDI sometimes increased with repeated depolarizations, reflecting buffer depletion with attendant slowing of RF/C waveform decay. Cells exhibiting peak currents ≤500 pA were not considered, as these cells demonstrated negligible RF/C transients, presumably reflecting insufficient trafficking of active channels. Solid-black-curve exponential fits were similar, with SC exponential fit (initial phase: 200 ms (10%), 880 ms (90%); recovery phase: 500 ms (80%), 2500 ms (20%)), and RF/C fit (initial phase: 200 ms (10%), 880 ms (90%); recovery phase: 400 msec (70%), 2000 ms (30%)). Dashed black curve, continuation of initial phase SC exponential fit, which asymptotes at lower horizontal black line in middle. c, Schematic of various classes of CaV2.2/TN-XL species in TIRF volume. Blue shading indicates region of excitation by evanscent wave in TIRF volume.
Forward transform estimates of nanodomain Ca2+ amplitudes
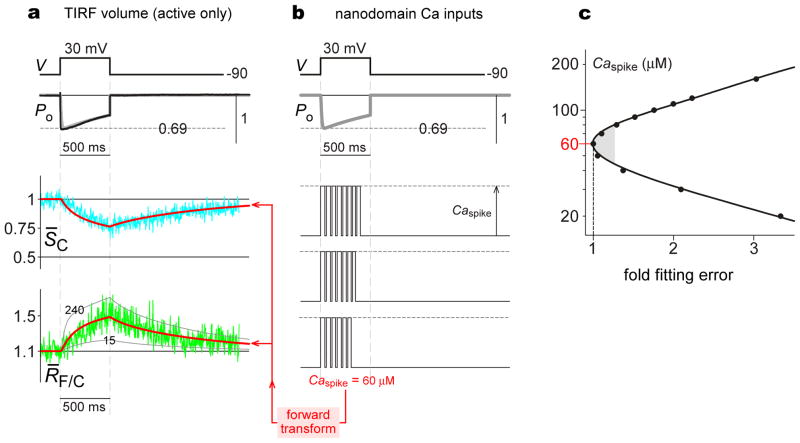
As a prelude to more rigorous assessment of CaV2.2/TN-XL responses to nanodomain signals, we averaged signals from multiple responsive cells dialyzed with 10 mM EGTA. The mean responses (Fig. 5b) were similar to those of the exemplar, with nearly identical characteristic time constants. This reproducibility encouraged us to quantitatively scrutinize these averaged waveforms. In particular, while the robust responses observed here under elevated Ca2+ buffering do indicate an appreciable fraction of active channels within the TIRF volume (Fig. 5c, active), the data do not exclude the possibility of a still substantial fraction of channels that are present in the plasmalemma but fail to open (Fig. 5c, silent), or resident within submembranous vesicles within the TIRF volume (Fig. 5c, intracellular). Such silent and/or intracellular channels would contribute static background fluorescence that could complicate quantitative interpretation. An indication of this scenario comes from exponential extrapolation of the SC waveform decline (Fig. 5b, dashed curve), which asymptotes in the range of ~0.8. By contrast, under the assumption that all channels are active, simulations of the forward transform in Figs. 4e and 4f indicate that, over a large range of plausible nanodomain Ca2+ pulse amplitudes (≥ 35 μM), SC waveforms would asymptote near Ca2+-saturating levels of ~0.5 (Supplementary Figure S2, Supplementary Methods), as specified by the experimentally determined 1/α0 value (defined in Table 1). Hence, the experimentally deduced asymptote of ~0.8 (Fig. 5b) suggests that ~40% of CaV2.2/TN-XL channels are active within the TIRF volume, while ~60% of channels are intracellular or silent. Cognizant of this configuration, we correct SC and RF/C waveforms to reflect only active channels, simply by subtracting ~60% of the baseline amplitude of CFP (SC) and FRET (SF) signals, and taking a ratio of these subtracted signals to obtain a corrected RF/C. Fig. 6a shows the corrected signals (S̄C, R̄F/C), averaged over multiple cells. These waveforms were then suitable for making inferences about nanodomain Ca2+.
Figure 6. Estimates of nanodomain Ca2+ amplitude from CaV2.2/TN-XL responses.
a, Average CaV2.2/TN-XL responses to nanodomain Ca2+ signals, corrected to reflect signal of active channels alone. Top, black trace plots average whole-current, rescaled to open probability PO by normalizing peak current during 30-mV pulse to 0.69. Gray trace, exponential fit to PO waveform. Middle, cyan trace plots average S̄C, corrected CFP signal. Red trace, forward transform prediction of experimental S̄C using Caspike = 60 μM. Bottom, Green trace plots average R̄F/C, corrected FRET-ratio signal. Red trace, forward transform prediction of experimental R̄F/C using Caspike = 60 μM. Gray traces, transform prediction with Caspike as labeled. b, Nanodomain Ca2+ signals input to forward transform for prediction of CaV2.2/TN-XL responses in a. Top, PO waveform reproduced from a. Middle, Schematic of stochastic Ca2+ pulses in nanodomain. Bottom, Forward transform box with parameters in Table 1. c, Caspike estimation criteria. Presumed Caspike values plotted versus fold-fitting error (sum of squared deviations between fit and data, normalized to sum with Caspike = 60 μM). Cusp of plot denotes Caspike = 60 μM as best fit. Shaded zone specifies approximate confidence region, where fits yield errors within 25% of minimum.
That nanodomain Ca2+ signals take the form of equi-amplitude Ca2+ pulses synchronized to the millisecond opening of channels is widely accepted10,12 (cartoon, Fig. 6b); however, the critical amplitude of such pulses (Caspike) has only been inferred via simulations of Ca2+ diffusion12–16. To estimate Caspike empirically, we analyzed the corrected CaV2.2/TN-XL responses (Fig. 6a) using the forward transform in Figs. 4e and 4f. The experimental waveforms might initially appear difficult to conceptualize, since each TN-XL sees a different stochastic record of Ca2+ pulses driven by single-channel openings. However, because the response of each TN-XL is slow (Table 1, compare Figs. 4b, 4c) relative to the millisecond duration of individual Ca2+ spikes, we can adopt a previously established kinetic simplification10 that permits the Ca2+-driven forward reaction rates in Fig. 4e to be treated, not as stochastic entities that fluctuate with each Ca2+ pulse, but as smoothly changing entities k01·Caspike·PO(t) and k23·Ca2spike·PO(t). Importantly, these entities are to the first order equal for all active CaV2.2/TN-XL sensors, such that the collective output from multiple sensors, as displayed in Fig. 6a, would approximate that for any individual sensor on average. Thus, using our forward transform (Figs. 4e and 4f) to predict experimental waveforms becomes simple. Single-channel data (Fig. 3e) permit average whole-cell currents (Fig. 5b) to be rescaled as an open probability PO waveform (Figs. 6a and 6b); this PO drives straightforward numerical integration of the differential equations corresponding to the transform in Fig. 4e; and this integration yields predictions of experimental waveforms, with the magnitude of Caspike being the only unknown (Supplementary Methods). While presumed Caspike values of 15 or 240 μM yield predicted R̄F/C trajectories (Fig. 6a, gray smooth curves) that are patently inconsistent with the experimental curve, Caspike = 60 μM produces an impressive fit to data (smooth red curves). Indeed, error analysis (Fig. 6c) confirms 60 μM as a best estimate of Caspike. As a conservative lower limit, we performed transform analysis on uncorrected waveforms (Fig. 5b), yielding a still-large estimated Caspike ~15 μM (Supplementary Figure S3, Supplementary Methods). Fits to uncorrected data were notably inferior, further prompting us to favor Caspike estimates from the background-corrected data in Fig. 6a. For these data, the shading in Fig. 6c encompasses fits with errors similar to the minimum, yielding an approximate Caspike range of 45–80 μM. This estimate, combined with determination of the underlying single-channel current amplitude, can potentially furnish valuable insight into Ca2+ diffusion properties within the nanodomain itself (Fig. 7).
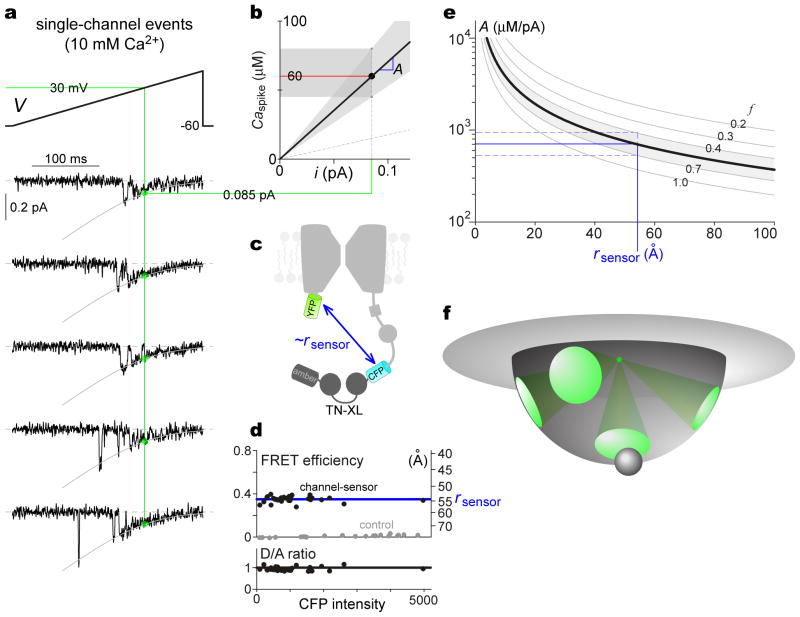
Figure 7. Potential fenestrated Ca2+ egress within CaV2.2 channel nanodomain.
a, On-cell single-channel ramp experiments, except 10 mM Ca2+ is charge carrier to accord with whole-cell experiments; 700-Hz lowpass filtering. Voltages adjusted to align single-channel activation with whole-cell activation curves. Exemplars from single patch, with openings to GHK relation (gray curve). Unitary current i at 30 mV (green circles), 0.085 pA in 5 patches. b, Gain factor A, slope of Caspike versus i relation. Shading, various estimates of A over confidence range of Caspike. Sloped dashed line, lower-limit estimate of A using uncorrected data. c, Schematic, FRET construct to estimate rsensor. Substituting circularly permuted amber for circularly permuted Citrine of TN-XL maintains sensor-channel configuration. d, Estimating rsensor via FRET. Each black symbol, individual HEK293 cell expressing construct in c. Two outputs for each such cell obtained via 33-FRET analysis54 of fluorescence images: FRET efficiency and ratio of donor (CFP) to acceptor (YFP) molecules (D/A ratio), both plotted versus CFP intensity (∝ construct expression level). Data from cells exhibiting D/A ratio ~1 (as expected from c) are displayed and analyzed (bottom subpanel, black line at unity). Invariant FRET efficiency with increasing CFP intensity (top subpanel) excludes spurious FRET, facilitating interpretation of FRET efficiency (~0.35, blue line, top subpanel, left axis) as indicative of rsensor ~55 Å (top subpanel, right axis). Gray symbols, control cells expressing YFP and CFP separately, yielding efficiencies ~0. e, A versus sensor distance from channel mouth (rsensor), calculated with Neher-Stern equation (Eq. 1), using parameters13,60 DCa = 0.4 μm2 ms−1 and kBon = 10−3μM−1 ms−1 for EGTA. Gray curves, plots with f values as labeled. Mapping A from b, with rsensor from d, yields f ~0.53 (black curve) and range (0.4–0.7, shading). f, Schematic, idealized fenestrated Ca2+ egress from inner channel mouth. Green cones, permissive radial diffusion paths from source (green dot), summing to f ~ 0.53. Target molecule (gray ball) within/near portal enjoys preferential signaling.
DISCUSSION
The fusion of CaV2.2 channels to the genetically encoded Ca2+ sensor TN-XL here furnishes a new tool for detecting submembranous Ca2+ signals near these ‘N-type’ Ca2+ channels (Fig. 1b). Our particular application concerns the nanodomain Ca2+ transients accompanying individual channel openings and closings. These transients have eluded direct experimental comment until the recent advent of TIRF microscopy to image single-channel activity30,31, and the availability of targetable chemical-fluorescent Ca2+ indicators38. Here we demonstrate an alternative approach exploiting TIRF/patch-clamp electrophysiology and our CaV2.2/TN-XL fusion. This tactic yields a first empirical estimate of the amplitude of nanodomain Ca2+ pulses (Caspike). This entity figures crucially in the Ca2+ activation of co-localized channels and enzymes2, evoked release of nearby neurotransmitter vesicles25, and excitation-transcription coupling8,9.
A limitation of the present study concerns the incomplete targeting of active CaV2.2/TN-XL channels to the surface membrane in the TIRF volume, necessitating approximate correction of static background fluorescence. The potential error in such correction renders our Caspike determination as a coarse approximate estimate. Nonetheless, the approach taken will prove valuable as means to improve channel trafficking arise, and GECI technology progresses.
With this proviso, we consider other long-sought-after Ca2+ channel signaling properties that could, in principle, be accessed with our strategy. First, we estimate the gain factor A, defined as the ratio of Caspike to unitary current i fluxing through individual Ca2+ channels12. As such, the magnitude of A is crucial for local Ca2+ channel signaling to downstream nanodomain targets. Given knowledge of Caspike, we could estimate A itself, so long as the i were known under the conditions of Caspike determination (Fig. 6, with 10 mM Ca2+ as the charge carrier). The single-channel data thus far (Figs. 3c, 3d) were obtained with 90 mM Ba2+ to facilitate resolution of open probability; these data could not be utilized here. The relevant single-channel data for parameter A specification would entail several-fold smaller signals25,52. Nonetheless, despite signals near the limits of detection, we acquired well-resolved unitary currents under these conditions (Fig. 7a). These data yield i = 0.085 ± 0.003 pA (n = 5 patches) at 30 mV (as in Fig. 6), resembling values recorded in native CaV2.2 channels25. Combining i with our estimates of Caspike (center ~60 μM; range 45–80 μM), we obtain A ~700 μM/pA (Fig. 7b, shaded regions).
Second, if the distance from the cytoplasmic mouth of the channel to the sensor (rsensor) were also known, nearfield Ca2+ sensing could comment powerfully on Ca2+ diffusion within the nanodomain itself. We therefore gauge rsensor from FRET measured between the CFP of our CaV2.2/TN-XL fusion, and YFP fused to the base of the channel amino terminus (Fig. 7c). To eliminate crosstalk, YFP within TN-XL itself is replaced with the inert analog amber53. FRET efficiency was thus determined54 (Fig. 7d), yielding an estimated rsensor ~55 Å (Methods).
With both parameter A and rsensor in hand, we scrutinize nanodomain Ca2+ diffusion via classic point-source Ca2+ diffusion, as given by a generalized Neher-Stern equation13,16
| (1) |
where rsensor is the distance of the TN-XL sensor from the point source of Ca2+ influx, DCa is the diffusion coefficient of free Ca2+, F is Faraday’s constant, kBon is the on rate for Ca2+ binding to EGTA, and BT is the internal EGTA concentration (10 mM). The parameter f, specifying the fraction of half-infinite space into which radial Ca2+ diffusion occurs, has always been set at unity (Ca2+ diffuses into a full half-infinite space), absent any evidence to the contrary. Intriguingly, our estimates of A and rsensor square poorly with the traditional view (Fig. 7e, dashed curve with f = 1), but fit well with f ~ 0.53 (0.53 × 2π steradians, solid curve). This outcome may represent an early functional indication of fenestrated Ca2+ egress from the channel into cytoplasm (Fig. 7f), according with crystallographic structures of K channels55.
Thus, nanodomain Ca2+ diffusion could focus Ca2+ through particular molecular geometries within the channel-signaling complex, raising the possibility of enhanced preferential signaling to target molecules near or within exit portals (Fig. 7f, gray ball). Indeed, while prior studies elegantly suggest that distinctive unitary current amplitudes i render CaV2.2 channels favorable for triggering vesicle fusion25, it is also plausible that differing A and f values also factor into such optimization. The latter possibilities represent an intriguing, but nearly unexplored realm of Ca2+ biology. Though other interpretations are certainly viable, further empirical estimates of Caspike, parameter A, and rsensor will likely aid in this field of inquiry.
Methods
Molecular biology
CaV2.2/TN-XL (CaV2.2 is of human origin (NM000718)) fusion construct was made by PCR, using fusion primers. Pfu polymerase (Strategene) was used for fidelity. To make CaV2.2/TN-XL, we first generated a CaV2.2 channel with the C-terminus truncated at the 2180th amino acid and without the stop codon, CaV2.2Δ2180 stopless/XbaI in pcDNA3.1. This was made by cutting CaV2.2 with Xho I and Xba I, and replacing the resulting fragment with the PCR product amplified from CaV2.2 between the Xho I and 2180th amino acid sites using forward primer CGCATCAGTTACAATGACATG, and reverse primer CTGTCTAGAAGCACCAGATGTTGA CAGCA. Next, TN-XL was PCR-amplified with an Xba I site upstream of CFP (ATG removed) and a Nhe I/Spe I site downstream of the stop codon of Citrine. The forward and reverse primers used were CGGCCGCCACCTCTAGAGTGAGC, and CAGACTAGTGCTAGCTTAGTCCTC GATGTTGTGGC respectively. Finally, the TN-XL PCR fragment was spliced into the CaV2.2Δ2180 stopless/XbaI construct at the Xba I site to give our CaV2.2/TN-XL construct. For the variant construct in Fig. 7c, the circularly permuted Citrine in TN-XL was replaced with circularly permuted amber53, and YFP fused to the amino terminus of CaV2.2 after removal of the first 81 amino acids22 (nearly the entire amino terminus).
Verification by complete sequence analysis of regions subjected to PCR was performed.
HEK293 cell culture and transfection
HEK293 cells were cultured on No-1 25 mm glass cover slips (Bellco glass) in 10 cm plates. These slips were coated overnight with 0.01% (wt/v; diluted 1:5 in 0.1 M Borate buffer) poly-D-lysine (Sigma), and washed with ddH20 on the day cells were seeded onto the slips. The poly-D-lysine coating facilitates proper whole-cell voltage control at the membrane/glass interface56, and also increases electrostatic attraction between the glass surface and cells, thus favoring cell attachment and optimal TIRF imaging. Cells were transiently transfected, using calcium-phosphate precipitation54, with CaV2.2/TN-XL, rat brain β2a54, and rat brain α2δ54 (10 μg each). CaV2.2 and β2a-CFP were substituted for CaV2.2/TN-XL and β2a respectively in some experiments. Cotransfection of channels with a number of molecules did not appreciably improve channel trafficking in our system: PI3K and Akt/PKB57, ORL receptors58, and dominant-negative dynamin59. TN-XL and TN-XL-Ras sensors were transfected using FuGENE6 (Roche).
Electrophysiology
Whole-cell and single-channel current records were obtained at room temperature 1–3 d post-transfection using patch-clamp amplifiers Axopatch 200B and Axopatch 200A (Axon Instruments), respectively.
For whole-cell recordings, the cells were bathed in external solution containing (in mM): TEA-MeSO3, 140; HEPES (pH 7.4 with TEA-OH), 10; and CaCl2, 10; at 300 mOsm, adjusted with glucose. The internal solution contained (in mM): Cs-MeSO3, 114–135; CsCl2, 5; EGTA, 1 or 10; MgCl2, 1; MgATP, 4; and HEPES (pH 7.4 with CsOH), 10; 295 mOsm with Cs-MeSO3. In some experiments, 10 μM Fluo 4FF and 2.5 μM Alexa 568 (Invitrogen, Molecular Probes) were included for ratiometric Ca2+ determination. Electrodes were pulled from borosilicate glass capillaries(WPI MTW 150-F4) and had pipette resistances ranging from 1.5–2.5 MΩ before 75% series resistance compensation. Voltage pulses were applied at 90 sec intervals. Currents were filtered at 5 kHz, and digitized at 25 kHz. Leak and capacitance transients subtracted by P/8 protocol. Data acquired and analyzed with custom MATLAB scripts (Mathworks, Natick, MA).
Single-channel recordings were all conducted in the on-cell configuration. The bath contained (in mM): K Glutamate, 132; KCl, 5; NaCl, 5; MgCl2, 3; EGTA, 2; glucose, 10; and HEPES (pH 7.4 adjusted with KOH), 20; at 300 mOsm adjusted with glucose. This bath solution zeroed the membrane potential. The pipette solution contained, (in mM): BaCl2, 90; TEA-MeSO3, 20; HEPES (pH 7.4 adjusted with TEA-OH), 10; at 300 mOsm, adjusted with TEA-MeSO3. In some experiments, 10 mM CaCl2 was substituted for BaCl2 and TEA-MeSO3 correspondingly increased. To minimize electrical noise, 5–20 MΩ patch pipettes were pulled from ultra-thick-walled (2 mm outer diameter, 1.16 mm inner diameter) borosilicate glass (BF200-116-10, Sutter Instruments), and coated with Sylgard. Seal resistance was ~80 GΩ. Voltage pulses applied at repetition intervals of 5–15 s; data sampled at 70 μsec intervals, and filtered at 2 kHz (Fig. 3c, d) or 700 Hz (Fig. 7a).
TIRF microscopy
On the day of experiments, the cover slips on which cells were cultured were mounted on a custom bath chamber for TIRF imaging. Fluorescence measurements of single cells were performed on a Nikon Eclipse TE2000U microscope, equipped with a Plan Apo 60 × TIRF objective (NA 1.45 oil immersion). Nikon immersion oil, with nd = 1.515 (23 °C) was used. For TN-XL imaging, CFP excitation was delivered by a 16-mW Melles Griot 56ICS 442-nm diode laser. For Fluo 4FF/Alexa 568 imaging, excitation was delivered by a 40-mW Melles Griot IMA100 argon laser featuring a 514-nm line. For TN-XL imaging, the optical path diagrammed in Supplementary Figure S4 was used, as described below. A high-resolution CoolSNAP HQ CCD camera (Photometrics), driven by IPLab (Scanalytics) scientific imaging software, was used for cell orientation and verification under TIRF microscopy. Fast quantitative data, averaged across single cells, were instead acquired via PMTs, as follows. Single-cell fluorescent signals was selected by the image-plane pinhole in a D-104C dual-channel photometer (PTI), and sent to a dichroic mirror (510dclp) which divides the fluorescence emission signals into CFP and FRET signals. These two signals passed through their respective emitters (HQ485/30m and HQ545/40m, Chroma), before being captured by two Hamamatsu R1527 PMTs. Fluorescent signals were filtered at 2 kHz and sampled at 25 kHz, and reflect the activity of several thousand CaV2.2/TN-XL molecules. Shutter control, dark current subtraction, and data acquisition were controlled by custom MATLAB programs. For Fluo 4FF/Alexa 568 imaging, the optical path was modified slightly. Single-cell fluorescence was isolated via the same pinhole and then sent to a dichroic mirror (545 dclp) which parsed signals into Fluo 4FF and Alexa 568 signals. These two signals passed through their respective emitters (545/40 nm bandpass and HQ580 nm lp, Chroma), before being captured by the same PMTs. The apparent Kd for Fluo 4FF/Alexa 568 ratios under our recording conditions was 20 μM, as determined in live HEK293 cells dialyzed with Ca2+ dye and a series of calibrated [Ca2+] internal solutions.
Widefield FRET microscopy
33-FRET efficiency determination, along with estimation of the ratio of donor to acceptor molecules, was performed within individual live HEK293 cells imaged through a image-plane pinhole according to CFP, FRET, and YFP filter cubes54. Moreover, rsensor in Fig. 7d was estimated using a Forster distance of 49 Å54.
Immunoblots
HEK293 cells expressing CaV2.2/TN-XL or TN-XL-Ras were washed and harvested with PBS, centrifuged, and resuspended in lysis buffer (20 mM Tris-HCl [pH 7.5], 300 mM sucrose, 1 mM DTT and protease inhibitor cocktail (Roche)). Lysis was completed with freeze-thaw cycles and sonication. Membrane proteins were separated by initial centrifugation (16,000g ×10 min at 4°C), followed by solubilizing the resulting pellet in 1% Triton X-100. This solution was then subjected to centrifugation (16,000g ×10 min at 4°C), yielding membrane proteins in the supernatant. Membrane proteins were denatured (95°C ×1 min) upon dilution with TGS buffer (Bio-Rad), and resolved by 5% SDS-PAGE with no CaCl2 added to the gel. Proteins were transferred to nitrocellulose membranes (Bio-Rad) by cooled transfer (Tris/Glycine transfer buffer/pH 8.3 ×19 h). After blocking, membranes were sequentially incubated with rabbit polyclonal anti-GFP antibody (Abcam Inc., 1:5000 dilution as directed by manufacturer) and HRP-conjugated secondary antibody (Sigma, 1:2400 dilution as recommended by manufacturer). Protein bands were visualized with enhanced chemiluminescence (Pierce ECL, Thermo Scientific).
Data Analysis and Modeling
Single-channel ramp data were analyzed for CaV2.2/TN-XL unitary current and open probability using custom MATLAB programs10. For the analysis, all patches contained 1 or 2 active channels. TIRF fluorescent signals from CaV2.2/TN-XL were modeled using custom MATLAB scripts, using approaches detailed in Supplementary Methods. Generalization of the Neher-Stern equation (Eq. 1) for f values other than unity was accomplished by considering mass balance and substituting Q1/f for Q1 in Eq. 6 of the original Stern paper13.
Supplementary Material
Acknowledgments
Manu Ben Johny contributed valuable insights to the work. Michael Tadross created acquisition software, as well as advising on numerical simulations and Fluo 4FF imaging. The custom bath chamber for mounting cover slips was a kind gift from Luis Fernando Santana, University of Washington. Supported by a grant from the NIMH (R01 MH65531 to D.T.Y.), and from the NHLBI (Kirschstein-NRSA T32 HL07227-35 to I.E.D.).
Footnotes
Author contributions
L.H.T. established the TIRF/patch-clamp apparatus, designed and performed research, analysed data, and wrote the paper. I.E.D. performed extensive electrophysiological experiments, FRET imaging, and data analysis. W.Y. performed western blots and molecular biology. M.M. and O.G. guided use of TN-XL. D.T.Y. conceived experiments and analyses, established and performed numerical simulations, analysed data, made figures, and wrote the paper.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Parekh AB. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci. 2011;36:78–87. doi: 10.1016/j.tibs.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Bautista DM, Lewis RS. Modulation of plasma membrane calcium-ATPase activity by local calcium microdomains near CRAC channels in human T cells. J Physiol. 2004;556:805–817. doi: 10.1113/jphysiol.2003.060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkefeld H, et al. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 5.Hudmon A, et al. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans RM, Zamponi GW. Presynaptic Ca2+ channels--integration centers for neuronal signaling pathways. Trends Neurosci. 2006;29:617–624. doi: 10.1016/j.tins.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 8.Ma H, Groth RD, Wheeler DG, Barrett CF, Tsien RW. Excitation-transcription coupling in sympathetic neurons and the molecular mechanism of its initiation. Neurosci Res. 2011;70:2–8. doi: 10.1016/j.neures.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolmetsch R. Excitation-transcription coupling: signaling by ion channels to the nucleus. Sci STKE. 2003;2003:PE4. doi: 10.1126/stke.2003.166.pe4. [DOI] [PubMed] [Google Scholar]
- 10.Tadross MR, Dick IE, Yue DT. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell. 2008;133:1228–1240. doi: 10.1016/j.cell.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 12.Sherman A, Keizer J, Rinzel J. Domain model for Ca2(+)-inactivation of Ca2+ channels at low channel density. Biophys J. 1990;58:985–995. doi: 10.1016/S0006-3495(90)82443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern MD. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992;13:183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- 14.Simon SM, Llinas RR. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985;48:485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogelson AL, Zucker RS. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J. 1985;48:1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klingauf J, Neher E. Modeling buffered Ca2+ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 19.Sugimori M, Lang EJ, Silver RB, Llinas R. High-resolution measurement of the time course of calcium-concentration microdomains at squid presynaptic terminals. Biol Bull. 1994;187:300–303. doi: 10.2307/1542286. [DOI] [PubMed] [Google Scholar]
- 20.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 21.Liang H, et al. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–960. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- 22.Dick IE, et al. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature. 2008;451:830–834. doi: 10.1038/nature06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borst JG, Sakmann B. Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. J Physiol. 1998;506 (Pt 1):143–157. doi: 10.1111/j.1469-7793.1998.143bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley EF. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993;11:1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- 25.Weber AM, et al. N-type Ca2+ channels carry the largest current: implications for nanodomains and transmitter release. Nat Neurosci. 2011;13:1348–1350. doi: 10.1038/nn.2657. [DOI] [PubMed] [Google Scholar]
- 26.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 27.Schermelleh L, Heintzmann R, Leonhardt H. A guide to super-resolution fluorescence microscopy. J Cell Biol. 2010;190:165–175. doi: 10.1083/jcb.201002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song LS, Sham JS, Stern MD, Lakatta EG, Cheng H. Direct measurement of SR release flux by tracking ‘Ca2+ spikes’ in rat cardiac myocytes. J Physiol (Lond) 1998;512:677–691. doi: 10.1111/j.1469-7793.1998.677bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Axelrod D, Thompson NL, Burghardt TP. Total internal inflection fluorescent microscopy. J Microsc. 1983;129:19–28. doi: 10.1111/j.1365-2818.1983.tb04158.x. [DOI] [PubMed] [Google Scholar]
- 30.Demuro A, Parker I. Imaging single-channel calcium microdomains. Cell Calcium. 2006;40:413–422. doi: 10.1016/j.ceca.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang MC, et al. 3D structure of the skeletal muscle dihydropyridine receptor. J Mol Biol. 2002;323:85–98. doi: 10.1016/s0022-2836(02)00890-2. [DOI] [PubMed] [Google Scholar]
- 33.Synge EH. A suggested method for extending microscopic resolution into the ultramicroscopic region. Philosophical Magazine. 1928;6:356–362. [Google Scholar]
- 34.Blinks JR, Wier WG, Hess P, Prendergast FG. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40:1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- 35.Brini M, et al. Nuclear Ca2+ concentration measured with specifically targeted recombinant aequorin. Embo J. 1993;12:4813–4819. doi: 10.1002/j.1460-2075.1993.tb06170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montero M, et al. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. Embo J. 1995;14:5467–5475. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 38.Tour O, et al. Calcium Green FlAsH as a genetically targeted small-molecule calcium indicator. Nat Chem Biol. 2007;3:423–431. doi: 10.1038/nchembio.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadley RW, Lederer WJ. Properties of L-type calcium channel gating current in isolated guinea pig ventricular myocytes. J Gen Physiol. 1991;98:265–285. doi: 10.1085/jgp.98.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyawaki A, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 41.Tallini YN, et al. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci U S A. 2006;103:4753–4758. doi: 10.1073/pnas.0509378103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heim N, Griesbeck O. Genetically encoded indicators of cellular calcium dynamics based on troponin C and green fluorescent protein. J Biol Chem. 2004;279:14280–14286. doi: 10.1074/jbc.M312751200. [DOI] [PubMed] [Google Scholar]
- 43.Mori MX, Erickson MG, Yue DT. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 2004;304:432–435. doi: 10.1126/science.1093490. [DOI] [PubMed] [Google Scholar]
- 44.Mank M, et al. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys J. 2006;90:1790–1796. doi: 10.1529/biophysj.105.073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colecraft HM, Brody DL, Yue DT. G-protein inhibition of N- and P/Q-type calcium channels: distinctive elementary mechanisms and their functional impact. J Neurosci. 2001;21:1137–1147. doi: 10.1523/JNEUROSCI.21-04-01137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noceti F, et al. Effective gating charges per channel in voltage-dependent K+ and Ca2+ channels. J Gen Physiol. 1996;108:143–155. doi: 10.1085/jgp.108.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones LP, DeMaria CD, Yue DT. N-type calcium channel inactivation probed by gating-current analysis. Biophys J. 1999;76:2530–2552. doi: 10.1016/S0006-3495(99)77407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tay LH, Griesbeck O, Yue DT. Live-Cell Transforms between Ca2+ Transients and FRET Responses for a Troponin-C-Based Ca2+ Sensor. Biophys J. 2007;93:4031–4040. doi: 10.1529/biophysj.107.109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang K, Colecraft HM. Mechanism of auxiliary {beta}-subunit-mediated membrane targeting of L-type (CaV1.2) channels. J Physiol. 2011;589:4437–4455. doi: 10.1113/jphysiol.2011.214247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linse S, Helmersson A, Forsen S. Calcium binding to calmodulin and its globular domains. J Biol Chem. 1991;266:8050–8054. [PubMed] [Google Scholar]
- 51.Martin SR, Andersson Teleman A, Bayley PM, Drakenberg T, Forsen S. Kinetics of calcium dissociation from calmodulin and its tryptic fragments. A stopped-flow fluorescence study using Quin 2 reveals a two-domain structure. Eur J Biochem. 1985;151:543–550. doi: 10.1111/j.1432-1033.1985.tb09137.x. [DOI] [PubMed] [Google Scholar]
- 52.Chaudhuri D, Issa JB, Yue DT. Elementary mechanisms producing facilitation of Cav2.1 (P/Q-type) channels. J Gen Physiol. 2007;129:385–401. doi: 10.1085/jgp.200709749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koushik SV, Chen H, Thaler C, Puhl HL, 3rd, Vogel SS. Cerulean, Venus, and VenusY67C FRET reference standards. Biophys J. 2006;91:L99–L101. doi: 10.1529/biophysj.106.096206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca(2+) channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 55.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 56.Blunck R, Starace DM, Correa AM, Bezanilla F. Detecting rearrangements of shaker and NaChBac in real-time with fluorescence spectroscopy in patch-clamped mammalian cells. Biophys J. 2004;86:3966–3980. doi: 10.1529/biophysj.103.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viard P, et al. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- 58.Altier C, et al. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- 59.Green EM, Barrett CF, Bultynck G, Shamah SM, Dolmetsch RE. The tumor suppressor eIF3e mediates calcium-dependent internalization of the L-type calcium channel CaV1.2. Neuron. 2007;55:615–632. doi: 10.1016/j.neuron.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kits KS, de Vlieger TA, Kooi BW, Mansvelder HD. Diffusion barriers limit the effect of mobile calcium buffers on exocytosis of large dense cored vesicles. Biophys J. 1999;76:1693–1705. doi: 10.1016/S0006-3495(99)77328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.