Summary
Background
The biogenesis of autophagosomes, the hallmark of autophagy, depends on the function of the autophagy-related (Atg) proteins and the generation of phosphatidylinositol-3-phosphate (PtdIns3P) at the phagophore assembly site (PAS), the location where autophagosomes arise. The current model is that PtdIns3P is involved primarily in the recruitment of Atg proteins to the PAS and that once an autophagosome is complete, the Atg machinery is released from its surface back into the cytoplasm and reused for the formation of new vesicles.
Results
We have identified a PtdIns3P phosphatase, Ymr1, that is essential for the normal progression of both bulk and selective types of autophagy. This protein is recruited to the PAS at an early stage of formation of this structure through a process that requires both its GRAM domain and its catalytic activity. In the absence of Ymr1, Atg proteins fail to dissociate from the limiting membrane of autophagosomes, and these vesicles accumulate in the cytoplasm.
Conclusions
Our data thus reveal a key role for PtdIns3P turnover in the regulation of the late steps of autophagosome biogenesis and indicate that the disassembly of the Atg machinery from the surface of autophagosomes is a requisite for their fusion with the vacuole.
Introduction
Macroautophagy (hereafter called autophagy) is a transport pathway conserved among eukaryotes that ensures cellular homeostasis during stress conditions like nutrient deprivation. It is essential in a growing number of physiological processes [1, 2] and is associated with the pathophysiology of numerous diseases including cancer and neurodegeneration [3]. Via autophagy, cytoplasmic structures are sequestered into large double-membrane vesicles termed autophagosomes before being released into the lumen of mammalian lysosomes, or plant and yeast vacuoles, to be degraded by hydrolases, allowing the resulting metabolites to be used as either a source of energy or building blocks [4]. This transport function is also exploited to deliver a subset of vacuolar hydrolases to the vacuole in yeast [5] and to secrete specific proteins into the extracellular space in mammalian cells [6].
Upon autophagy induction, autophagy-related (Atg) proteins are recruited in a precise hierarchical order to a perivacuolar structure called the phagophore assembly site (PAS) [7]. According to the current model, the concerted action of the Atg proteins at the PAS first leads to the formation of a membranous cistern known as the phagophore and then mediates its expansion and closure to form an autophagosome. The current view that still remains to be experimentally demonstrated is that autophagosomes are ready to fuse with the lysosome/vacuole once the Atg machinery is released from the surface of the sealed vesicles into the cytoplasm [8, 9]. Autophagosome formation also relies on the generation of phosphatidylinositol-3-phosphate (PtdIns3P) on the autophagosomal membranes by a protein complex containing the PtdIns 3 kinase Vps34 [10]. Although the function of PtdIns3P in autophagy is still poorly understood, this lipid is essential for recruitment of effector proteins such as the Atg18/WIPI protein family members [11–15]. The amount and spatial localization of PtdIns3P in intracellular membranes is tightly regulated by the concerted action of phosphoinositide kinases and phosphatases. Various PtdIns3P phosphatases of the myotubularin (MTMR) protein family, e.g., Jumpy (MTMR14), MTMR3, MTMR6, and MTMR7, act as negative regulators of autophagy in higher eukaryotes [16–18]. Although the PtdIns3P phosphatase Ymr1 is the unique MTMR protein existing in yeast [19], the phosphoinositide phosphatases Sac1 and the synaptojanin-like phosphatases Sjl2/Inp52 and Sjl3/Inp53 are also able to dephosphorylate PtdIns3P in this organism [19]. In this study, we have explored the role of PtdIns3P turnover in autophagy and determined that the clearance of this lipid from the surface of autophagosomes by Ymr1 is required for the release of Atg proteins and completion of these vesicles.
Results
Ymr1 Is Essential for Selective and Nonselective Types of Autophagy
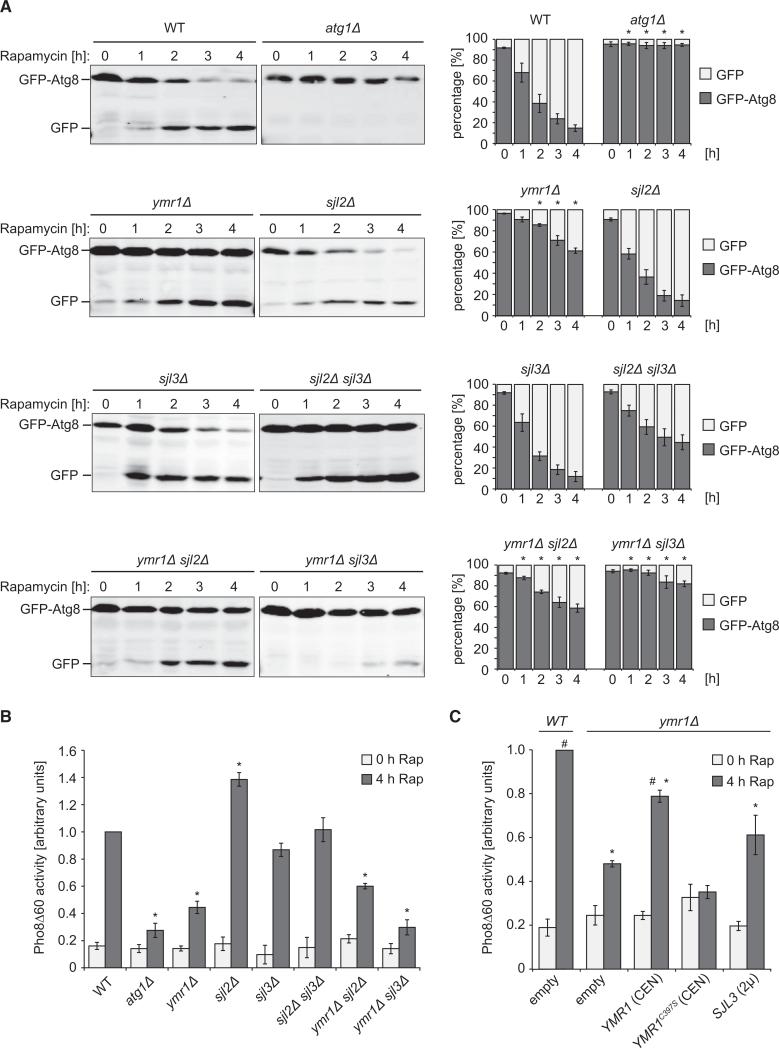
Because MTMR proteins are required for autophagy in mammals, we wondered whether Ymr1 is also involved in autophagy. The functionality of this pathway was tested in a strain lacking YMR1 by using the GFP-Atg8 processing assay [20]. As expected, in wild-type (WT) cells subjected to autophagy conditions, the amounts of free GFP increased over time, whereas no free GFP was detected in the atg1Δ strain, which displays a defect in autophagosome formation (Figure 1A). A significant delay in autophagy flux was observed in ymr1Δ compared to WT cells. In the absence of either SJL2 or SJL3, in contrast, the GFP-Atg8 processing kinetics was essentially the same as the WT (Figure 1A). Because yeast PtdIns3P phosphatases are functionally redundant [21], we wondered whether these proteins could complement each other during autophagy. When either SJL2 and SJL3 or YMR1 and SJL2 were simultaneously deleted, the GFP-Atg8 processing was not significantly different from the WT. The knockout of both YMR1 and SJL3, however, caused an almost complete block in autophagy (Figure 1A). Autophagy was additionally tested with the quantitative Pho8Δ60 assay [22]. This enzymatic assay confirmed the GFP-Atg8 processing assay data and also revealed that autophagy was not reduced, but upregulated, when SJL2 was deleted (Figure 1B). We decided to continue our investigations with the ymr1Δ strain, which displays similar cellular PtdIns3P levels as WT cells and thus, unlike the ymr1Δ sjl3Δ double mutant, does not exhibit general defects in membrane trafficking and signaling [21, 23]. By using standard protocols [24, 25], we determined that Ymr1 is also required in selective types of autophagy such as mitophagy and pexophagy, where excess mitochondria and peroxisomes, respectively, are degraded (Figure S1 available online). Together, these results reveal that Ymr1 is necessary for the normal progression of both bulk and selective types of autophagy.
Figure 1. PtdIns3P Phosphatases Are Essential for Autophagy.
(A) WT (SEY6210), atg1Δ (WHY1), ymr1Δ (YMR1Δ), sjl2Δ (JGY130), sjl3Δ (JGY131), sjl2Δ sjl3Δ (JGY132), ymr1Δ sjl2Δ (BPY06), and ymr1Δ sjl3Δ (BPY01) strains carrying the pCuGFPAtg8(416) plasmid were incubated with rapamycin (Rap) to induce autophagy and culture aliquots were collected at intervals of 1 hr. GFP-Atg8 cleavage was determined by western blot with anti-GFP antibodies, and the percentages of GFP-Atg8 and GFP were plotted.
(B) WT (YTS159), atg9Δ (FRY300), ymr1Δ (ECY190), sjl2Δ (ECY194), sjl3Δ (ECY189), sjl2Δ sjl3Δ (ECY192), ymr1Δ sjl2Δ (ECY200), and ymr1Δ sjl3Δ (ECY193) cells expressing Pho8D60 were grown and treated as in (A) before measuring Pho8Δ60 activity.
(C) The WT (YTS159) or the ymr1Δ (ECY190) strain harboring pRS426 (empty), pYMR1(416) [YMR1(CEN)], pYMR1MUT(416) [YMR1C397S(CEN)], or pRSSJL3(426) [SJL3(2μ)] plasmids were processed as in (B).
The graphs represent the average of three experiments ± SEM. The asterisks indicate a significant difference with the WT, while the number sign shows a significant difference with the ymr1Δ strain harboring pRS426 (p values < 0.05). See also Figure S1.
Ymr1 Phosphatase Activity Is Required during Autophagy
To test the importance of Ymr1 enzymatic activity during autophagy, we generated the phosphatase-dead mutant Ymr1C397S [26]. The Pho8Δ60 and GFP-Atg8 processing assays showed that the ymr1Δ strain carrying the YMR1C397S allele had a severe impairment in autophagy flux, whereas the control cells expressing YMR1 displayed no defect (Figure 1C and data not shown). This result demonstrates that the PtdIns3P phosphatase activity of Ymr1 is crucial for autophagy.
Although the SJL3 deletion exacerbated the autophagy defect of the ymr1Δ strain (Figures 1A and 1B), SJL3 overexpression was unable to rescue the phenotype of ymr1Δ cells (Figure 1C). This result appears to indicate that Sjl3 cannot substitute for Ymr1 during autophagy, but it cannot be excluded that excessively high Sjl3 levels could have a negative effect on this pathway.
Ymr1 Localizes to the PAS
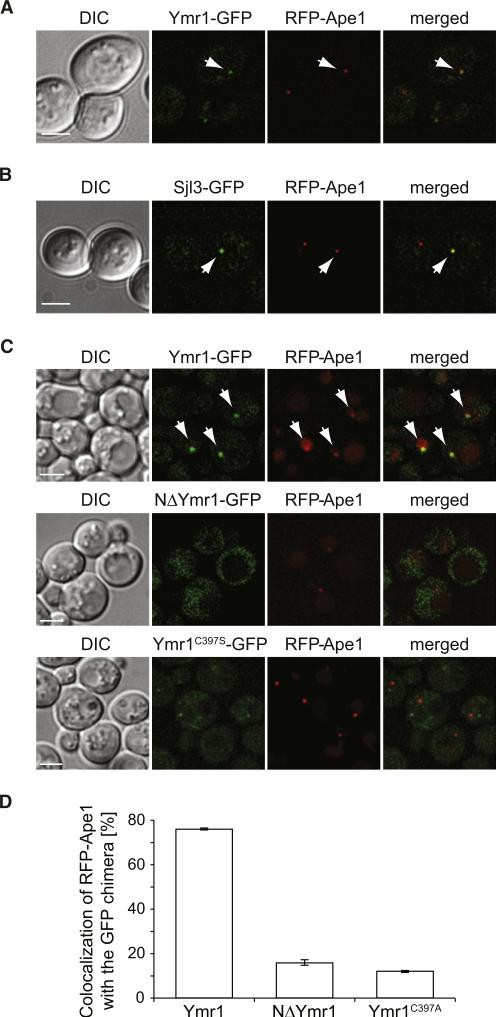
Next, we explored the subcellular distribution of genomically GFP-tagged Ymr1. Under autophagy conditions, this fusion protein was distributed to cytoplasmic puncta, and one of them, prominent and perivacuolar, occasionally colocalized with the PAS marker proteins RFP-Ape1 and mChe-Atg8 (Figures 2A and 2C and data not shown) [7]. In agreement with the fact that the additional deletion of SJL3 exacerbates the autophagy defect of the ymr1Δ strain, we also found that Sjl3 associated with the PAS under the same conditions (Figure 2B). These data show that Ymr1 (and Sjl3) is recruited to the PAS, where it presumably executes a direct role in autophagy.
Figure 2. The PH-GRAM Domain and Phosphatase Activity Are Required for Ymr1 Localization to the PAS.
(A) WT cells expressing RFP-Ape1 and Ymr1-GFP (ECY202) were incubated with rapamycin for 3 hr before collecting images.
(B) Cells expressing RFP-Ape1 and Sjl3-GFP (AVY070) were treated and imaged as in (A).
(C) The ymr1Δ RFP-Ape1 (ECY207) strain carrying integrated pYMR1GFP(406) (Ymr1-GFP), pNΔYMR1GFP(406) (NΔYmr1-GFP), or pYMR1C397SGFP(406) (Ymr1C397S-GFP) were starved for 3 hr in SD-N medium before imaging. Arrows highlight colocalization. DIC, differential interference contrast.
(D) Quantification of the degree of RFP-Ape1 colocalization with the GFP-tagged proteins in the samples imaged in (C). The graphs represent the average of two experiments ± SEM.
Scale bars represent 5 μm. See also Figure S2.
We next analyzed which protein domains are necessary for the recruitment of Ymr1 to autophagosomal membranes. A truncated form of Ymr1 lacking the N-terminal PH-GRAM domain [21], i.e., NΔYmr1-GFP, was mainly dispersed in the cytoplasm and showed no colocalization with RFP-Ape1 (Figures 2C and 2D). Similar results were obtained with the phosphatase-dead version of Ymr1, indicating that the PH-GRAM domain and a functional catalytic site are required for Ymr1 to associate with the PAS.
The deletion of Atg proteins also had a negative effect on the recruitment of Ymr1-GFP to the PAS (Figure S2), which was especially evident for cells lacking ATG13, a key organizational factor of the PAS [7]. This result suggests that Ymr1 associates with the PAS at an early formation stage through a process that is favored by an intact Atg machinery and/or progression of autophagosome biogenesis. Finally, Ymr1 was absent from complete autophagosomes accumulated by deleting VAM3 [27], showing that Ymr1 dissociates from these vesicles upon completion (Figure S2).
Autophagosomes Accumulate in the Cytoplasm of ymr1Δ Cells
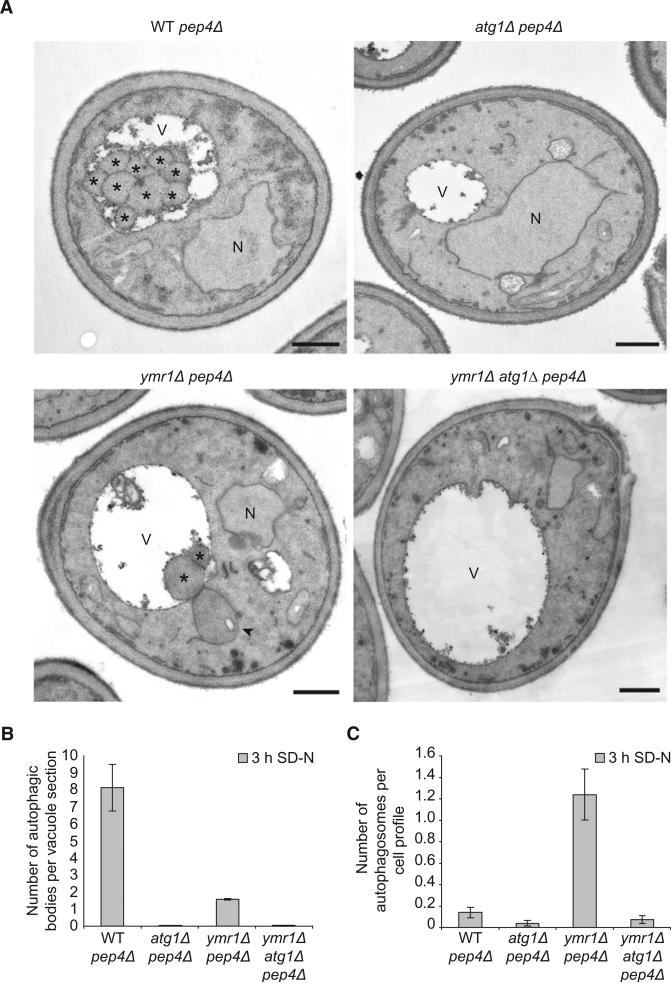
Electron microscopy (EM) analysis of nitrogen-starved ymr1Δ cells lacking Pep4, the main vacuolar protease [28], also revealed a severe autophagy defect, because only a few autophagic bodies accumulated in the vacuole of this strain in comparison to the pep4Δ control strain (Figures 3A and 3B). This examination also revealed that ymr1Δ pep4Δ cells accumulated large vesicles in the cytoplasm that resembled autophagosomes (Figures 3A and S3A, arrowhead) [29]. These structures were completely absent in the pep4Δ and atg1Δ pep4Δ control strains, and also in the ymr1Δ atg1Δ pep4Δ mutant indicating a probable autophagosomal origin (Figures 3A and 3C). The autophagic nature of these vesicles was additionally confirmed by fluorescence microscopy with the autophagosomal marker protein mChe-Atg8. Whereas WT and atg1Δ strains exposed to rapamycin occasionally displayed a single fluorescent punctum representing the PAS (Figures S3B–S3D), the autophagosomes accumulated in the vam3Δ knockout were visualized as multiple Atg8-positive puncta [27], which dramatically decreased in number when vesicle biogenesis was blocked by the additional deletion of ATG1 (Figures S3B–S3D). A similar situation was observed in the ymr1Δ strain; the incubation with rapamycin led to an increase in the number of mChe-Atg8 puncta, but to a lesser extent than the vam3Δ knockout, while the ymr1Δ atg1Δ strain displayed no or one mChe-Atg8 puncta per cell (Figures S3B–S3D). Together, these analyses indicate that the vesicles accumulated when the PtdIns3P turnover is blocked are autophagosomes.
Figure 3. Electron Microscopy Analysis of the ymr1Δ Strain.
WT pep4Δ (TVY1), atg1Δ pep4Δ (YTS113), ymr1Δ pep4Δ (AVY059), and ymr1Δ atg1Δ pep4Δ (ECY191) cells were grown in rich medium to early log phase and then transferred into SD-N medium for 3 hr before processing the samples for EM as described in the Experimental Procedures.
(A) Micrographs of starved cells. Autophagic bodies are marked with an asterisk, while an autophagosome is highlighted with an arrowhead. N, nucleus; V, vacuole. Scale bars represent 500 nm.
(B) Quantification of the autophagic bodies.
(C) Quantification of autophagosome accumulation.
In (B) and (C), the results are expressed as the average number of autophagic bodies per vacuole and autophagosomes per cell section, respectively. Error bars represent the SD in the counting of two different grids.
See also Figure S3.
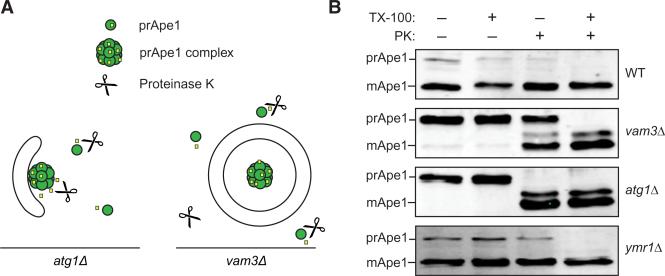
To distinguish whether these structures are sealed autophagosomes or elongated, but still unsealed, late phagophores, we performed a protease protection assay (Figure 4) [30]. The prApe1 oligomer, an autophagosome cargo, from starved WT and atg1Δ cells was entirely processed by proteinase K independently from the presence of the detergent TX-100 (Figure 4B; note that in the WT, most of the Ape1 has reached the vacuole and is already processed, so that only the small cytoplasmic population can be analyzed). In the vam3Δ strain, in contrast, the part of prApe1 resistant to proteinase K represents the pool enclosed in the autophagosome interior, which was completely processed after membrane solubilization with TX-100 (Figure 4B). Similarly, a subpopulation of prApe1 from the ymr1Δ strain was also resistant to proteinase K treatment, revealing that the autophagosomes accumulated in the absence of Ymr1 are closed.
Figure 4. Closed Autophagosomes Accumulate in the Absence of Ymr1.
(A) Schematic explaining the principle of the prApe1 protease protection assay.
(B) The WT (SEY6210), vam3Δ (CWY40), atg1Δ (WHY1), and ymr1Δ (YMR1Δ) cells were converted to spheroplasts and starved for 3 hr in SD-N. Total cell extracts from lysed spheroplasts were centrifuged and the pellet fraction was either not treated, or mixed with proteinase K (PK) and/or TX-100 detergent before being incubated on ice for 30 min. After protein precipitation, samples were analyzed by immunoblots with anti-prApe1 antibodies [38]. The experiment was repeated four times with identical results.
In sum, the ultrastructural analyses and the protease protection assay show that the large vesicles accumulated in the cytoplasm in the absence of YMR1 are autophagosomes.
Atg Proteins Remain Associated with the Autophagosomes Accumulated in the ymr1Δ Strain
Several Atg proteins are abundantly present on phagophores but absent or in lower amounts on autophagosomes, indicating that they release from the autophagosome surface into the cytosol when these vesicles are completed [9, 31, 32]. We therefore wondered whether the accumulation of autophagosomes in ymr1Δ cells could be caused by a dissociation defect of Atg proteins from these vesicles, which could potentially lead to an inhibition of their fusion with the vacuole.
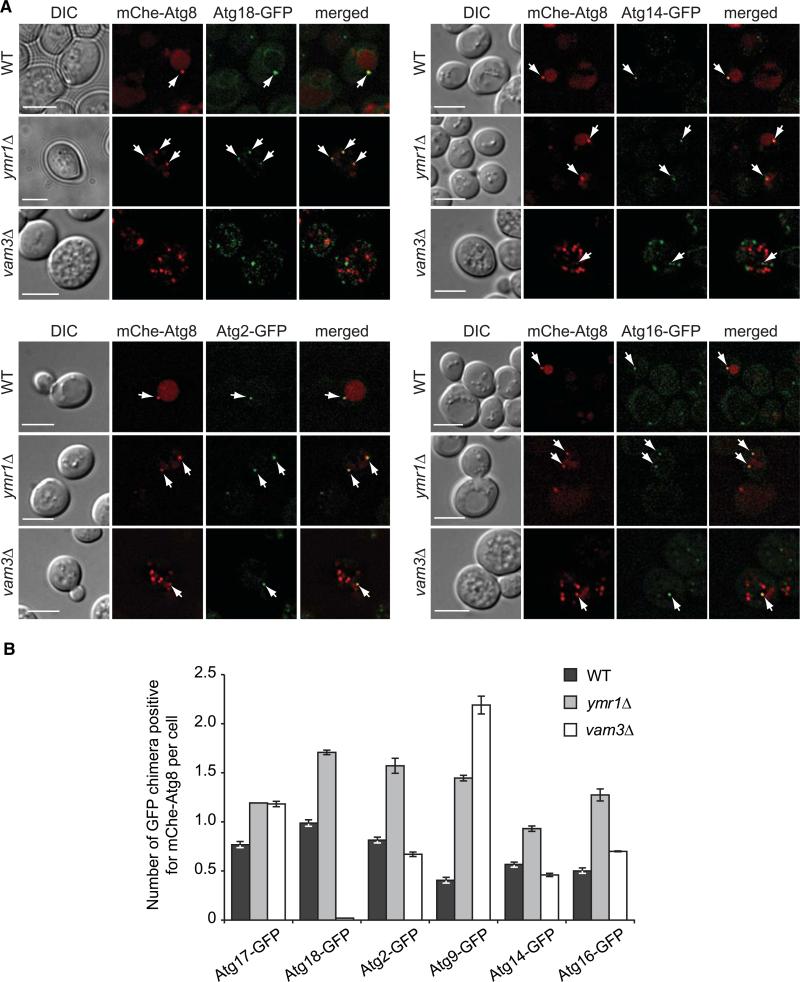
For analysis of their distribution, endogenously GFP-tagged versions of Atg2, Atg9, Atg14, Atg16, Atg17, and Atg18 were colocalized with mChe-Atg8 under autophagy-inducing conditions (Figures 5 and S4). The number of GFP-tagged Atg puncta associated with mChe-Atg8 (Figure 5B) and the percentage of colocalization of mChe-Atg8 with each fluorescent fusion protein (Figure S4B) were determined in the ymr1Δ strain and compared to those obtained with WT and vam3Δ cells, where mChe-Atg8 labels the PAS and autophagosomes, respectively. The autophagosomes accumulated in the ymr1Δ mutant were positive for all the Atg proteins tested, and the colocalization percentages of mChe-Atg8 were almost identical to those obtained with WT cells, but higher compared to vam3Δ cells (Figures 5B and S4B). Additionally, the number of Atg protein-positive puncta associated with mChe-Atg8 per cell was also higher in the absence of YMR1 than in WT and vam3Δ cells (Figure 5B). These results show that Atg proteins are abundantly present on the PAS and autophagosomes accumulated in ymr1Δ cells but not on “ready-to-fuse” mature autophagosomes.
Figure 5. Atg Proteins Remain Associated with Autophagosomes in the ymr1Δ Strain.
WT, ymr1Δ, and vam3Δ strains expressing endogenous Atg2-GFP (PSY102, ECY155, and ECY183), Atg9-GFP (FRY162, ECY153, and AVY078) Atg14-GFP (PSY142, ECY162, and ECY184), Atg16-GFP (KTY148, ECY157, and ECY185), Atg17-GFP (ECY167, ECY169, and ECY172), or Atg18-GFP (PSY62, ECY137, and ECY147) and the pCumCheAtg8(415) plasmid were treated with rapamycin for 3 hr and imaged.
(A) Fluorescence microscopy images of the various strains. Arrows highlight colocalizations. DIC, differential interference contrast. Scale bars represent 5 μm.
(B) Quantification of the experiments presented in (A) and Figure S4A by determining the average number of GFP chimera positive for mCheAtg8 per cell. Error bars represent the SEM.
See also Figure S4.
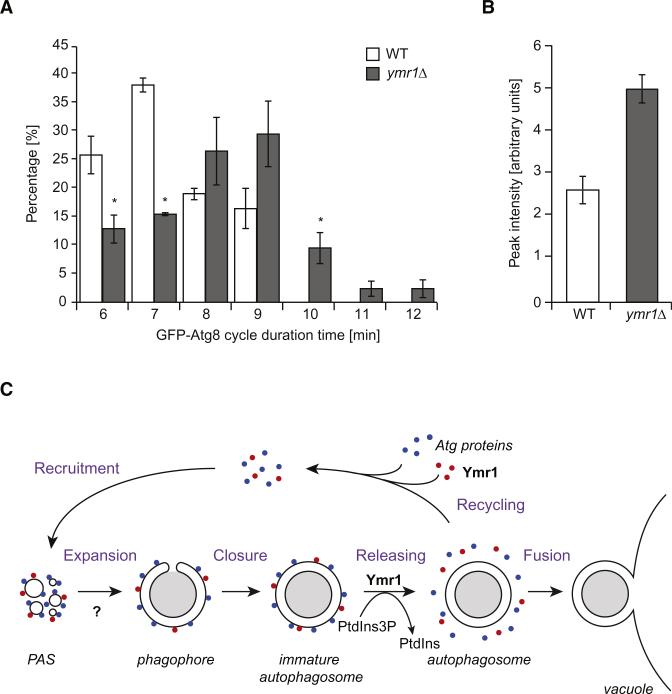
During autophagosome formation, Atg8 is present onto both sides of the expanding phagophores, and upon double-membrane vesicle completion, the Atg8 pool on the surface of these structures is released in the cytoplasm while the internal Atg8 pool remains trapped [31]. Thus, Atg8 protein levels periodically change at the PAS, probably reflecting the process of elongation/completion during autophagosome biogenesis [33]. Because the absence of Ymr1 affects the dissociation of Atg proteins from completed autophagosomes (Figure 5), we explored whether the dynamic association of Atg8 with the PAS was also altered in this background. As previously reported, the fluorescence intensity of GFP-Atg8 at the PAS initially increases, then gradually decreases and finally disappears [33]. While GFP-Atg8 dots exhibited a periodic change of PAS intensity with duration ranging from 6 to 7 min in WT cells, the GFP-Atg8 cycle in the absence of Ymr1 often displayed a longer duration pattern, i.e., from 6 to 12 min (Figure 6A). We additionally determined the intensity of GFP-Atg8 puncta in the WT and ymr1Δ strains. Since the average GFP-Atg8 cycle time is 7.3 min for WT and 8.3 min for ymr1Δ, we focused on a pool displaying a 7 min cycle from WT and 8 min from ymr1Δ. The relative peak intensity in ymr1Δ was about two times of that seen in the WT, i.e., 5.1 versus 2.7 (Figure 6B). These results indicate that Ymr1 is required for efficient Atg8 dissociation from the PAS, and in its absence this event is delayed leading to increased levels of Atg8 at this location.
Figure 6. Atg8 Dynamic Association with the PAS Is Impaired in the Absence of YMR1.
(A) PtdIns3P turnover is important for Atg8 dissociation from the PAS. WT and ymr1Δ cells expressing endogenous GFP-Atg8 (MZY089 and MZY090) were exposed for 3 hr to rapamycin before being analyzed by microscopy. The cycle of GFP-Atg8 at the PAS was determined by quantification of the time elapsed from the appearance of a GFP-Atg8 punctum until it disappears. All data points came from four independent experiments; error bars indicate the SEM, and asterisks show significant differences.
(B) Atg8 accumulates at the PAS in the absence of YMR1. The fluorescence intensity was calculated as described in the Experimental Procedures. Data points came from three independent experiments and error bars indicate the SEM.
(C) Proposed model describing the role of Ymr1 during autophagosome biogenesis. This phosphatase is recruited at the early stage of PAS formation, when all the Atg proteins start to assemble following a hierarchical order at this site [7]. The Atg machinery first mediates the formation of the phagophore and then expands it into an immature autophagosome. The release of the Atg proteins and Ymr1, a step that is at least in part regulated through the hydrolysis of PtdIns3P into PtdIns by Ymr1 and probably required for the recycling of the Atg machinery, leads to the completion of the autophagosome, which is then ready to fuse with the vacuole. Our data cannot exclude a function of Ymr1 during the initial phases of the Atg machinery assembly at the PAS (question mark).
Together, these data show that Atg proteins are released from the surface of autophagosomes when they are completed and that the PtsIns3P turnover is required for this event.
Discussion
The generation of PtdIns3P by the Vps34 lipid kinase is a key event required during, at least, the initial steps of autophagosome formation [7]. Here, we reveal that dephosphorylation of PtdIns3P by the PtdIns3P phosphatase Ymr1 is also crucial for the normal progression of autophagy. Ymr1 has a direct role in autophagy because this protein localizes to the PAS. Importantly, ymr1Δ cells do not display additional trafficking impairments that could indirectly affect autophagy [21]. While the ymr1Δ mutant exhibits a partial but important defect in autophagy, this pathway is completely inhibited when YMR1 and SJL3 are simultaneously deleted. This indicates a certain degree of redundancy among these phosphatases in autophagy, which has been already reported for other cellular processes [19]. This notion is also supported by the fact that Sjl3 can also localize to the PAS and that the cellular levels of PtdIns3P in the ymr1Δ sjl3Δ double knockout are exacerbated while those in the ymr1Δ strain are indistinguishable from those in the WT [21].
Ymr1 association with the PAS is favored by an intact Atg machinery and requires the Ymr1 N-terminal PH-GRAM motif. Because this domain can mediate membrane association by binding either phosphoinositides [34, 35] or proteins [36], additional investigations are necessary to unveil the type of interactions used for Ymr1 recruitment to the PAS. While the phosphatase-dead version of Ymr1 is still detected as punctate structures, probably endosomes [21], the localization of this protein to the PAS requires its phosphatase activity (Figure 2C). Jumpy association with autophagosomal membranes, in contrast, does not depend on its catalytic activity [16]. While the cause of this phenotype is unclear, one speculative idea is that Ymr1 modulation of PtdIns3P levels at the PAS also provides a regulatory loop for its recruitment to this location.
The ymr1Δ cells accumulate autophagosomes onto which the Atg proteins are still present. Because the Atg proteins (except Atg8) cannot be detected on the “ready-to-fuse” autophagosomes seen in the vam3Δ strain, our data suggest that the Atg machinery has to be released from complete autophagosomes to allow their fusion with vacuoles. The PtdIns3P turnover mediated by Ymr1 would have a key role in this regulation (Figure 6C) by preventing the premature and potentially harmful fusion of phagophores with the vacuole. A plausible hypothesis is that the clearance of PtdIns3P triggers the dissociation of those Atg proteins that bind to this lipid, which in turn leads to the disassembly of all the Atg machinery including Ymr1. This could appear to not be an essential step in autophagy because ymr1Δ cells display only a partial defect in autophagosome fusion with vacuoles, but we think that the presence of Sjl3 prevents a complete block. Interestingly, a role for PtdIns3P turnover in the regulation of organelle maturation is not unprecedented and is required for the formation of multivesicular bodies (MVBs) [19, 37].
Although previous studies show mammalian MTMR phosphatases such as Jumpy and MTMR3 acting as negative regulators in autophagy [16, 17], our data point to Ymr1 being a positive regulator of yeast autophagy. The functional overlap between phosphoinositide phosphatases could explain the apparent discrepancy between our data and those in mammals. It is possible that the implication of some PtdIns3P phosphatases as positive autophagy regulators has been overlooked in higher eukaryotes because phenotypes have only been scrutinized in cells where MTMR proteins were individually knocked down [16]. Nonetheless, MTMR phosphatases seem to have a conserved molecular role in yeast and mammalian autophagy by promoting the dissociation of Atg proteins from autophagosomal membranes (Figure 6C) [16, 17]. Accordingly, PtdIns3P phosphatases could have opposite effects on autophagy progression depending on when they act during autophagosome biogenesis. By functioning at early steps like the mammalian Jumpy and MTMR3 [16, 17], they would have a negative effect on autophagy through the inhibition of Atg machinery recruitment to the PAS/phagophore. According to our data, the deletion of SJL2 has a positive effect on autophagy, suggesting that this enzyme could be the yeast paralog of Jumpy and MTMR3, but this needs further investigation. In contrast, by acting in the release of Atg proteins from the surface of autophagosomes, the PtdIns3P phosphatases could have a positive role on autophagy progression promoting the fusion of these vesicles with vacuoles/lysosomes. This is the function in autophagy that we propose for Ymr1, and MTMR3 could also have a similar role in mammalian cells because its overexpression reduces autophagosome size, suggesting a possible premature release of the Atg machinery [17].
Because Ymr1 associates with the PAS we cannot exclude that this phosphatase regulates other steps of autophagosome biogenesis prior to vesicle completion. Hence, autophagosomes appear to be formed less efficiently in the ymr1Δ strain than in WT cells; i.e., the total number of autophagosomes and autophagic bodies in the mutant is lower than the number of autophagic bodies detected in the WT, indicating a partial defect of autophagy progression. While this could be caused by the impaired recycling of Atg proteins from complete autophagosomes for reuse, a defect in the PAS or phagophore formation and expansion could also explain this phenotype. Electron microscopy of ymr1Δ cells, however, has failed to detect an accumulation of expanded phagophores and, consequently, if there is a defect, this is probably at an early stage of PAS and phagophore assembly, where Jumpy and MTMR3 also appear to act [16, 17].
Ymr1 is one of the few proteins involved in autophagosome biogenesis that is required for a step beyond the initial nucleation that leads to PAS organization. Because Ymr1 is present at the PAS, its activity is probably spatially and temporally highly regulated. Future studies are required to identify the factors involved in the control of Ymr1 function during autophagy and how PtdIns3P turnover mediates the release of the Atg machinery from autophagosomes.
Experimental Procedures
Experimental procedures are described in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
The authors thank Christian Ungermann and Scott Emr for reagents, and Christian Ungermann and Ingrid Jordens for critically reading the manuscript. F.R. is supported by ZonMW-VIDI (917.76.329), ECHO (700.59.003), ALW Open Program (821.02.017), and DFG-NWO cooperation (DN 82-303) grants. E.C. is supported by an ALW Open Program grant (817.02.023) to B.H. and F.R. D.J.K. and M.Z. are supported by NIH grant GM53396 to D.J.K.
Footnotes
Supplemental Information
Supplemental Information includes Supplemental Experimental Procedures, four figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2012.06.029.
References
- 1.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 5.Lynch-Day MA, Klionsky DJ. The Cvt pathway as a model for selective autophagy. FEBS Lett. 2010;584:1359–1366. doi: 10.1016/j.febslet.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 2011;30:4701–4711. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki K, Kubota Y, Sekito T, Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12:209–218. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Ohsumi Y. Current knowledge of the pre-autophagosomal structure (PAS). FEBS Lett. 2010;584:1280–1286. doi: 10.1016/j.febslet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Noda T, Fujita N, Yoshimori T. The late stages of autophagy: how does the end begin? Cell Death Differ. 2009;16:984–990. doi: 10.1038/cdd.2009.54. [DOI] [PubMed] [Google Scholar]
- 10.Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polson HE, de Lartigue J, Rigden DJ, Reedijk M, Urbé S, Clague MJ, Tooze SA. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy. 2010;6:506–522. doi: 10.4161/auto.6.4.11863. [DOI] [PubMed] [Google Scholar]
- 12.Lu Q, Yang P, Huang X, Hu W, Guo B, Wu F, Lin L, Kovács AL, Yu L, Zhang H. The WD40 repeat PtdIns(3)P-binding protein EPG-6 regulates progression of omegasomes to autophagosomes. Dev. Cell. 2011;21:343–357. doi: 10.1016/j.devcel.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Barth H, Meiling-Wesse K, Epple UD, Thumm M. Autophagy and the cytoplasm to vacuole targeting pathway both require Aut10p. FEBS Lett. 2001;508:23–28. doi: 10.1016/s0014-5793(01)03016-2. [DOI] [PubMed] [Google Scholar]
- 14.Meiling-Wesse K, Barth H, Voss C, Eskelinen EL, Epple UD, Thumm M. Atg21 is required for effective recruitment of Atg8 to the preautophagosomal structure during the Cvt pathway. J. Biol. Chem. 2004;279:37741–37750. doi: 10.1074/jbc.M401066200. [DOI] [PubMed] [Google Scholar]
- 15.Guan J, Stromhaug PE, George MD, Habibzadegah-Tari P, Bevan A, Dunn WA, Jr., Klionsky DJ. Cvt18/Gsa12 is required for cytoplasm-to-vacuole transport, pexophagy, and autophagy in Saccharomyces cerevisiae and Pichia pastoris. Mol. Biol. Cell. 2001;12:3821–3838. doi: 10.1091/mbc.12.12.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergne I, Roberts E, Elmaoued RA, Tosch V, Delgado MA, Proikas-Cezanne T, Laporte J, Deretic V. Control of autophagy initiation by phosphoinositide 3-phosphatase Jumpy. EMBO J. 2009;28:2244–2258. doi: 10.1038/emboj.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taguchi-Atarashi N, Hamasaki M, Matsunaga K, Omori H, Ktistakis NT, Yoshimori T, Noda T. Modulation of local PtdIns3P levels by the PI phosphatase MTMR3 regulates constitutive autophagy. Traffic. 2010;11:468–478. doi: 10.1111/j.1600-0854.2010.01034.x. [DOI] [PubMed] [Google Scholar]
- 18.Dowling JJ, Low SE, Busta AS, Feldman EL. Zebrafish MTMR14 is required for excitation-contraction coupling, developmental motor function and the regulation of autophagy. Hum. Mol. Genet. 2010;19:2668–2681. doi: 10.1093/hmg/ddq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2007;1771:353–404. doi: 10.1016/j.bbalip.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheong H, Klionsky DJ. Biochemical methods to monitor autophagy-related processes in yeast. Methods Enzymol. 2008;451:1–26. doi: 10.1016/S0076-6879(08)03201-1. [DOI] [PubMed] [Google Scholar]
- 21.Parrish WR, Stefan CJ, Emr SD. Essential role for the myotubularin-related phosphatase Ymr1p and the synaptojanin-like phosphatases Sjl2p and Sjl3p in regulation of phosphatidylinositol 3-phosphate in yeast. Mol. Biol. Cell. 2004;15:3567–3579. doi: 10.1091/mbc.E04-03-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiesli P, Bernays R, Brändle M, Zwimpfer C, Seiler H, Zapf J, Spinas GA, Schmid C. Effect of pituitary surgery in patients with acromegaly on adiponectin serum concentrations and alanine aminotransferase activity. Clin. Chim. Acta. 2005;352:175–181. doi: 10.1016/j.cccn.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Parrish WR, Stefan CJ, Emr SD. PtdIns(3)P accumulation in triple lipid-phosphatase-deletion mutants triggers lethal hyper-activation of the Rho1p/Pkc1p cell-integrity MAP kinase pathway. J. Cell Sci. 2005;118:5589–5601. doi: 10.1242/jcs.02649. [DOI] [PubMed] [Google Scholar]
- 24.Hutchins MU, Veenhuis M, Klionsky DJ. Peroxisome degradation in Saccharomyces cerevisiae is dependent on machinery of macroautophagy and the Cvt pathway. J. Cell Sci. 1999;112:4079–4087. doi: 10.1242/jcs.112.22.4079. [DOI] [PubMed] [Google Scholar]
- 25.Kanki T, Kang D, Klionsky DJ. Monitoring mitophagy in yeast: the Om45-GFP processing assay. Autophagy. 2009;5:1186–1189. doi: 10.4161/auto.5.8.9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streuli M, Krueger NX, Tsai AY, Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc. Natl. Acad. Sci. USA. 1989;86:8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darsow T, Rieder SE, Emr SD. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J. Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Vaart A, Griffith J, Reggiori F. Exit from the Golgi is required for the expansion of the autophagosomal phagophore in yeast Saccharomyces cerevisiae. Mol. Biol. Cell. 2010;21:2270–2284. doi: 10.1091/mbc.E09-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krick R, Bremer S, Welter E, Schlotterhose P, Muehe Y, Eskelinen EL, Thumm M. Cdc48/p97 and Shp1/p47 regulate autophagosome biogenesis in concert with ubiquitin-like Atg8. J. Cell Biol. 2010;190:965–973. doi: 10.1083/jcb.201002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J. Cell Biol. 1999;147:435–446. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geng J, Baba M, Nair U, Klionsky DJ. Quantitative analysis of autophagy-related protein stoichiometry by fluorescence microscopy. J. Cell Biol. 2008;182:129–140. doi: 10.1083/jcb.200711112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsujita K, Itoh T, Ijuin T, Yamamoto A, Shisheva A, Laporte J, Takenawa T. Myotubularin regulates the function of the late endosome through the gram domain-phosphatidylinositol 3,5-bisphosphate interaction. J. Biol. Chem. 2004;279:13817–13824. doi: 10.1074/jbc.M312294200. [DOI] [PubMed] [Google Scholar]
- 35.Lorenzo O, Urbé S, Clague MJ. Analysis of phosphoinositide binding domain properties within the myotubularin-related protein MTMR3. J. Cell Sci. 2005;118:2005–2012. doi: 10.1242/jcs.02325. [DOI] [PubMed] [Google Scholar]
- 36.Choudhury P, Srivastava S, Li Z, Ko K, Albaqumi M, Narayan K, Coetzee WA, Lemmon MA, Skolnik EY. Specificity of the myotubularin family of phosphatidylinositol-3-phosphatase is determined by the PH/GRAM domain. J. Biol. Chem. 2006;281:31762–31769. doi: 10.1074/jbc.M606344200. [DOI] [PubMed] [Google Scholar]
- 37.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mari M, Griffith J, Rieter E, Krishnappa L, Klionsky DJ, Reggiori F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J. Cell Biol. 2010;190:1005–1022. doi: 10.1083/jcb.200912089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.