Abstract
The functions of Beclin-1 in macroautophagy, tumorigenesis and cytokinesis are thought to be mediated by its association with the PI3K-III complex. Here, we describe a new role for Beclin-1 in mitotic chromosome congression that is independent of the PI3K-III complex and its role in autophagy. Beclin-1 depletion in HeLa cells leads to a significant reduction of the outer kinetochore proteins CENP-E, CENP-F and ZW10, and, consequently, the cells present severe problems in chromosome congression. Beclin-1 associates with kinetochore microtubules and forms discrete foci near the kinetochores of attached chromosomes. We show that Beclin-1 interacts directly with Zwint-1—a component of the KMN (KNL-1/Mis12/Ndc80) complex—which is essential for kinetochore–microtubule interactions. This suggests that Beclin-1 acts downstream of the KMN complex to influence the recruitment of outer kinetochore proteins and promotes accurate kinetochore anchoring to the spindle during mitosis.
Keywords: Beclin-1, chromosome congression, kinetochore assembly, mitosis, Zwint-1
INTRODUCTION
The mammalian class III phosphatidylinositol 3-kinase (PI3K-III) complex is involved in numerous membrane-trafficking events, cytokinesis and autophagy, and has been implicated in immunity, development, tumour suppression, lifespan extension and neurodegeneration [[1,2,3,4,5]. The core of the PI3K-III complex encompasses three proteins, the kinase VPS34, the regulatory kinase VPS15 and Beclin-1, and other regulatory proteins associate with the core complex [[5,6,7,8] depending on the particular cell function it is executing. Interestingly, monoallelic depletion of Beclin-1 reportedly causes chromosomal disorders such as aneuploidy and double-minute chromosomes [[9]. An open question is whether these phenotypes are a direct consequence of deficient autophagy, or whether they reflect other unidentified functions of Beclin-1. Indeed, there is growing evidence suggesting non-autophagic roles for ‘autophagy’ proteins [[10]. As aneuploidy is caused by errors in chromosome segregation, we asked in this study whether Beclin-1 might be directly involved in mitosis, beyond its already characterized role in cytokinesis (as part of the PI3K-III complex). We find that Beclin-1 does indeed have a mitosis-specific function. Beclin-1 depletion leads to severe chromosome congression defects, associated with a reduction in several outer kinetochore components, including ZW10, CENP-E and CENP-F. We also detect a direct interaction between Beclin-1 and the outer kinetochore component Zwint-1. None of these functions is compromised by depletion of other subunits of the PI3K-III complex, suggesting that they are specific to Beclin-1.
RESULTS AND DISCUSSION
To examine the involvement of Beclin-1 in cell cycle progression, we depleted Beclin-1 by small interfering RNA (siRNA) in HeLa cells (Fig 1A). Beclin-1 depletion led to an increased number of cells with 4n DNA content (56%) compared to control cells (25%), as determined by flow cytometry (Fig 1A, upper panel), together with the appearance of multinucleated cells (as previously reported [[3]). Importantly, the phenotype was significantly rescued by expression of a haemagglutinin (HA)-Beclin-1 construct resistant to the siRNA (Fig 1A, lower panel). These results suggested that Beclin-1 knockdown impaired progression through G2/M as well as cytokinesis. To differentiate between G2 and mitotic delay, we analysed the ability of cells depleted of Beclin-1 to resume mitosis following release from a nocodazole block. Whereas 40% of control cells reached G1 within 2 h of release, more than 60% of Beclin-1-depleted cells remained in mitosis even 8 h after release (Fig 1B). Similar results were obtained with a second siRNA targeting Beclin-1 (supplementary Fig S1A,B online). Cytological quantification of mitotic stages, using histone H3 phosphorylated on serine 10 (H3Ser10P) as a marker of mitosis, confirmed that Beclin-1 depletion significantly increased the mitotic index to 9.7% (compared to 3.4% in control) (Fig 1C), and most of these mitotic cells (88.5%) appeared to be in prometaphase (Fig 1D). In fact, we observed a 20-fold increase of cells with several non-aligned chromosomes around recognizable metaphase plates compared to the controls (Fig 1E; supplementary Fig S1C,D online). No obvious problems in nuclear envelope breakdown or overall centrosome and spindle organization were evident in Beclin-1 depleted cells (supplementary Fig S2 online). Altogether, these results show a major role for Beclin-1 in mitotic progression, particularly during prometaphase.
Figure 1.
Beclin-1 is required for proper mitotic progression. (A) Western blot of Beclin-1 and FACS profiles following siRNA treatment in HeLa cells and cells stably expressing a HA-Beclin-1 construct resistant to the siRNA targeting endogenous Beclin-1. (B) FACS profiles of cells treated with indicated siRNA following nocodazole blockage and release. (C,D) Quantification of cells in mitosis (C) and quantification of each step of mitosis (D) by H3Ser10P staining for Control and Beclin-1 siRNA-treated cells. Error bars represent standard deviations (s.d.) (n=number of counted cells) calculated on three independent experiments. Two and three asterisks indicate significant results (P=0.0028 and P=0.0006, respectively). (E) Knockdown of Beclin-1 increases the number of mitotic cells with misaligned chromosomes. siRNA-treated HeLa cells were fixed in PFA, immunolabelled for α-tubulin (green) and stained with DAPI (blue). White arrows indicate misaligned chromosomes. Quantification of cells with misaligned chromosomes is shown at the right (n=number of counted cells). Three asterisks indicate significant results (P=0.0001). Scale bar, 10 μm. DAPI, 4′,6-diamidino-2-phenylindole; HA, haemagglutinin; PFA, paraformaldehyde; siRNA, small interfering RNA.
We then investigated the function of Beclin-1 in chromosome alignment. To visualize both the microtubules and the chromosomes in living cells, we generated a HeLa cell line stably expressing YFP-α-tubulin and the histone H2B-Cherry (Fig 2A). In this cell line transfected with control siRNA, mitosis proceeded from nuclear envelope breakdown to anaphase in about 60 min, with chromosomes perfectly aligned on the metaphase plate (Fig 2A,G). Following Beclin-1 depletion, progression from nuclear envelope breakdown to anaphase in these cells took much longer (110 min in Fig 2C, 190 min in Fig 2D,G) than the 60 min seen in controls (Fig 2A,G). This delay in anaphase onset is regulated by the spindle checkpoint, as co-depleting Beclin-1 and the checkpoint protein MAD2 (Fig 2B) resulted in rapid progression through mitosis (Fig 2F–G). We observed three different classes of mitotic progression among the Beclin-1-depleted cells (Fig 2C–E). In the first class (7 of 23 cells), although prometaphase took longer, all the chromosomes ultimately aligned on the metaphase plate and anaphase proceeded normally (Fig 2C). In the second class (7 of 23 cells), anaphase began after a prolonged prometaphase even though some chromosomes had not yet congressed to the metaphase plate. Anaphases in these cells were abnormal with frequent lagging chromatids (Fig 2D). Finally, in the third class (9 of 23 cells), chromosomes failed to align, and after a long delay, the cells exited mitosis without undergoing a clear anaphase (Fig 2E). The relative frequency of the three different classes depended on the efficiency of Beclin-1 knockdown (Fig 2H). The more complete the Beclin-1 depletion, the more cells that underwent mitotic exit without proper anaphase (Fig 2H).
Figure 2.
Beclin-1 depletion prolongs prometaphase by delaying chromosome congression. (A–H) Time-lapse films of HeLa cells stably expressing YFP-α-tubulin and H2B-Cherry and treated with the indicated siRNA. The pictures are acquired every 10 min. Time lapse starts at nuclear envelope breakdown. (A) Mitosis in control cells. (B) Western blot of whole-cell lysates from Control, Beclin-1 and Beclin-1+Mad2 siRNA-treated cells. (C–E) Three different classes of mitotic progression were observed upon Beclin-1 depletion. Higher magnification of lagging chromatids in the anaphase of Beclin-1-depleted cells is shown on the right of (D). (F) The prometaphase delay is dependent on MAD2 and the spindle checkpoint. Cells treated simultaneously with siRNA targeting MAD2 and Beclin-1 rapidly exit mitosis. (G) Average duration of mitosis in different classes of Beclin-1-depleted cells, compared to control and MAD2 depletions. n=number of counted cells in three independent experiments. Error bars on graphs represent s.d. (H) The frequency of the different Beclin-1 phenotypes depended on the efficiency of Beclin-1 knockdown. Mitotic progression in cells treated with increasing concentrations of Beclin-1 siRNA was followed by video-microscopy. Left panel: western blot of Beclin-1 upon RNAi treatment; right panel: quantification of the appearance of the three different mitotic progression patterns, that is, prolonged mitosis with normal anaphase (as C), with lagging chromatids (as D) and mitotic exit without clear anaphase (as E) upon increased Beclin-1 knockdown experiments. NEB, nuclear envelop breakdown; RNAi, RNA interference; siRNA, small interfering RNA; YFP, yellow fluorescent protein.
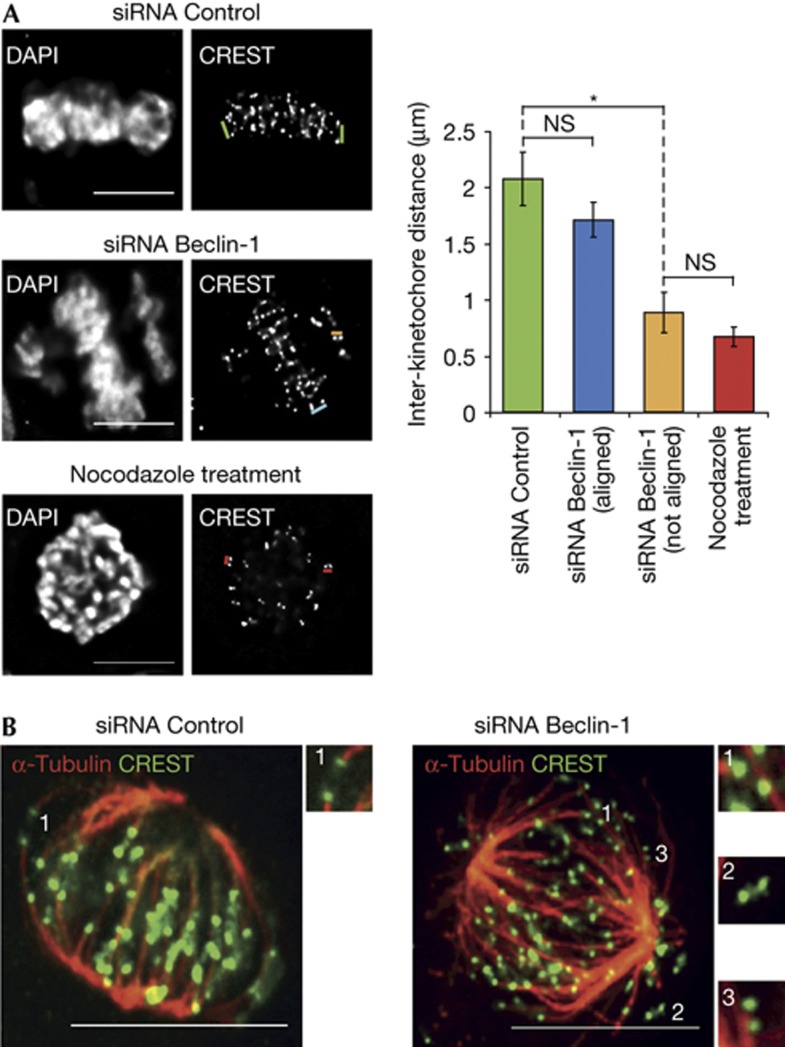
Defects in chromosome alignment suggest impairment of chromosome attachment or failure to correct attachment errors [[11]. The tension exerted by microtubules on properly attached kinetochores increases the distance between sister kinetochores [[11]. To assess the role of Beclin-1 in kinetochore–microtubule (K-MT) attachment, we measured the distance between sister kinetochores using CREST antisera, which recognize inner kinetochore proteins and the constitutive centromeric proteins CENP-A, B and C (Fig 3A). A significant reduction in inter-kinetochore distance of non-aligned chromosomes in Beclin-1-depleted cells (0.9 μm) was observed compared to that of aligned chromosomes in the same cells (1.7 μm) or in control cells (2.1 μm) (Fig 3A). This reduced tension was comparable to the loss of tension in nocodazole-treated control cells (0.7 μm; Fig 3A). Together with the time-lapse video-microscopy (Fig 2C–E), this latter result suggested that Beclin-1 depletion is impairing the ability of kinetochores to form proper K-MT attachments in a timely manner, providing an explanation for the observed chromosome alignment problems. On average, each Beclin-1-depleted cell had nine kinetochore pairs (range 3–15) that failed to align. To more closely examine K-MT interactions, control and Beclin-1-depleted cells were briefly cooled to 4°C to reveal the cold-stable k-fibres that specifically retain mitotic chromosomes. Control cells showed proper localization of the CREST signal at the terminal end of k-fibres, indicative of bi-oriented chromosomes (Fig 3B, number 1). In contrast, in Beclin-1-depleted cells, mono-oriented or totally unattached chromosomes were frequently observed (Fig 3B, numbers 2 and 3, respectively), indicating that Beclin-1 depletion delays the establishment of stable K-MT linkages. Nevertheless, as most chromosomes do align, and with near-normal inter-kinetochore tension (Fig 3), one plausible interpretation of our data is that in normal mitosis, Beclin-1 promotes an earlier stage in K-MT interactions, but is not required once mature, load-bearing K-MT attachments develop.
Figure 3.
Beclin-1 knockdown affects kinetochore–microtubule attachment. (A) Inter-kinetochore distance is reduced in unaligned chromosomes of Beclin-1-depleted cells. The distance between each kinetochore pair was quantified in siRNA-treated and control cells treated with nocodazole for 3 h before fixation. Cells were stained with anti-CREST antibody and DAPI. Quantification of the distance between kinetochore pairs (indicated by coloured bars) is reported at the bottom and done on sister kinetochores present in the same focal plane. One asterisk indicates significant result (P=0.0365). Graphs represent three independent experiments (error bars=s.d.). (B) Depletion of Beclin-1 leads to defects in K-MT attachment. siRNA-treated cells were cooled on ice before fixation to reveal stable k-fibres and then stained with anti-α-tubulin (red) and anti-CREST (green) antibodies. Numbers point to magnified areas and indicate the mode of attachment of k-fibres to kinetochores (1, bi-oriented; 2, unattached; 3, mono-oriented kinetochores). Scale bar, 10 μm. DAPI, 4′,6-diamidino-2-phenylindole; NS, not significant; siRNA, small interfering RNA.
We next examined the levels of several kinetochore components by immunofluorescence in Beclin-1-depleted cells (Fig 4). We found severe reductions of CENP-E (to 13.4% of control levels) and CENP-F (17.6%), as well as a moderate reduction of ZW10 (35.3%) (Fig 4A–C). We also found a reduction in the amount of MAD2 (28%) on unattached kinetochores (Fig 4D), consistent with the reduction in ZW10, which is required for its recruitment [[12]. As our earlier experiments (Fig 2F) showed that the prometaphase delay in Beclin-1-depleted cells is dependent on the spindle checkpoint, this reduced amount of MAD2 must still be adequate for checkpoint function. Indeed, cytoplasmic levels of MAD2 are unaffected when Beclin-1 is depleted (Fig 4D). HEC1 (Ndc80) and KNL-1, two important components of the KMN network, which forms the microtubule-binding module of the kinetochore [[13], are still properly recruited to unattached kinetochores in the absence of Beclin-1 (Fig 4E,F, respectively). Importantly, Beclin-1 depletion does not significantly affect overall expression levels of CENP-E, CENP-F, Zwint-1, ZW10, MAD2, HEC1 or KNL-1 (Fig 4G). Thus, the reduced kinetochore levels of CENP-E, CENP-F, ZW10 and, to a lesser extent, MAD2 reflect an important role for Beclin-1 in their recruitment or maintenance.
Figure 4.
Beclin-1 depletion reduces targeting of several outer kinetochore proteins. Control and Beclin-1-depleted cells were treated with nocodazole before fixation, and labelled with the indicated antibody. (A) CENP-E and CREST. Two asterisks indicate significant results, P=0.0053. (B) CENP-F and CREST. Three asterisks indicate significant results, P=0.0006. (C) ZW10 and CREST. Three asterisks indicate significant results, P=0.0007. (D) MAD2 and CREST. Two asterisks indicate significant results, P=0.0032. (E) HEC1 and CREST. (F) KNL-1 and CREST. On the right, magnified areas and quantification of the fluorescence intensity of the protein of interest normalized to the fluorescence intensity of CREST staining are shown. Graphs represent three independent experiments (error bars=s.d.). Scale bar, 10 μm. (G) Western blot of whole-cell lysates from control and Beclin-1 siRNA-treated cells. siRNA, small interfering RNA.
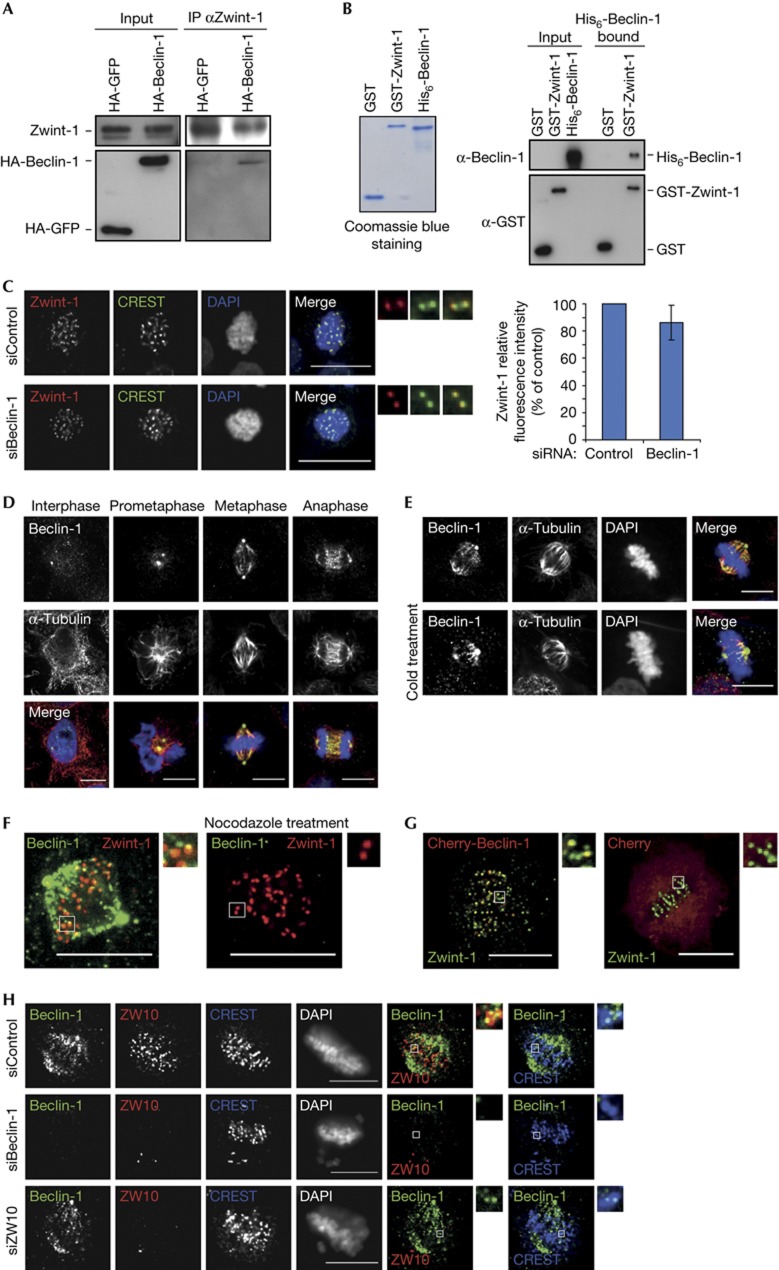
One possible clue to understanding the mechanism by which Beclin-1 is influencing kinetochore structure comes from an earlier report [[14] suggesting that a possible partner of Beclin-1 was Zwint-1, a component of the KMN network [[12, [15]. Perturbation of the KMN complex can reduce levels of other outer kinetochore components, including CENP-F, CENP-E, ZW10 and the spindle checkpoint protein MAD2 [[12, [16,17,18,19,20,21]. We confirmed the interaction between Zwint-1 and Beclin-1, as HA-tagged Beclin-1 transiently expressed in HeLa cells could be co-immunoprecipitated with antibodies to endogenous Zwint-1 (Fig 5A). Moreover, this interaction appeared to be direct, as recombinant purified His6-Beclin-1 interacted with recombinant purified GST-Zwint-1 (Fig 5B). However, Beclin-1 depletion had no effect on Zwint-1 kinetochore levels in nocodazole-arrested cells (Fig 5C), suggesting that Beclin-1 acts downstream of the KMN complex to influence the recruitment of outer kinetochore proteins.
Figure 5.
Beclin-1 interacts directly with Zwint-1 and localizes to the mitotic apparatus. (A) Immunoprecipitation of endogenous Zwint-1 in HeLa cell extracts expressing HA-Beclin-1 or HA-GFP. Input is 3.5% of the immunoprecipitated material. (B) GST-Zwint-1 or GST-beads were used to pull down recombinant purified His6-Beclin-1. (Left) Coomassie-blue-stained gel of the purified recombinant proteins (1 μg). (Right) A western blot of GST proteins and His6-Beclin-1 in the input and bound fractions. His6-Beclin-1 in the input represents 4% of the amount used for the pull-down. (C) Zwint-1 levels do not change in Beclin-1-depleted cells. Cells were treated with nocodazole before fixation. On the right, magnified individual kinetochore pairs stained for Zwint-1 (red) and CREST (green), and quantification of Zwint-1 levels normalized to CREST signal from three independent experiments (error bars=s.d.). (D) Beclin-1 is associated with both the centrosome and the mitotic spindle during mitosis. HeLa cells were permeabilized before fixation and immunostained for Beclin-1 (green), α-tubulin (red) and DNA (DAPI, blue). (E) Beclin-1 is retained on the k-fibres. HeLa cells were cooled on ice before permeabilization and fixation, and stained with anti-α-tubulin (red) and anti-Beclin-1 (green) antibodies. (F) Beclin-1 forms discrete foci in the vicinity of the outer kinetochore in a microtubule-dependent manner. Untreated cells (left) and nocodazole-treated cells (right) were pre-extracted and fixed before labelling with Beclin-1 (green) and Zwint-1 (red) antibodies. (G) The fluorescent-tagged Cherry-Beclin-1 forms foci in the vicinity of the outer kinetochore. HeLa cells transfected with indicated constructs were pre-extracted and fixed before labelling with Zwint-1 (green) antibody. Magnified areas are shown on the right. (H) Beclin-1 localization close to the kinetochore does not depend on the ZW10 protein. Cells treated with Control, Beclin-1 and ZW10 siRNA were pre-extracted and fixed before labelling with Beclin-1 (green), CREST (blue) and ZW10 (red) antibodies. DNA was visualized with DAPI. (C–H) Scale bar, 10 μm. DAPI, 4′,6-diamidino-2-phenylindole; DNA, deoxyribonucleic acid; GFP, green fluorescent protein; GST, glutathione S-transferase; HA, haemagglutinin.
Although Beclin-1 was not known to be a component of the mitotic apparatus, our immunostaining studies revealed that Beclin-1 did in fact mark spindle microtubules including cold-stable k-fibers during mitosis, as well as the centrosomes throughout the cell cycle (Fig 5D,E). In addition, weak but discrete foci of Beclin-1 could be seen in the vicinity of the outer kinetochores of attached chromosomes (Fig 5F). Importantly, transient transfection of the fluorescent-tagged Cherry-Beclin-1 in HeLa cells also revealed a few kinetochore-associated Beclin-1 spots (Fig 5G). However, Beclin-1’s presence near kinetochores was dependent on microtubules, as nocodazole-treated cells showed no Beclin-1 localization at the kinetochore (Fig 5F). Depletion of ZW10 had no effect on Beclin-1 localization, either on the microtubules or near the outer kinetochore (Fig 5H), even though Beclin-1 depletion reduces ZW10 kinetochore levels, suggesting that Beclin-1 acts upstream of the Rod-ZW10-Zwilch (RZZ) complex to promote accurate kinetochore anchoring to the spindle during mitosis.
Importantly, this new mitosis-specific function of Beclin-1 is not related to its role in autophagy, nor is it shared by any of its partners within the PI3K-III complex, as we saw no similar effect on mitotic progression in cells depleted of VPS34, VPS15, UVRAG, ATG5, LC3b or Bif-1 (supplementary Fig S3A–C online). Nor did depletion of VPS34, VPS15, ATG5 or LC3b have any effect on kinetochore levels of CENP-E, CENP-F and ZW10 in nocodazole-arrested cells, or on Beclin-1 localization during mitosis (supplementary Fig S3D–F and H online). In addition, none of the PI3K-III subunits labelled the mitotic spindle, although they did label the mid-body during cytokinesis (supplementary Fig S3G online). These results are also consistent with a report showing that the complex of VPS34 and Beclin-1 disassembles early in mitosis, following Cdk1 phosphorylation of VPS34 [[22]. This mitosis-specific function of Beclin-1 could partly explain an earlier report showing that Beclin-1 mono-allelic depletion in mouse primary cells caused chromosome instability, leading to double-minute chromosome and aneuploid cells [[9].
Altogether, our data support a model where Beclin-1, possibly by interacting with Zwint-1, contributes to the recruitment or maintenance of key proteins at the outer kinetochore. Although it is not obvious how Beclin-1 might influence outer kinetochore protein levels if it is itself not a bona fide component of the unattached outer kinetochore, a very similar behaviour has been reported for the RanGAP1–RanBP2 complex [[23, [24]. In fact, depletion of RanBP2 leads to a similar set of problems affecting chromosome congression, and similarly reduces kinetochore levels of CENP-E, CENP-F, ZW10 and MAD2 (among others). The shared phenotypes of RanBP2 and Beclin-1 depletions suggest that they might be acting through the same pathway. Future work will be required to explore in depth the role of Beclin-1 within this pathway.
METHODS
For detailed protocols, see supplementary information online.
Cell culture, transfections, siRNA and mammalian expression vectors. SiRNA (10 nM) transfections were performed on HeLa cells using Lipofectamine RNAiMAX (Invitrogen), according to the reverse transfection procedure described by the manufacturer. HeLa cells were transfected using the FUGENE-6 reagent (Roche Diagnostics) as described by the manufacturer. HeLa cells constitutively expressing the YFP-α-tubulin and the H2B-Cherry histone were generated to monitor mitotic progression in living cells.
Fluorescence microscopy. Immunofluorescence microscopy was performed on HeLa cells grown on coverslips and transfected with siRNA when indicated. To block cells in prometaphase, cells were treated for 3 h before fixation with 3 μg/ml nocodazole (Sigma-Aldrich). Depending on the antibodies used, cells were fixed either with methanol at −20 °C for 5 min or with 4% paraformaldehyde (PFA) in PBS for 25 min. For Beclin-1 and MAD2 stainings, cells were permeabilized in PBS/0.1% bovine sera albumin/0.05% saponin (Sigma-Aldrich) before fixation with 4% PFA in PBS for 25 min. To test the stability of MT capture at kinetochores, before fixation, cells were incubated for 5 min at 4°C in cold PBS, to destabilize most non-kinetochore MT. For immunofluorescence with Zwint-1, CENP-F, CENP-E and ZW10 antibodies, cells were pre-extracted for 2 min at 37°C in PEM (100 mM K-PIPES, pH 6.8, 5 mM EGTA, 2 mM MgCl2)–0.5% triton, and fixed in PEM/0.1% triton/4% PFA for 25 min, as described [[12].
Flow cytometry analysis. Forty-eight hours after siRNA transfection, cells were washed with PBS, harvested and fixed in 70% ethanol. After fixation, cells were washed in PBS and then stained with propidium iodide (Sigma-Aldrich) for 30 min at 37°C in a buffer containing 20 mM HEPES, 160 mM NaCl, 1 mM EGTA, 0.2 mg/ml RNAse A and 50 μg/ml propidium iodide.
Immunoprecipitation assay and pull-down assays. Coimmunoprecipitations were performed by incubating indicated whole-cell extracts for 4 h at 4°C with anti-Zwint-1 antibody coupled with protein G sepharose (GE Healthcare). Pull-down experiments were performed by incubating 400 ng of GST and GST-Zwint-1 (Abnova) proteins, and fixed on glutathione-sepharose beads (GE Healthcare), with 500 ng of purified His6-Beclin-1 in the interaction buffer (20 mM Tris HCl pH 8, 250 mM NaCl, 0.25% NP-40) for 2 h at 4°C.
Quantification of kinetochore-bound proteins. To quantify the amount of kinetochore-bound protein, the average pixel intensities from 10 kinetochores or more on three nocodazole-treated cells were measured using Image J software and background pixel intensities subtracted.
Statistical analysis. Statistical significance was analysed by paired two-tailed Student’s t-test and expressed as a P value. The number of analysed samples (n) is indicated. All experiments were performed as three independent experiments. When indicated, standard deviations are represented as scale bars on graphs.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank S. Emiliani, F. Margottin-Goguet and C. Transy for helpful discussions, P. Bourdoncle and B. Durel from the Imaging Facility of Cochin Institute for technical assistance, the Almouzni lab (Institut Curie, Paris) for the CREST autoantibody, the Malim lab for the expression vector HA-GFP, and E. Ségéral for the GST protein. S.F. was supported by the Ministère Français de l’enseignement supérieur et de la Recherche, and Fondation pour la Recherche Médicale, A.G. by a fellowship from SIDACTION. This work is funded by the ANR-07-JCJC-0102 programme and by ANRS.
Author contributions: S.F., A.G., M.G. and K.J. conceived, designed and performed the experiments. R.E.K. conceived and designed some experiments. C.B.T. conceived and designed the experiments, and supervised the study. All authors analysed the data and contributed to the writing of the manuscript, with major writing contributions from A.G., R.E.K. and C.B.T.
Footnotes
The authors declare that they have no conflict of interest.
References
- Yang Z, Klionsky DJ (2010) Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 22: 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoresen SB, Pedersen NM, Liestol K, Stenmark H (2010) A phosphatidylinositol 3-kinase class III sub-complex containing VPS15, VPS34, Beclin 1, UVRAG and BIF-1 regulates cytokinesis and degradative endocytic traffic. Exp Cell Res 316: 3368–3378 [DOI] [PubMed] [Google Scholar]
- Sagona AP, Nezis IP, Pedersen NM, Liestol K, Poulton J, Rusten TE, Skotheim RI, Raiborg C, Stenmark H (2010) PtdIns(3)P controls cytokinesis through KIF13A-mediated recruitment of FYVE-CENT to the midbody. Nat Cell Biol 12: 362–371 [DOI] [PubMed] [Google Scholar]
- He C, Levine B (2010) The Beclin 1 interactome. Curr Opin Cell Biol 22: 140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburk SF, Wang QJ, Yue Z (2010) The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond. Trends Cell Biol 20: 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z (2009) Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol 11: 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K et al. (2009) Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Bio 11: 385–396 [DOI] [PubMed] [Google Scholar]
- Abrahamsen H, Stenmark H, Platta HW (2012) Ubiquitination and phosphorylation of Beclin 1 and its binding partners: tuning class III phosphatidylinositol 3-kinase activity and tumor suppression. FEBS Lett 586: 1584–1591 [DOI] [PubMed] [Google Scholar]
- Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E (2007) Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev 21: 1367–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S, Malhotra V (2013) Non-autophagic roles of autophagy-related proteins. EMBO Rep 14: 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezi L, Musacchio A (2009) Sister chromatid tension and the spindle assembly checkpoint. Curr Opin Cell Biol 21: 785–795 [DOI] [PubMed] [Google Scholar]
- Kops GJ, Kim Y, Weaver BA, Mao Y, McLeod I, Yates JR 3rd, Tagaya M, Cleveland DW (2005) ZW10 links mitotic checkpoint signaling to the structural kinetochore. J Cell Biol 169: 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Desai A (2008) Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol 9: 33–46 [DOI] [PubMed] [Google Scholar]
- Behrends C, Sowa ME, Gygi SP, Harper JW (2010) Network organization of the human autophagy system. Nature 466: 68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuse C, Iwasaki O, Kiyomitsu T, Goshima G, Toyoda Y, Yanagida M (2004) A conserved Mis12 centromere complex is linked to heterochromatic HP1 and outer kinetochore protein Zwint-1. Nat Cell Biol 6: 1135–1141 [DOI] [PubMed] [Google Scholar]
- Starr DA, Saffery R, Li Z, Simpson AE, Choo KH, Yen TJ, Goldberg ML (2000) HZwint-1, a novel human kinetochore component that interacts with HZW10. J Cell Sci 113(Pt 11): 1939–1950 [DOI] [PubMed] [Google Scholar]
- Lin YT, Chen Y, Wu G, Lee WH (2006) Hec1 sequentially recruits Zwint-1 and ZW10 to kinetochores for faithful chromosome segregation and spindle checkpoint control. Oncogene 25: 6901–6914 [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR 3rd, Oegema K, Desai A (2004) A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev 18: 2255–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H et al. (2004) Human Zwint-1 specifies localization of Zeste White 10 to kinetochores and is essential for mitotic checkpoint signaling. J Biol Chem 279: 54590–54598 [DOI] [PubMed] [Google Scholar]
- Bomont P, Maddox P, Shah JV, Desai AB, Cleveland DW (2005) Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J 24: 3927–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Mancini MA, Chang KH, Liu CY, Chen CF, Shan B, Jones D, Yang-Feng TL, Lee WH (1995) Characterization of a novel 350-kilodalton nuclear phosphoprotein that is specifically involved in mitotic-phase progression. Mol Cel Biol 15: 5017–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T et al. (2010) Negative regulation of Vps34 by Cdk mediated phosphorylation. Mol Cell 38: 500–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Liu ST, Jablonski SA, Yen TJ, Dasso M (2004) The RanGAP1–RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr Biol: CB 14: 611–617 [DOI] [PubMed] [Google Scholar]
- Salina D, Enarson P, Rattner JB, Burke B (2003) Nup358 integrates nuclear envelope breakdown with kinetochore assembly. J Cell Biol 162: 991–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.