Abstract
Propagation of tau pathology is linked with progressive neurodegeneration, but the mechanism underlying trans-synaptic spread of tau is unknown. We show that stimulation of neuronal activity, or AMPA receptor activation, induces tau release from healthy, mature cortical neurons. Notably, phosphorylation of extracellular tau appears reduced in comparison with intracellular tau. We also find that AMPA-induced release of tau is calcium-dependent. Blocking pre-synaptic vesicle release by tetanus toxin and inhibiting neuronal activity with tetrodotoxin both significantly impair AMPA-mediated tau release. Tau secretion is therefore a regulatable process, dysregulation of which could lead to the spread of tau pathology in disease.
Keywords: calcium, neurodegeneration, phosphorylation, receptors, tau
INTRODUCTION
In Alzheimer’s disease and related disorders, characteristic neuropathological depositions of the cytoskeletal protein tau spread progressively from the entorhinal cortex to anatomically connected brain regions [1, 2]. Moreover, injection of brain extracts from mice expressing P301S mutant tau into brains of transgenic mice expressing wild-type tau induced tau pathology that spread from the site of injection to adjacent brain regions [3]. The mechanisms controlling neuronal transmission of tau pathology are unknown, but one possibility is that tau, the primary component of neurofibrillary tangles, is released following neuronal death, allowing it to be taken up by neighbouring cells [4]. However, in two lines of transgenic mice overexpressing a single isoform of mutant tau (P301L), known to cause fronto-temporal dementia, inter-neuronal transfer of mutant tau appears to occur before any marked neurodegeneration [5, 6]. Moreover, tau pathology appeared to cross synapses; monosynaptic spread leading to the induction of tau pathology in neighbouring entorhinal neurons, and trans-synaptic spread causing tau pathology to appear in hippocampal pyramidal neurons. This suggests that propagation of tau pathology is an active process, associated with synapses, and might not be due solely to the release of tau from dying neurons. This indicates therefore that tau release from healthy neurons could be a physiological process that might be disrupted in diseased brain.
Several recent studies suggest that tau, which is viewed primarily as a microtubule-associated protein, is released from viable cells, often in association with membrane-bound exosomes or vesicles [7,8,9,10,11]. However, the majority of these studies measured the tau content in culture medium from cell lines overexpressing exogenous human tau, or from non-mammalian neurons. While the physiological relevance of these observations is not certain, these findings are supported by the recent observation that endogenous tau is released from induced pluripotent stem cell-derived human neurons in the absence of marked cell death [10]. Taken together, these observations imply that tau release from cells might be a physiologically regulated process. However, the mechanism underlying neuronal tau release is not yet understood.
We have examined the mechanisms underlying endogenous tau release from mature cortical neurons in culture. We show here that tau is released from neurons in the absence of cell death and that this process is regulated by neuronal activity. We provide evidence that stimulation of AMPA, but not NMDA, receptors increases tau release through a mechanism that is dependent on calcium and exocytosis of pre-synaptic vesicles, and that secreted tau is largely non-exosomal. These data suggest that altered tau release is likely to occur in response to regional changes in neuronal excitability in the Alzheimer’s brain, and that secreted tau might underlie the propagation of tau pathology in tauopathies. Therefore, targeting tau release could be explored as a new therapeutic approach for the treatment of Alzheimer’s disease and related tauopathies.
RESULTS AND DISCUSSION
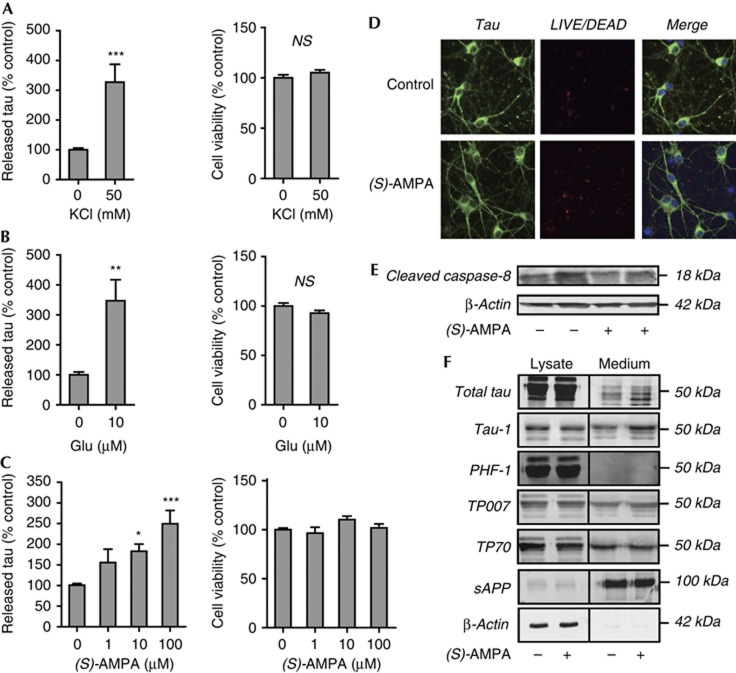
To determine whether stimulation of neuronal activity results in the release of endogenous tau, we treated primary cortical cultures with 50 mM KCl and tau release was quantified using a sensitive and specific sandwich enzyme-linked immunosorbent assay (ELISA) [12]. The amount of tau detected in the culture medium of treated neurons was significantly increased by KCl (Fig 1A, left). 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium (MTS) assay (Fig 1A, right) showed that tau release induced by KCl occurred in the absence of any changes in cell viability. Next, to investigate whether excitatory stimulation of neurotransmitter receptors regulates tau release, cultured cortical neurons were treated with glutamate (10 μM) for 30 min. Glutamate treatment significantly increased tau secretion (Fig 1B, left) in the absence of cytotoxicity (Fig 1B, right). To determine which glutamatergic receptors are involved in the regulation of tau release, neurons were treated with non-toxic concentrations (1, 10 and 100 μM) [13] of the AMPA receptor agonist (S)-AMPA for 30 min. We found that AMPA receptor stimulation dose dependently increased tau release, with 10 μM and 100 μM (S)-AMPA significantly increasing the amount of tau detected in the culture medium when compared with control cells (Fig 1C, left). We verified that AMPA treatment did not affect neuronal viability, as determined by MTS assay (Fig 1C, right) and LIVE/DEAD assay (Fig 1D). We also found that (S)-AMPA treatment does not activate the initiator caspase, caspase-8, as the amount of active caspase-8 fragment, p18, was unchanged in treated neurons when compared with controls (Fig 1E). This finding indicates that tau release in response to (S)-AMPA treatment is not evoked by the induction of neuronal stress.
Figure 1.
Neuronal activity stimulates tau release from neurons. (A) KCl depolarization (50 mM; 30 min), (B) glutamate treatment (10 μM Glu; 30 min) or (C) (S)-AMPA (1 μM, 10 μM and 100 μM; 30 min) increase tau release without altering neuronal viability. Tau release was quantified by ELISA and cell viability was determined by MTS (A–C) or (D) LIVE/DEAD assays. Neurons in (D) were visualized using antibody against tau (green) and nuclei were stained with Hoechst 33352 (blue). Red labelling indicates dead cells that have taken up LIVE/DEAD dye. (E) Activity of initiator caspase-8 was determined by immunoblot analysis of the active 18 kDa fragment, p18. (S)-AMPA treatment (100 μM, 4 h) does not result in activation of caspase-8, demonstrating that (S)-AMPA treatment does not induce neuronal stress under these conditions. (F) Tau released from neurons is largely dephosphorylated. Western blots show that intracellular tau is labelled by all tau antibodies. Extracellular tau is positive for Tau-1 but negative for PHF-1, indicating dephosphorylation at Ser199/202/Thr205 and Ser396/404. Extracellular tau is positive for antibodies directed against both the N and C termini (TP007 and TP70, respectively), indicating that released tau is not truncated. (S)-AMPA (100 μM, 4 h) increases extracellular tau, without affecting sAPP secretion. Absence of β-actin in the medium confirms the lack of contamination by intracellular protein. Values represent mean±s.e.m. (N=6); *P<0.05, **P<0.01; ***P<0.001. ELISA, enzyme-linked immunosorbent assay; Glu, glutamate; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium; NS, not significant; (S)-AMPA, (S)-2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)-propanoic acid; sAPP, soluble amyloid precursor protein.
Examination of conditioned medium on western blots confirmed that untreated neurons secrete a modest amount of tau, and that the amount of extracellular tau was substantially increased on (S)-AMPA application (Fig 1F). Neuronal culture medium was enriched for amyloid precursor protein (APP), significant amounts of which are secreted from neurons [14], but APP secretion was not increased by (S)-AMPA. The culture medium was devoid of β-actin, confirming that the presence of tau in medium was not owing to contamination by intracellular protein from degenerating neurons.
We next examined the phosphorylation state of the released tau and found that extracellular tau in neuronal culture medium has characteristics of dephosphorylation, including reduced mobility on SDS–polyacrylamide gel electrophoresis (Fig 1F). Moreover, immunodetection of extracellular tau with phospho-specific antibodies that detect tau phosphorylated at Ser396/404 (PHF-1) or non-phosphorylated tau at Ser199/202/Thr205 (Tau-1) demonstrate that tau collected from the medium of primary neurons is largely dephosphorylated (Fig 1F). These findings are in agreement with a previous report that extracellular tau released following death of neuroblastoma cells is dephosphorylated at several epitopes, possibly owing to the activity of an extracellular phosphatase [15]. Furthermore, Chai et al [10] report that heterologous tau secreted from non-neuronal cells is not highly phosphorylated on threonine 181. To investigate whether cell stress that results in altered tau phosphorylation might impact on its release, we pretreated neurons with 0.1 μM Aβ1–42 for 24 h before stimulating with KCl (50 mM, 30 min). Neurons treated with Aβ1–42 contained more intracellular tau phosphorylated at the Tau-1 epitope (supplementary Fig 1A) but this did not result in any change in the amount of tau released (supplementary Fig 1B). Furthermore, Aβ1–42 pretreatment before KCl stimulation of neurons resulted in an intermediate amount of tau release, which was not statistically different from either untreated or KCl-stimulated neurons (supplementary Fig 1B). In a previous study, we have also shown that tau associated with the neuronal plasma membrane is largely dephosphorylated, which suggests that this pool of dephosphorylated, non-microtubule-associated tau might be released by neurons [16]. In addition to phosphorylation status, we examined whether tau released by neurons was truncated at either the amino or carboxy termini. We found that antibodies directed against either a tau N-terminal epitope (TP007) or C-terminal epitope (TP70) both detected extracellular tau (Fig 1F), indicating that the tau released from neurons is intact. This finding is in contrast to those of Plouffe et al [17], who report that overexpression of tau in Hela cells results in release of C-terminally cleaved tau. In their study, tau secretion was apparently enhanced by either phosphorylation or cleavage of intracellular tau, as determined by measuring tau release in cells overexpressing phospho-mimic mutant or truncated tau species. These authors suggest that such increased tau phosphorylation and cleavage, as occurs in Alzheimer’s disease [18], could lead to tau accumulation in cerebrospinal fluid. Taken together, these studies suggest that changes in tau phosphorylation could be involved in modulating its release from neurons.
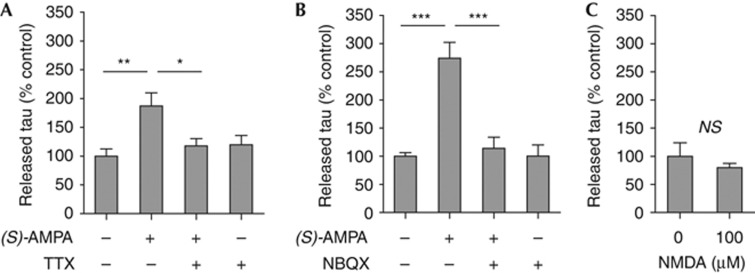
To determine whether neuronal activation is responsible for (S)-AMPA-stimulated tau release, we pre-treated neurons with the sodium channel blocker tetrodotoxin (TTX; 2 μM) for 6 h before (S)-AMPA treatment. TTX significantly prevented (S)-AMPA-mediated tau release (Fig 2A). Furthermore, treatment with the specific AMPA receptor antagonist 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo[f]quinoxaline-2,3-dione (NBQX; 100 μM) also inhibited (S)-AMPA stimulation of tau release (Fig 2B). In contrast, NMDA receptor stimulation (NMDA; 100 μM) for 30 min did not induce tau release (Fig 2C), although a similar concentration of NMDA was shown to activate NMDA receptors in cultured neurons [19]. These data suggest that tau release is induced by stimulation of specific receptors and that neuronal tau release is a regulatable physiological process that is mediated by neuronal activity.
Figure 2.
(S)-AMPA stimulation of tau release depends on neuronal activity. (A) Inhibition of neuronal activity (2 μM TTX) or (B) blockade of AMPA receptors (100 μM NBQX) prevents (S)-AMPA-induced (100 μM, 30 min) release of tau. (C) NMDA (100 μM; 30 min) receptor stimulation does not affect the amount of extracellular tau. Values represent mean±s.e.m. N=6; *P<0.05, **P<0.01; ***P<0.001. NBQX, 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo[f]quinoxaline-2,3-dione; NMDA, N-methyl-D-aspartate; NS, not significant; (S)-AMPA, (S)-2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)-propanoic acid; TTX, tetrodotoxin.
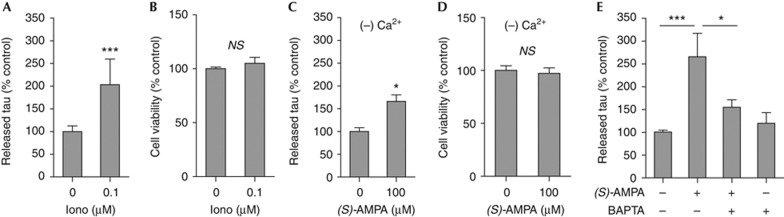
As AMPA receptor stimulation generates action potentials that result in increased pre-synaptic calcium concentrations, evoking vesicle release [20], we hypothesized a role for calcium in regulating neuronal tau release. To determine whether changes in intracellular calcium are necessary for stimulation of tau secretion, we first treated neurons with an ionophore. Ionomycin (0.1 μM) mimicked the stimulatory effect of (S)-AMPA on tau secretion (Fig 3A), without affecting neuronal viability (Fig 3B). To determine whether (S)-AMPA-induced tau release is dependent on calcium influx, we treated neurons with (S)-AMPA in calcium-free culture medium. Stimulation of tau release by (S)-AMPA was significantly reduced in the absence of calcium (Fig 3C), without having any effect on neuronal viability (Fig 3D). (S)-AMPA-induced tau release was not completely abolished in calcium-free medium, suggesting that calcium release from intracellular stores on stimulation could also have a role in modulating neuronal tau release. Therefore, neurons were treated with the cell-permeable calcium chelator BAPTA-AM at a concentration that has been shown not to affect neuronal viability [21]. Application of 1 μM BAPTA-AM before (S)-AMPA substantially reduced the amount of tau release stimulated by (S)-AMPA (Fig 3E). These findings indicate that alteration in intraneuronal calcium, such as occurs on neuronal stimulation, evokes tau release from primary cortical neurons.
Figure 3.
(S)-AMPA-induced neuronal tau release is regulated by intracellular calcium. (A) Tau secretion is induced by Iono (0.1 μM, 30 min), without any change in neuronal viability (B; MTS assay). (C) Treatment of neurons with the calcium chelator BAPTA-AM (1 μM) prevents (S)-AMPA (100 μM, 30 min) stimulation of tau release. (D) Tau release from neurons in calcium-free medium is blunted, although it remains significantly increased following (S)-AMPA (100 μM, 30 min), in the absence of cytotoxicity (E; MTS assay). Values represent mean±s.e.m. (N=6); *P<0.05, ***P<0.001. BAPTA-AM, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraaceticacid-tetra(acetoxymethyl)ester; Iono, ionomycin; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium; NS, not significant; (S)-AMPA, (S)-2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)-propanoic acid.
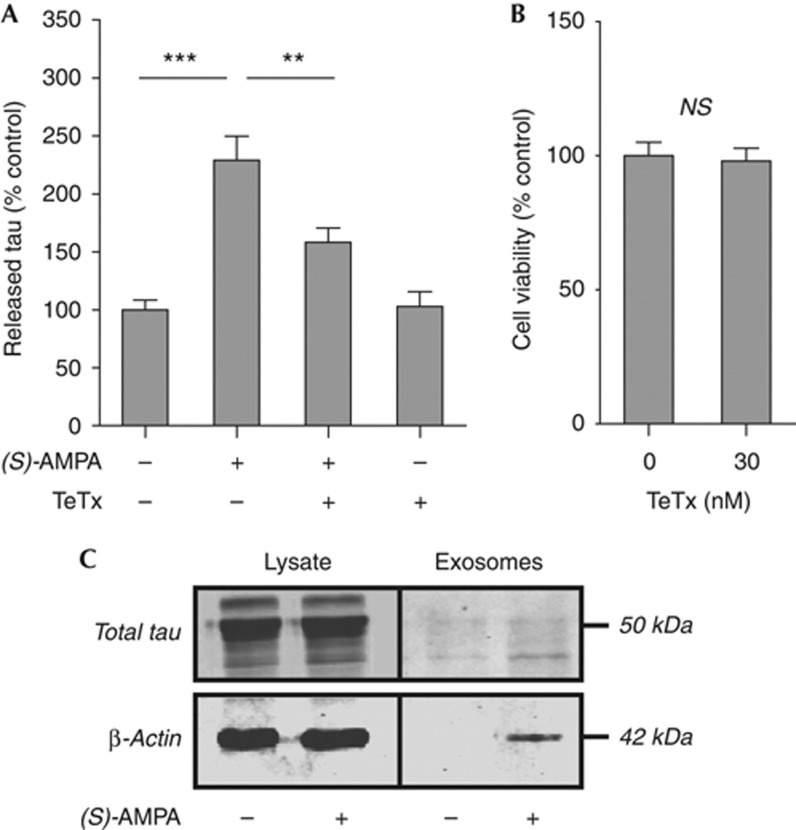
In vivo models of tauopathy suggest that inter-neuronal tau transfer might occur trans-synaptically [5, 6]. Therefore, we examined whether AMPA-stimulated tau release from neurons is dependent on secretion of pre-synaptic vesicles. Neurons were treated with tetanus toxin (TeTx), which cleaves synaptobrevin, thereby blocking synaptic vesicle secretion [22], before addition of (S)-AMPA. TeTx (30 nM, 1 h) significantly attenuated (S)-AMPA-induced extrusion of tau from neurons (Fig 4A) in the absence of neurotoxicity (Fig 4B). However, TeTx treatment did not completely block AMPA-dependent tau secretion, suggesting that further mechanisms might also govern tau release. Nevertheless, these results indicate that AMPA-stimulated tau release is dependent, at least in part, on pre-synaptic vesicle secretion.
Figure 4.
Tau release is mediated by a pre-synaptic mechanism. (A) Neurons treated with 30 nM TeTx (1 h) before (S)-AMPA (100 μM, 30 min) show significantly reduced tau release. (B) TeTx does not influence neuronal viability (MTS assay). (C) Tau is barely detectable in exosomes isolated from control or (S)-AMPA-stimulated (100 μM, 4 h) neurons. Values represent mean±s.e.m. (N=6); **P<0.01, ***P<0.001. MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium; NS, not significant; (S)-AMPA, (S)-2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)-propanoic acid; TeTx, tetanus toxin.
Studies in cells overexpressing exogenous tau have suggested that tau release might be mediated by exosomes and/or membrane vesicles [7,8,9,]. However, although stimulating exosome release from cortical neurons with (S)-AMPA did increase the amount of β-actin detected in the exosomal fraction [23], the amount of tau in exosomes was barely detectable (Fig 4C). In agreement with this finding, previous reports show that tau is not detected in exosomes isolated from primary cortical neurons or tau-overexpressing neuroblastoma cells [24]. However, other reports suggest that in neuroblastoma cells exogenously expressing tau, extracelluar tau might be both exosomal and non-exosomal [9]. Our data indicate that AMPA receptor-mediated release of endogenous tau is unlikely to occur through neuronal exosome secretion. Significantly, this finding also distinguishes the mechanisms governing physiological tau release from the unconventional protein secretion of other proteins involved in neurodegeneration. For example, α-synuclein, although also reported to be released through a calcium-dependent process, is detected in exosomes secreted from neuroblastoma cells [25].
It is apparent, therefore, that release of endogenous tau from neurons is a physiological process, dysregulation of which could have a role in the spreading of tau pathology through Alzheimer’s disease brain [26]. Moreover, our results indicate that the transmission of tau pathology in animal models of neurodegenerative tauopathies, in which disease-associated tau mutations are expressed in transgenic mouse brain, could result from the pre-synaptic release of soluble tau on neuronal stimulation [5, 6]. Interestingly, clusters of hyperactive neurons are detected in proximity to plaques in the brain of a mouse model of Alzheimer’s disease in which mutant forms of APP and presenilin-1 are expressed [27]. Although it has not yet been examined, it is plausible therefore that tau release could be exacerbated in the affected brain areas.
We conclude therefore that release of tau from cortical neurons is a physiological and regulatable process that is mediated by neuronal activity, and this is likely to occur through a pre-synaptic mechanism rather than by extrusion of exosomes. Our results strengthen the argument that, in addition to stabilization of microtubules, tau has other, previously undescribed roles, including activation of post-synaptic muscarinic receptors and participation in inter-neuronal signalling [16, 28, 29]. Indeed, extracellular tau has been demonstrated to directly activate muscarinic acetylcholine receptors [30], suggesting a direct role in cell–cell signalling. Furthermore, synaptic release of tau might result in its uptake by post-synaptic neurons, and alterations in this process could account for trans-synaptic tau transmission in Alzheimer’s disease.
METHODS
Reagents. The following compounds were obtained from Tocris Bioscience (Bristol, UK): (s)-α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ((S)-AMPA); 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide disodium salt (NBQX); and N-methyl-D-aspartic acid (NMDA). TTX was purchased from Merck Chemicals Ltd (Nottingham, UK). TeTx, purified from Clostridium tetani, was obtained from Calbiochem (Merck, Feltham, UK). Ionomycin and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) were obtained from Sigma-Aldrich (Gillingham, UK). Synthetic Aβ1–42 was purchased from rPeptide (Bogart, GA, USA) and was dissolved in 0.2% NH4OH before use. All compounds were dissolved in deionized water except ionomycin and BAPTA-AM, which were prepared in dimethyl sulphoxide. In experiments using ionomycin or BAPTA-AM, an equal amount of dimethyl sulphoxide was added to all treatment groups. All stock solutions were stored at −20°C.
Cell culture. Primary cortical neuronal cultures were prepared from embryonic day 18 Sprague–Dawley rat embryos (Charles River, Margate, UK) as described previously [16]. Animals were maintained and treated in accordance with the Animals (Scientific Procedures) Act, 1986, following approval by the local ethical review committee. All experiments were performed on neurons cultured for 14 days in vitro.
Cell viability assay. Cell viability was assessed using the Celltiter 96 Aqueous non-radioactive cell proliferation (MTS) assay (Promega, Southampton, UK). Primary rat cortical neurons were cultured in 12-well plates and treated as described above. After removal of the medium, CellTiter 96 Aqueous One Solution Reagent was diluted in Neurobasal medium and added to each well, according to the manufacturer’s instructions. Neuronal viability was determined by measuring absorbance at 490 nm using a Victor3 multilabel plate reader (Perkin Elmer, Cambridge, UK). Analysis of neuronal death using the Live/Dead Cell Viability Assay (Life Technologies Ltd, Paisley, UK) was performed according to the manufacturer’s instructions. Cells were additionally immunolabelled with an antibody against tau (rabbit polyclonal; DAKO, Glostrup, Denmark) to visualize neuronal morphology, and nuclei were stained with Hoechst 33352 [31].
Tau ELISA. Culture medium was removed from neurons and centrifuged at 12,000g for 10 min at 4°C to remove cell debris. Tau content in the medium was determined by ELISA, as described previously [12]. Absorbance was read at 450 nm with a Victor3 multilabel plate reader (Perkin Elmer).
Concentration of extracellular tau. Rat cortical neurons (70–80% confluence) were treated with the indicated compounds diluted in Hanks’ Balanced Salt Solution (Life Technologies Ltd). Following treatment, Hanks’ Balanced Salt Solution was collected and centrifuged at 12,000g for 10 min at ambient temperature and the supernatant was concentrated ∼50-fold using Amicon Ultra-0.5 Centrifugal Filter Units (Ultracel-30 membrane; Millipore, Watford, UK).
Gel electrophoresis and western blotting. Samples were heated at 100°C for 10 min in Laemmli SDS–polyacrylamide gel electrophoresis sample buffer, and centrifuged at 12,000g for 10 min at ambient temperature. Equal amounts of protein were loaded onto 10% (w/v) SDS–polyacrylamide gels. Separated proteins were blotted onto nitrocellulose membranes (Whatman, Maidstone, UK) and blocked in 5% (w/v) non-fat milk/0.05% (v/v) Tween-20 in phosphate-buffered saline for 1 h. After blocking, membranes were incubated overnight at 4°C in blocking solution containing primary antibody. The antibodies used were directed against total tau (rabbit polyclonal; DAKO), β-actin (mouse monoclonal; Abcam, Cambridge, UK), PHF-1 (phospho Ser396/404, mouse monoclonal; gift from P. Davies), Tau-1 (dephospho Ser199/202, mouse monoclonal; Millipore), TP007 (tau N terminus; rabbit polyclonal [19]), TP70 (tau C terminus; rabbit polyclonal [32]), APP (mouse monoclonal; Millipore) and active caspase-8 (rabbit polyclonal; Cell Signaling, Danvers, USA). After washing three times in phosphate-buffered saline containing 0.05% (v/v) Tween-20, blots were incubated with IRDye800-conjugated goat anti-rabbit (Rockland, Gilbertsville, PA, USA), Alexa-Fluor680-conjugated goat anti-mouse (Life Technologies Ltd) or horseradish peroxidase-conjugated donkey anti-rabbit (GE Healthcare, Little Chalfont, UK; for caspase-8 blots) secondary antibodies for 1 h at ambient temperature. Proteins were visualized using the Odyssey imaging system (LI-COR Biosciences, Cambridge, UK), or enhanced chemiluminescence (for caspase-8). Scanned images were analysed using ImageJ (http://rsb.info.NIH.gov/nih-image/).
Exosome preparation. Exosomes were prepared from treated and untreated rat cortical neurons as described [33]. Briefly, conditioned medium was collected and centrifuged at 1,000g for 10 min at 4°C, and the supernatant was centrifuged at 12,000g for 20 min at 4°C, to remove cell debris. Exosomes were pelleted from the resulting supernatant by centrifugation at 120,000g for 1 h at 4°C.
Data analysis. Statistical analyses were performed using Excel and GraphPad Prism 5.0 (Graph Pad Software, La Jolla, CA, USA). One-way analysis of variance was used to determine differences between groups (significance level, P<0.05) with post hoc analysis using Dunnett’s or Tukey’s multiple comparison tests. Data are presented as mean±s.e.m. and each experiment was performed in triplicate (N=6 per condition).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Professor Peter Davies (Albert Einstein College of Medicine), for generously providing tau antibodies, and Professor K. Peter Giese (King’s College London), for critical reading of the manuscript. This work was supported by grants from the Henry Smith Charity, Alzheimer’s Research UK and The Wellcome Trust.
Author contributions: A.M.P., E.C.P. and D.H.W.L. performed the research and analysed the data. A.M.P., W.N. and D.P.H. designed the research and wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: 239–259 [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70: 960–969 [DOI] [PubMed] [Google Scholar]
- Clavaguera F et al. (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11: 909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI (2009) Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284: 12845–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A et al. (2012) Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73: 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K (2012) Trans-synaptic spread of tau pathology in vivo. PLoS One 7: e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim W, Li Z, Hall GF (2012) Accumulation of vesicle-associated human tau in distal dendrites drives degeneration and tau secretion in an in situ cellular tauopathy model. Int J Alzheimers Dis 2012: 172837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Garcia-Garcia E, Royo F, Falcon-Perez JM, Avila J (2012) Proteostasis of tau. Tau overexpression results in its secretion via membrane vesicles. FEBS Lett 586: 47–54 [DOI] [PubMed] [Google Scholar]
- Saman S et al. (2012) Exosome-associated Tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287: 3842–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Dage JL, Citron M (2012) Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol Dis 48: 356–366 [DOI] [PubMed] [Google Scholar]
- Karch CM, Jeng AT, Goate AM (2012) Calcium phosphatase calcineurin influences tau metabolism. Neurobiol Aging 34: 374–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatterer SD, Tremblay MA, Acker CM, Davies P (2011) Neuronal c-Abl overexpression leads to neuronal loss and neuroinflammation in the mouse forebrain. J Alzheimers Dis 25: 119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Trelles R, Solana-Lopez A, Fernandez-Gonzalez JR, Novelli A, Fernandez-Sanchez MT (1999) Terfenadine induces toxicity in cultured cerebellar neurons: a role for glutamate receptors. Amino Acids 16: 59–70 [DOI] [PubMed] [Google Scholar]
- Wang C, Wurtman RJ, Lee RK (2000) Amyloid precursor protein and membrane phospholipids in primary cortical neurons increase with development, or after exposure to nerve growth factor or Abeta(1-40). Brain Res 865: 157–167 [DOI] [PubMed] [Google Scholar]
- Diaz-Hernandez M, Gómez-Ramos A, Rubio A, Gómez-Villafuertes R, Naranjo JR, Miras-Portugal MT, Avila J (2010) Tissue-nonspecific alkaline phosphatase promotes the neurotoxicity effect of extracellular tau. J Biol Chem 285: 32539–32548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler AM, Usardi A, Evans CJ, Philpott KL, Noble W, Hanger DP (2012) Dynamic association of tau with neuronal membranes is regulated by phosphorylation. Neurobiol Aging 33: 431 e427–38 [DOI] [PubMed] [Google Scholar]
- Plouffe V et al. (2012) Hyperphosphorylation and cleavage at d421 enhance tau secretion. PLoS One 7: e36873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger DP, Anderton BH, Noble W (2009) Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med 15: 112–119 [DOI] [PubMed] [Google Scholar]
- Davis DR et al. (1995) The phosphorylation state of the microtubule-associated protein tau as affected by glutamate, colchicine and beta-amyloid in primary rat cortical neuronal cultures. Biochem J 309: 941–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y, Luccarelli J, Kim M, Wang M, Sulzer D (2009) Glutamate controls growth rate and branching of dopaminergic axons. J Neurosci 29: 11973–11981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan J, Galindo MF, Gonzalez-Garcia C, Cena V (2003) Role and regulation of p53 in depolarization-induced neuronal death. Neuroscience 122: 707–715 [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C (1992) Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359: 832–835 [DOI] [PubMed] [Google Scholar]
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R (2011) Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci 46: 409–418 [DOI] [PubMed] [Google Scholar]
- Santa-Maria I, Varghese M, Ksiezak-Reding H, Dzhun A, Wang J, Pasinetti GM (2012) Paired helical filaments from Alzheimer disease brain induce intracellular accumulation of Tau protein in aggresomes. J Biol Chem 287: 20522–20533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30: 6838–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K (2011) Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol 121: 589–595 [DOI] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O (2008) Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321: 1686–1689 [DOI] [PubMed] [Google Scholar]
- Gomez-Ramos A, Diaz-Hernandez M, Cuadros R, Hernandez F, Avila J (2006) Extracellular tau is toxic to neuronal cells. FEBS Lett 580: 4842–4850 [DOI] [PubMed] [Google Scholar]
- Reynolds CH et al. (2008) Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cgamma1, Grb2, and Src family kinases. J Biol Chem 283: 18177–18186 [DOI] [PubMed] [Google Scholar]
- Gomez-Ramos A, Diaz-Hernandez M, Rubio A, Miras-Portugal MT, Avila J (2008) Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol Cell Neurosci 37: 673–681 [DOI] [PubMed] [Google Scholar]
- Garwood CJ, Pooler AM, Atherton J, Hanger DP, Noble W (2011) Astrocytes are important mediators of Abeta-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis 2: e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion JP, Couck AM, Robertson J, Loviny TL, Anderton BH (1993) Neurofilament monoclonal antibodies RT97 and 8D8 recognize different modified epitopes in paired helical filament-tau in Alzheimer’s disease. J Neurochem 60: 1372–1382 [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM (2011) Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 42: 360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.