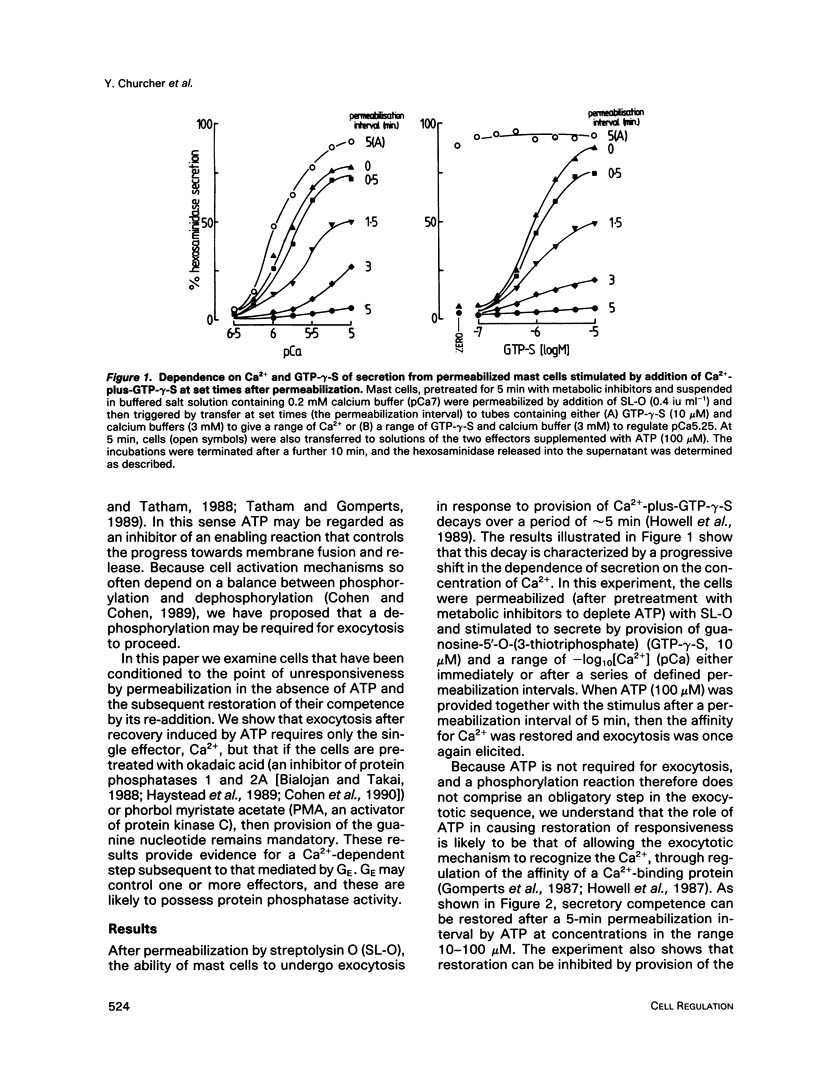
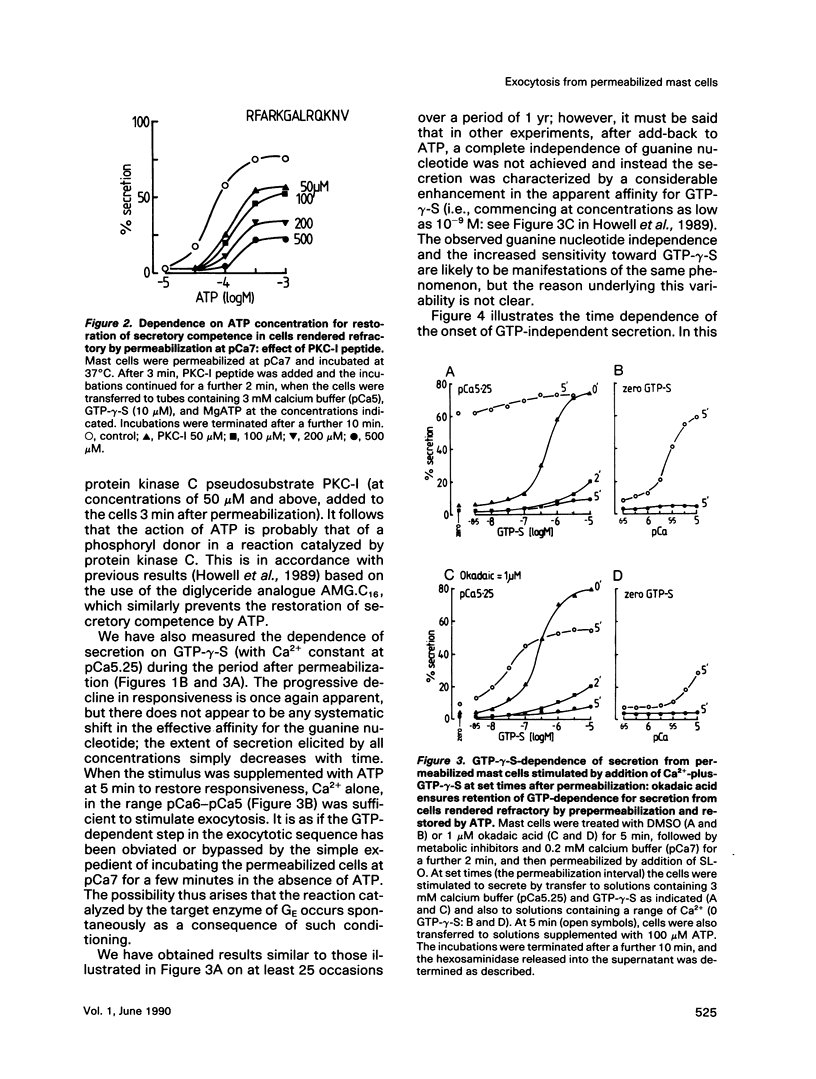
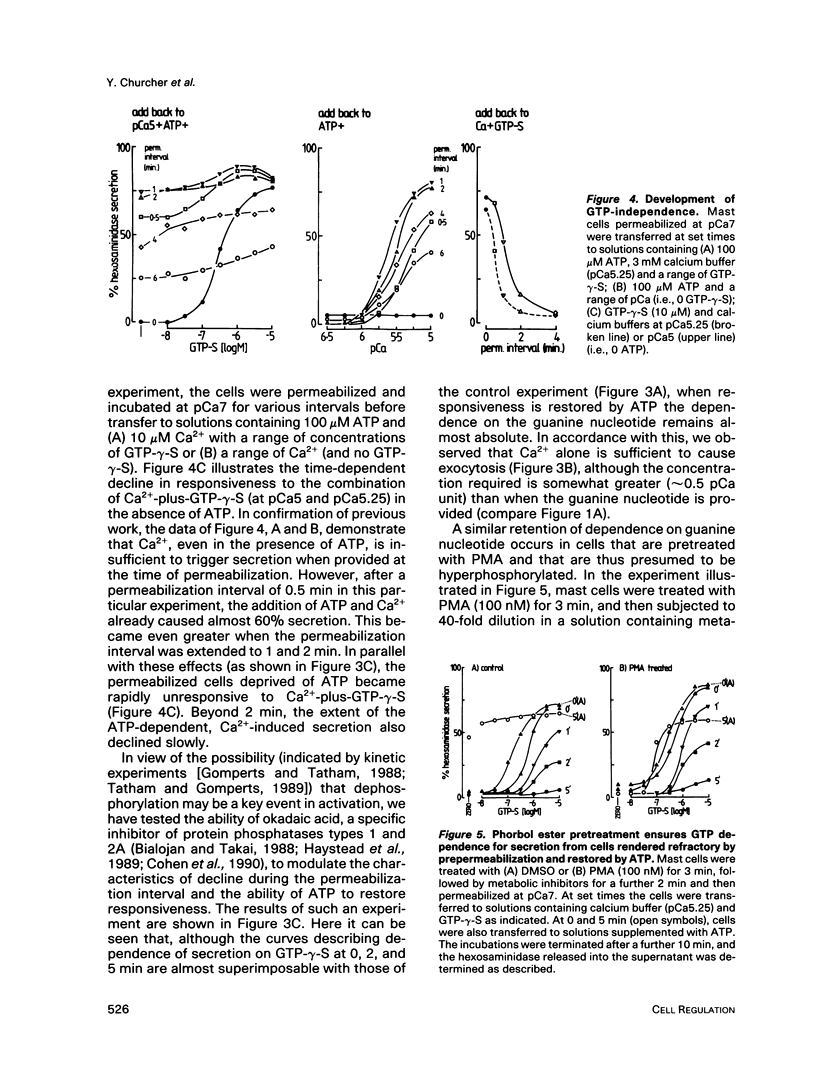
Abstract
Mast cells permeabilized by streptolysin O secrete histamine and lysosomal enzymes in response to provision of a dual effector system comprising Ca2+ and a guanine nucleotide (e.g., GTP-gamma-S2) at concentrations in the micromolar range. These are both necessary and together they are sufficient. There is no requirement for adenosine triphosphate (ATP) and hence no obligatory phosphorylation reaction in the terminal stages of the exocytotic pathway. When exocytosis is induced by Ca2(+)-plus-GTP-gamma-S (i.e., no ATP) added at times after permeabilization (the permeabilization interval), cellular responsiveness declines so that there is no response to provision of the two effectors (both at 10(-5)M) if they are initially withheld and then added after 5 min. Here we show that this decline in responsiveness is characterized by a time-dependent reduction in the effective affinity for Ca2+. Affinity for Ca2+ and hence secretory competence can then be restored if ATP is added alongside the stimulus. Unlike cells stimulated to secrete at the time of permeabilization, exocytosis from cells that have undergone the cycle of permeabilization-induced refractoriness followed by ATP-induced restoration can be triggered by Ca2+ alone: after such conditioning there is no requirement for guanine nucleotide. In contrast, dependence on guanine nucleotide remains mandatory in cells that have been pretreated (i.e., before permeabilization) with okadaic acid (understood to be an inhibitor of protein phosphatases 1 and 2A) or phorbol myristate acetate (an activator of protein kinase C). These results indicate that obligatory dependence on guanine nucleotide is retained when the cells are treated under conditions conducive to maintained phosphorylation. It is concluded that the exocytotic mechanism of permeabilized mast cells is enabled by a dephosphorylation reaction and that the effector of the guanosine triphosphate (GTP)-binding protein (G epsilon) that mediates exocytosis is likely to be a protein phosphate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Hexham J. M., Lucas S. C., Graves J. D., Cantrell D. A., Crumpton M. J. A protein kinase C pseudosubstrate peptide inhibits phosphorylation of the CD3 antigen in streptolysin-O-permeabilized human T lymphocytes. Biochem J. 1989 Jun 15;260(3):893–901. doi: 10.1042/bj2600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon R. A., Salminen A., Ruohola H., Novick P., Ferro-Novick S. The GTP-binding protein Ypt1 is required for transport in vitro: the Golgi apparatus is defective in ypt1 mutants. J Cell Biol. 1989 Sep;109(3):1015–1022. doi: 10.1083/jcb.109.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Beckers C. J., Balch W. E. Calcium and GTP: essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989 Apr;108(4):1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Brooks J. C., Brooks M. Protein thiophosphorylation associated with secretory inhibition in permeabilized chromaffin cells. Life Sci. 1985 Nov 18;37(20):1869–1875. doi: 10.1016/0024-3205(85)90003-7. [DOI] [PubMed] [Google Scholar]
- Brooks J. C., Treml S., Brooks M. Thiophosphorylation prevents catecholamine secretion by chemically skinned chromaffin cells. Life Sci. 1984 Jul 30;35(5):569–574. doi: 10.1016/0024-3205(84)90251-0. [DOI] [PubMed] [Google Scholar]
- Churcher Y., Gomperts B. D. ATP-dependent and ATP-independent pathways of exocytosis revealed by interchanging glutamate and chloride as the major anion in permeabilized mast cells. Cell Regul. 1990 Mar;1(4):337–346. doi: 10.1091/mbc.1.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D., Cockcroft S., Howell T. W., Nüsse O., Tatham P. E. The dual effector system for exocytosis in mast cells: obligatory requirement for both Ca2+ and GTP. Biosci Rep. 1987 May;7(5):369–381. doi: 10.1007/BF01362501. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. GE: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D., Tatham P. E. GTP-binding proteins in the control of exocytosis. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):983–992. doi: 10.1101/sqb.1988.053.01.113. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell T. W., Gomperts B. D. Rat mast cells permeabilised with streptolysin O secrete histamine in response to Ca2+ at concentrations buffered in the micromolar range. Biochim Biophys Acta. 1987 Feb 18;927(2):177–183. doi: 10.1016/0167-4889(87)90132-7. [DOI] [PubMed] [Google Scholar]
- Howell T. W., Kramer I. M., Gomperts B. D. Protein phosphorylation and the dependence on Ca2+ and GTP-gamma-S for exocytosis from permeabilised mast cells. Cell Signal. 1989;1(2):157–163. doi: 10.1016/0898-6568(89)90005-3. [DOI] [PubMed] [Google Scholar]
- Koffer A., Gomperts B. D. Soluble proteins as modulators of the exocytotic reaction of permeabilised rat mast cells. J Cell Sci. 1989 Nov;94(Pt 3):585–591. doi: 10.1242/jcs.94.3.585. [DOI] [PubMed] [Google Scholar]
- Kramer I. M., van der Bend R. L., Tool A. T., van Blitterswijk W. J., Roos D., Verhoeven A. J. 1-O-hexadecyl-2-Q-methylglycerol, a novel inhibitor of protein kinase C, inhibits the respiratory burst in human neutrophils. J Biol Chem. 1989 Apr 5;264(10):5876–5884. [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Colombo M. I., Stahl P. D. GTP gamma S stimulation of endosome fusion suggests a role for a GTP-binding protein in the priming of vesicles before fusion. Cell Regul. 1989 Nov;1(1):113–124. doi: 10.1091/mbc.1.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Novick P. J., Goud B., Salminen A., Walworth N. C., Nair J., Potenza M. Regulation of vesicular traffic by a GTP-binding protein on the cytoplasmic surface of secretory vesicles in yeast. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):637–647. doi: 10.1101/sqb.1988.053.01.073. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Satir B. H., Hamasaki T., Reichman M., Murtaugh T. J. Species distribution of a phosphoprotein (parafusin) involved in exocytosis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):930–932. doi: 10.1073/pnas.86.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Stecher B., Höhne B., Gras U., Momayezi M., Glas-Albrecht R., Plattner H. Involvement of a 65 kDa phosphoprotein in the regulation of membrane fusion during exocytosis in Paramecium cells. FEBS Lett. 1987 Oct 19;223(1):25–32. doi: 10.1016/0014-5793(87)80503-3. [DOI] [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Guanine nucleotides stimulate polyphosphoinositide phosphodiesterase and exocytotic secretion from HL60 cells permeabilized with streptolysin O. Biochem J. 1988 Mar 1;250(2):375–382. doi: 10.1042/bj2500375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham P. E., Gomperts B. D. ATP inhibits onset of exocytosis in permeabilised mast cells. Biosci Rep. 1989 Feb;9(1):99–109. doi: 10.1007/BF01117516. [DOI] [PubMed] [Google Scholar]
- Vilmart-Seuwen J., Kersken H., Stürzl R., Plattner H. ATP keeps exocytosis sites in a primed state but is not required for membrane fusion: an analysis with Paramecium cells in vivo and in vitro. J Cell Biol. 1986 Oct;103(4):1279–1288. doi: 10.1083/jcb.103.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner P. D., Vu N. D. Thiophosphorylation causes Ca2+-independent norepinephrine secretion from permeabilized PC12 cells. J Biol Chem. 1989 Nov 25;264(33):19614–19620. [PubMed] [Google Scholar]
- Zieseniss E., Plattner H. Synchronous exocytosis in Paramecium cells involves very rapid (less than or equal to 1 s), reversible dephosphorylation of a 65-kD phosphoprotein in exocytosis-competent strains. J Cell Biol. 1985 Dec;101(6):2028–2035. doi: 10.1083/jcb.101.6.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk W. J., van der Bend R. L., Kramer I. M., Verhoeven A. J., Hilkmann H., de Widt J. A metabolite of an antineoplastic ether phospholipid may inhibit transmembrane signalling via protein kinase C. Lipids. 1987 Nov;22(11):842–846. doi: 10.1007/BF02535541. [DOI] [PubMed] [Google Scholar]


